miR-21 promotes the differentiation of hair folliclederived neural crest stem cells into Schwann cells
2014-03-24YuxinNiKaizhiZhangXuejuanLiuTingtingYangBaixiangWangLiFuLanYanminZhou
Yuxin Ni, Kaizhi Zhang, Xuejuan Liu, Tingting Yang, Baixiang Wang, Li Fu, Lan A, Yanmin Zhou
1 Hospital of Stomatology, Jilin University, Changchun, Jilin Province, China
2 China-Japan Union Hospital, Jilin University, Changchun, Jilin Province, China
3 First Hospital of Jilin University, Changchun, Jilin Province, China
miR-21 promotes the differentiation of hair folliclederived neural crest stem cells into Schwann cells
Yuxin Ni1, Kaizhi Zhang2, Xuejuan Liu3, Tingting Yang1, Baixiang Wang1, Li Fu1, Lan A1, Yanmin Zhou1
1 Hospital of Stomatology, Jilin University, Changchun, Jilin Province, China
2 China-Japan Union Hospital, Jilin University, Changchun, Jilin Province, China
3 First Hospital of Jilin University, Changchun, Jilin Province, China
Hair follicle-derived neural crest stem cells can be induced to differentiate into Schwann cells in vivo and in vitro. However, the underlying regulatory mechanism during cell differentiation remains poorly understood. This study isolated neural crest stem cells from human hair follicles and induced them to differentiate into Schwann cells. Quantitative RT-PCR showed that microRNA (miR)-21 expression was gradually increased during the differentiation of neural crest stem cells into Schwann cells. After transfection with the miR-21 agonist (agomir-21), the differentiation capacity of neural crest stem cells was enhanced. By contrast, after transfection with the miR-21 antagonist (antagomir-21), the differentiation capacity was attenuated. Further study results showed that SOX-2 was an effective target of miR-21. Without compromising SOX2 mRNA expression, miR-21 can down-regulate SOX protein expression by binding to the 3′-UTR of miR-21 mRNA. Knocking out the SOX2 gene from the neural crest stem cells significantly reversed the antagomir-21 inhibition of neural crest stem cells differentiating into Schwann cells. The results suggest that miR-21 expression was increased during the differentiation of neural crest stem cells into Schwann cells and miR-21 promoted the differentiation through down-regulating SOX protein expression by binding to the 3′-UTR of SOX2 mRNA.
nerve regeneration; microRNA; stem cells; Schwann cells; SOX2; hair follicle; neural
crest stem cells; neurons; NSFC grant; neural regeneration
Funding: This work was supported by the National Natural Science Foundation of China, No. 81070855.
Ni YX, Zhang KZ, Liu XJ, Yang TT, Wang BX, Fu L, A L, Zhou YM. miR-21 promotes the differentiation of hair follicle-derived neural crest stem cells into Schwann cells. Neural Regen Res. 2014;9(8):828-836.
Introduction
The repair of injured peripheral nerve remains a great area of interest in neuroscience research. Recently, considerable attention has been paid to tissue engineering techniques, which are widely used in the repair of peripheral nerve injury (Gao et al., 2013; Marquardt and Sakiyama-Elbert, 2013; Pang et al., 2013). Schwann cells are part of the peripheral nervous system, and participate in nerve growth and regeneration, transmit nerve impulses, regulate the activity of motor nerves, and therefore are indispensable “seed cells” for peripheral nerve tissue engineering (Stefanescu et al., 2012; Nie et al., 2013). The majority of currently used Schwann cells are mainly the cells differentiated from autologous stem cells owing to the difficulties in harvesting autologous Schwann cells and the immunological rejection caused by allogeneic Schwann cells (Ren et al., 2012).
Neural crest stem cells, a kind of unique pluripotent stem cells in vertebrates, originate in the ectoderm at the margins of the neural tube in the embryonic stage and then develop into many tissues including the heart, smooth muscle and peripheral nerve (Hall, 2008; Sauka-Spengler and Bronner-Fraser, 2008; Huisman and Rivolta, 2012). Neural crest stem cells can be harvested from the heart, hair follicles, olfactory bulb and craniomaxillofacial tissue in adults (Achilleos and Trainor, 2012; Pelaez et al., 2013). Hair follicle-derived neural crest stem cells are mostly studied owing to the ease of harvesting and wide availability (Sieber-Blum and Grim, 2004). A previous study by our laboratory has shown that after induction by transforming growth factor-β1, hair follicle-derived neural crest stem cells can differentiate into smooth muscle cell-like cells expressing smooth muscle actin and calponin (Liu et al., 2013b). There is strong evidence that under the effects of different inducers, hair follicle-derived neural crest stem cells also possess the potential to differentiate into many different mature cell types including Schwann cells, melanocytes and neurons (El Seady et al., 2008; Sviderskaya et al., 2009; Lin et al., 2011; Dong et al., 2012). Accumulating evidence exists that hair follicle-derived neural crest stem cells, as an important source of Schwann cells, play a critical role in the repair of injured peripheral nerve by tissue engineering (Lin et al., 2009, 2011). However, the mechanism by which hair follicle-derived neural crest stem cells differentiateinto Schwann cells remains poorly understood and therefore brings dif fi culties for a better understanding of the regulation of Schwann cell differentiation (Bhatt et al., 2013). A recent study has shown that microRNAs (miRNAs), an important small molecule substance in vivo, play a crucial role in Schwann cell differentiation (Dugas and Notterpek, 2011).
miRNAs are a class of non-coding single-stranded small RNA molecules of 18-25 nucleotides that can bind to the 3′UTR of the mRNA molecules and regulate the protein expression of target gene (Amiel et al., 2012; Nana-Sinkam and Croce, 2013). Approximately 1,600 human miRNAs have been identified to participate in various pathophysiological processes, including stem cell differentiation, tumor formation and metastasis, cell apoptosis, in fl ammation and embryonic development (Garofalo et al., 2012; Kong et al., 2012; Rutnam and Yang, 2012; Koerner et al., 2013; Qi et al., 2013; Sethi et al., 2013). Studies using quantitative real time-PCR, microarray and northern blot technique have shown that miRNA expression alters during Schwann cell differentiation of precursor cells and subsequent myelination (Bremer et al., 2010; Verrier et al., 2010; Yun et al., 2010). After knocking out the Dicer1 gene (a key enzyme for miRNA biogenesis), Schwann cells do not have the capacity to form myelin sheaths, while after silencing Dicer1 gene expression, these cells greatly proliferated and cannot form myelin sheath with normal function (Dugas et al., 2010; Pereira et al., 2010). These fi ndings suggest that miRNAs likely regulate Schwann cell differentiation, myelination and peripheral nerve growth and development. Nevertheless, the miRNAs included in the regulation and the mechanisms by which miRNAs regulate Schwann cell differentiation need to be further studied.
miR-21 is a miRNA closely related to stem cell differentiation and neural regeneration (Ciof fi et al., 2010; Strickland et al., 2011). Yu et al. (2012a, b) reported that miR-21 expression was significantly increased in injured peripheral nerve tissue. However, a study using microarray technique has shown that miR-21 expression was gradually increased in myelinating Schwann cells of mouse sciatic nerve (Gokey et al., 2012). These fi ndings indicate that miR-21 is associated with Schwann cell differentiation and remyelination. However, to the best of our knowledge, the role of miR-21 in Schwann cell differentiation of stem cells and the underlying mechanisms are poorly understood. This study isolated neural crest stem cells from human hair follicle, induced them to differentiate into Schwann cells and detected miR-21 expression change using quantitative real time (RT)-PCR. In addition, intracellular miR-21 expression was interfered to investigate the effect of miR-21 expression on Schwann cell differentiation. This study further sought to fi nd the possible gene target of miR-21 responsible for regulating Schwann cell differentiation.
Materials and Methods
Materials
Human skin tissue containing hair follicles was provided by healthy adults (irrespective of sex and age) who were physically examined in the Department of Dermatology, Norman Bethune First Hospital, Jilin University, China. Informed consent was obtained from participants. Skin harvesting and use of samples was approved by the Ethics Committee, Jilin University, China. HEK-293 cells were purchased from Baoman Biotechnology Co., Ltd., Shanghai, China. Human Sox-2 shRNA lentiviral plasmids (sc-108080) and copGFP control lentiviral plasmids (sc-108084) were purchased from Santa Cruz Biotechnology, Dallas, TX, USA.
Isolation of hair follicle-derived neural crest stem cells
Fresh human skin tissue was harvested and then stored in ice-cold PBS. Neural crest stem cells were isolated using a previously published method (Krejci and Grim, 2010; Yu et al., 2010). Brie fl y, after several PBS washes and careful scraping of attached adipose tissue, skin tissue was cut into small blocks and then treated with Dulbecco’s Modified Eagle’s Medium (DMEM) containing 4.8 mg/mL dispase (Invitrogen, Grand Island, NY, USA) at 4°C overnight. Under a light microscope (Olympus, Shanghai, China), hair follicles were isolated from deciduous epidermis, collected and then washed with PBS. The hair follicles were treated twice with 0.25% trypsin/ethylenediamine tetraacetic acid (Invitrogen) for 30 minutes each. The resultant cell suspension was filtered with a 40-μm cell strainer (BD Falcon, Bedford, MA, USA). After counting, cells were seeded in a Petri dish, in which human embryonic stem cell medium (Gibco, Shanghai, China) containing 4 ng/mL basic fi broblast growth factor (Sino Biological, Beijing, China) was added. Cells were then incubated in a 5% CO2incubator at 37°C. Culture medium was renewed once every 3 days.
Screening of neural crest stem cells by fl ow cytometric cell sorting
Cells were collected, washed and then prepared into single cell suspension. Neural crest stem cells were screened using flow-cytometric cell sorting as described previously (Jiang et al., 2009; Yang and Xu, 2013). Briefly, cells were diluted with PBS to a fi nal concentration of 10-20 × 106cells/mL. They were treated with a mixture of human natural killer-1 and p75 mouse monoclonal antibody (1:100; BD Bioscience Pharmingen Inc., San Jose, CA, USA) in the tube at 4°C for 40 minutes. After washes with ice-cold PBS, cells were centrifuged at 4°C and 800 × g for 5 minutes. After re-suspension with PBS, cells were sorted by flow cytometry (BD Bioscience Pharmingen Inc.), and human natural killer-1/p75 double-positive cells (neural crest stem cells) were collected.
Induced differentiation of hair follicle-derived neural crest stem cells into Schwann cells
Neural crest stem cells were induced to differentiate into Schwann cells as published previously (Nie et al., 2013). Brie fl y, neural crest stem cells were collected and treated with MesenPRO medium (Invitrogen) containing 20 ng/mL neuregulin-1 (Sino Biological) in a 5% CO2incubator at 37°C for 28 days. The culture medium was replaced once every 2 days.
Oligonucleotide transfection
To investigate the effect of miR-21 on differentiation, 200 nmol/L of agomir-21, agomir-NC, antagomir-21 or antagomir-NC(Riobo, Guangzhou, China) were added into 1640 medium (Gibco) before induction. Agomir and antagomir were chemically modified with cholesterol-conjugated RNA molecules that could be easily transfected into cells without Lipofectamine 2000. Agomir and antagomir activated and inhibited endogenous miRNA, respectively.
Knock out of SOX2 gene from neural crest stem cells
Methods used for gene knock out were reported in our previous study (Liu et al., 2013b). Brie fl y, neural crest stem cells were seeded in a 24-well plate. When cells reached over 50% con fl uence, polybrebe (Santa Cruz Biotechnology) was added until a fi nal concentration of 5 μg/mL and then cells were transfected with human Sox-2 shRNA lentiviral plasmid (multiplicity of infection = 15). The plate was shaken once every 15 minutes to enhance the transfection ef fi cacy. Culture medium was then discarded the next day and cells were incubated for 1-2 days with 500 μL polybrene-free complete culture medium. Thereafter, the culture medium containing 8 μg/mL puromycin was replaced once every 3 days to kill non-transfected cells. Thus, the surviving cells were SOX2-KD-neural crest stem cells.
Group management
Prior to (0 week) and 1, 2, 3 and 4 weeks after induced differentiation, neural crest stem cells were collected and intracellular miR-21 expression was detected using quantitative real time (RT)-PCR. To investigate the effect of miR-21 expression on cell differentiation, prior to and 20 days after induction, neural crest stem cells were divided into five groups: namely agomir-21, agomir-NC, antagomir-21, antagomir-NC and control. Prior to and 20 days after induction, cells in the above four groups were treated with agomir-21, agomir-NC, antagomir-21 and antagomir-NC, respectively at a fi nal concentration of 200 μmol/L and cells in the last group were not treated. At 40 days of induction, cell differentiation in each group was observed using immuno fl uorescence staining and quantitative RT-PCR. To investigate the effect of miR-21 on SOX2 expression in neural crest stem cells, neural crest stem cells were seeded in a 12-well plate and then divided into fi ve groups: namely control, miR-21 mimic, mimic-NC, miR-21 inhibitor and inhibitor-NC. When cells reached over 50% confluence, miR-21 mimic, mimic-NC, miR-21 inhibitor and inhibitor-NC at a fi nal concentration of 200 μmol/L were added to the four groups, respectively. After incubation for 48 hours, SOX2 protein and mRNA expression was detected. To investigate whether SOX2 participates in miR-21 promotion of stem cell differentiation, neural crest stem cells were divided into antagomir-21 and antagomir-NC groups and cells in these two groups were treated with antagomir-21 and antagomir-NC, respectively, at a fi nal concentration of 200 μmol/L. SOX2-KD-neural crest stem cells were divided into antagomir-21 + SOX2 KD and antagomir-NC + SOX2 KD groups and treated with antagomir-21 and antagomir-NC, respectively, at a final concentration of 200 μmol/L. After induction for 40 days, the differentiation of neural crest stem cells was detected by immunofluorescence staining and quantitative RTPCR.
Detection of cell differentiation by fl ow cytometry
To investigate cell differentiation, neural crest stem cells were washed three times with PBS for 3 minutes at room temperature. Then cells were treated with 0.1% Triton for 10 minutes. After three PBS washes for 3 minutes each, cells were blocked with 10% serum for 1 hour, treated with mouse anti-human S100 monoclonal antibody labeled with FITC (BD Bioscience Pharmingen Inc.) and mouse anti-human glial fi brillary acidic protein monoclonal antibody labeled with Cy3 (BD Bioscience Pharmingen Inc.) at a ratio of 1:100 at 4°C. Thirty minutes later, cells were washed three times with PBS for 3 minutes each. Subsequently, cells were suspended with 0.3 mL PBS and analyzed with fl ow cytometry (BD Bioscience Pharmingen Inc.).
Detection of miR-21 and SOX2 mRNA expression by quantitative RT-PCR
miR-21 and SOX2 mRNA expression was detected by PCR according to a previously described method (Liu et al., 2013b). Briefly, total RNA was extracted using the Trizol method (Sigma-Aldrich, St. Louis, MO, USA) and cDNA synthesis was performed using EasyScript First-Strand cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China). cDNA (2.5 μL), 1 μL miR-21 specific primer (RiboBio, Guangzhou, Guangdong Province, China) or SOX2 mRNA primer, S100 and glial fi brillary acidic protein mRNA primer were mixed with TransStart™ SYBR Green qPCR Supermix (TransGen Biotech) and quantitative RT-PCR was performed to detect miR-21 and SOX2 mRNA expression. The green fl uorescence intensity of PCR product SYBR was detected using a PCR instrument (ABI, Grand Island, NY, USA). U6 was used as an internal control of miR-21 and GAPDH mRNA as an internal control of SOX2, S100 and glial fi brillary acidic protein mRNA.
Detection of SOX2 expression by western blot assay
Neural crest stem cells were collected, centrifuged and lysed for 30 minutes. Protein concentration was determined by the bicinchoninic acid method (Krejci and Grim, 2010). Each protein sample was subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, electrophoretically transferred onto a polyvinylidene fluoride, and blocked with 5% defatted milk for 2 hours. Each membrane was treated with rabbit anti-SOX2, GAPDH polyclonal antibody (Santa Cruz Biotechnology) at 1:2,000 dilution for 2 hours at room temperature, and rinsed with Tris-buffered saline with Tween, six times for 10 minutes each. After washing, membranes were treated with horseradish peroxidase-labeled goat anti-rabbit IgM (Santa Cruz Biotechnology) at 1:2,000 dilution for 2 hours at room temperature, and developed by enhanced chemiluminescence. GAPDH (Santa Cruz Biotechnology) was used as an internal control.
Detection of miR-21 effects on fl uorescence expression using Luciferase assay

Figure 1 Isolation of hair follicle-derived neural crest stem cells.
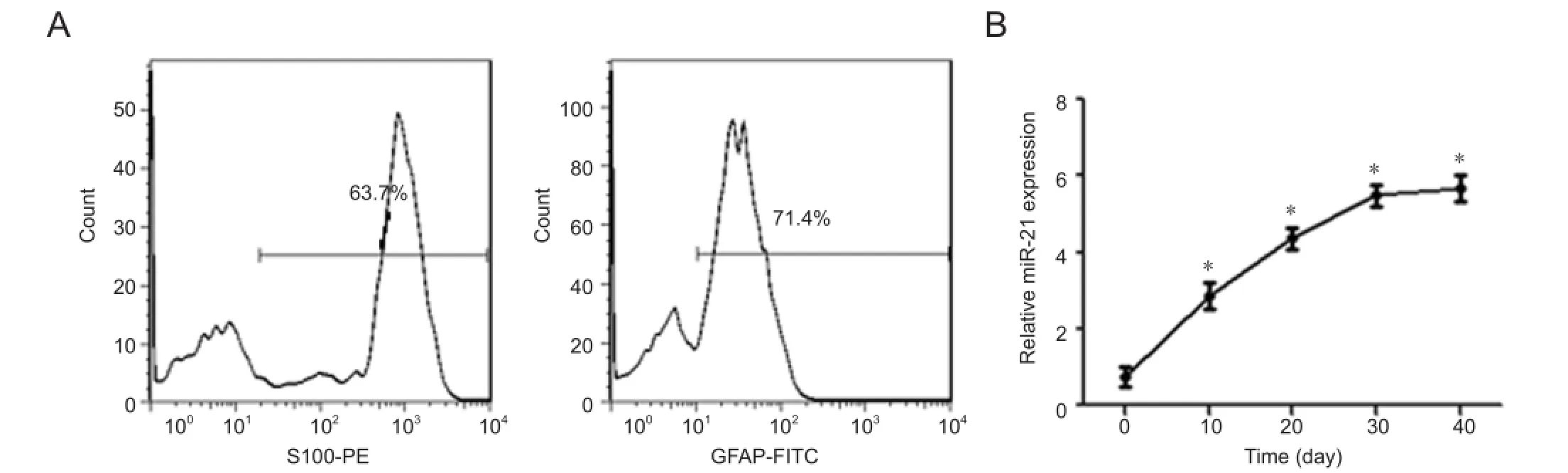
Figure 2 miR-21 expression during the differentiation of neural crest stem cells (NCSCs) into Schwann cells.
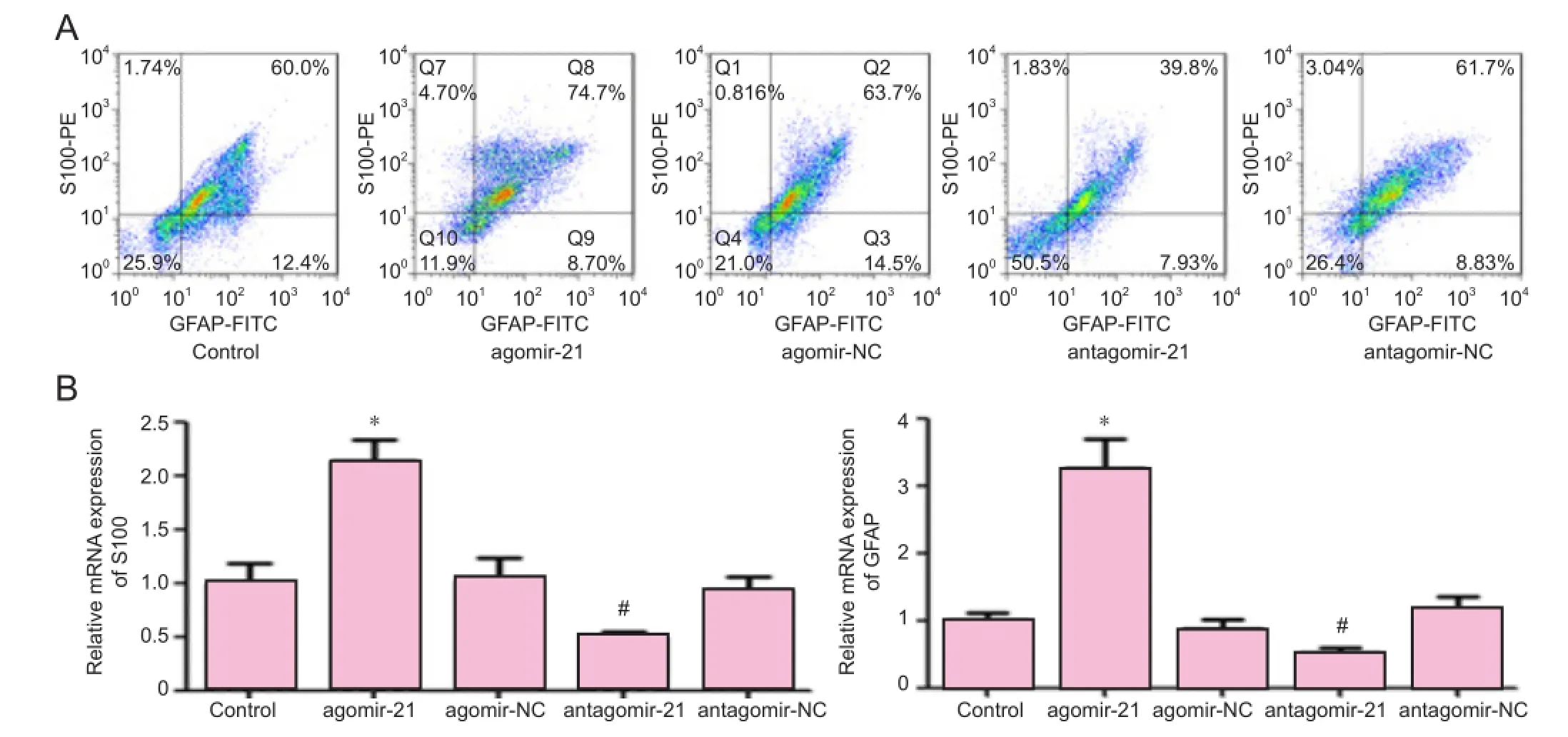
Figure 3 Regulatory role of miR-21 in the differentiation of neural crest stem cells.
To investigate the mechanism by which miR-21 promotes the differentiation of neural crest stem cells, we retrieved information for the miR-21 target from Pictar (http://pictar.mdc-berlin.de/cgi-bin/PicTar_vertebrate.cgi), Targetscan (http://www.targetscan.org/) and miRBase (www.mirbase. org) databases and found that a 7-mer-long sequence at the 3′UTR region of the SOX2 gene was matched to miR-21. To determine if this sequence could down-regulate SOX2 mRNA translation after binding to miR-21, the SOX2 mRNA 3′UTR containing this site was included in the luciferase reporter system (SOX2-3′UTR-wt) and simultaneously the mutational SOX2 mRNA 3′UTR was also inserted into this reporter system (SOX2-3′UTR-mut). miR-21 was transfected into HEK-293 cells together with luciferase empty vector, SOX2-3′UTR-wt or SOX2-3′UTR-mut. Subsequently, HEK-293 cells were cultured in RPMI 1640 culture medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (Thermo Fisher Scienti fi c Inc., Waltham, MA, USA) in a 5% CO2incubator at 37°C. Full length rat SOX2-3′UTR containing miR-21 binding site was ampli fi ed by PCR, and then cloned into the region between Hind III and Sac I sites in the downstream region and named SOX2-3′UTR-wt. After being subjected to point mutation in the Easy Mutagenesis System kit (TransGen Biotech), SOX2-3′UTR-wt was named as SOX2-3′UTR-mut. HEK293T cells were seeded in a 24-well plate and then divided into three groups: empty vector (400 ng empty vector was added), SOX2-3′UTR-wt (400 ng SOX2-3′UTR-wt plasmid was added) and SOX2-3′UTR-mut (400 ng SOX2-3′UTR-mut plasmid was added). In addition to lip2000 (Invitrogen), miR-21 and 20 ng pRL-TK (Ribobio, Guangzhou, China) were added as internal controls in each group. Two parallel wells were designated for each group, in which mimic-NC (Ribobio) rather than miR-21 mimic was added as negative control (miR-con). After transfection for 36 hours, cells were collected for fl uorescence intensity detection using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
Statistical analysis
All data were statistically processed using SPSS 18.0 software (SPSS, Chicago, IL, USA). Measurement data were expressed as mean ± SD. One-way analysis of variance and Student-Newman-Keuls test were used for comparison between groups. A level of P = 0.05 was considered statistically signi fi cant.
Results
Isolation and culture of hair follicle-derived neural crest stem cells
Human hair follicle bulges were inoculated in culture medium. Forty-eight hours later, a small number of shuttle-shaped cells appeared at the edge of hair follicle bulges, as shown in Figure 1A. When there were a large number of shuttle-shaped cells, they were digested with trypsin and stained with human natural killer-1 and p75 antibodies. Human natural killer-1/p75 double-positive cells were sorted by fl ow-cytometric cell sorting and accounted for 10% of all cells (Figure 1B). After neural crest stem cells were cultured in culture medium for 5 days, small typical clumps of stem cells formed (Figure 1C).
miR-21 expression during neural crest stem cell differentiation into Schwann cells
Flow-cytometric cell sorting showed that at day 30 of induction, 63.8 ± 3.7% of cells expressed S100 and 72.6 ± 4.9% of cells expressed glial fi brillary acidic protein. These results indicate that after induction by neuregulin-1, neural crest stem cells differentiated into Schwann cell-like cells (Figure 2A). Quantitative RT-PCR tests showed that miR-21 expression was detected at different time periods of induction. miR-21 expression was about two times higher at day 10 of induction compared with the level prior to induction and increased gradually thereafter (P < 0.05). This result demonstrates that miR-21 plays a regulatory role during neural crest stem cell differentiation into Schwann cells (Figure 2B).
miR-21 promoted neural crest stem cell differentiation into Schwann cells
After transfection with miR-21 agonist (agomir-21) or miR-21 antagonist (antagomir-21), neural crest stem cells were induced with 20 ng/mL neuregulin-1 to investigate whether miR-21 interference regulates Schwann cell differentiation. Results showed that after neuregulin-1 induction, 75% of agomir-21-transfected neural crest stem cells expressed both S100 and glial fi brillary acidic protein simultaneously, which was significantly higher than that in the corresponding negative control (agomir-NC) group (64%, P < 0.05). After neuregulin-1 induction, 41% of antagomir-21-transfected neural crest stem cells expressed both S100 and glial fi brillary acidic protein simultaneously, which was significantly lower than that in the corresponding negative control (antagomir-NC) group (62%, P < 0.05; Figure 3).
We also detected S100 and glial fibrillary acidic protein mRNA expression in each group using quantitative RTPCR. Results showed that after neuregulin-1 induction, S100 mRNA expression in the agomir-21 group and antagomir-21 group was signi fi cantly higher than that in the agomir-NC group (P < 0.05), but lower than that in the antagomir-NC group (P < 0.05). After neuregulin-1 induction, glial fi brillary acidic protein mRNA expressions in the agomir-21 group and antagomir-21 group were significantly higher than that in the agomir-NC group (P < 0.05), but lower than that in the antagomir-NC group (P < 0.05; Figure 3B). These fi ndings demonstrate that miR-21 can promote the differentiation of neural crest stem cells into Schwann cells.
miR-21 down-regulated SOX2 protein expression by binding to the 3′-UTR of SOX2 mRNA
To investigate the mechanism by which miR-21 regulates the differentiation of neural crest stem cells into Schwann cells, we retrieved databases to find the effective target of miR-21, and found that a 7-mer-long sequence at the 3′-UTR of SOX2 mRNA was matched to miR-21 (Figure 4A). To investigate whether this sequence can down-regulate SOX2 mRNA translation by binding to miR-21, the SOX2 mRNA 3′UTR containing this site was included in the luciferase reporter system (SOX2-3′UTR-wt) and simultaneously the mutational SOX2 mRNA 3′UTR (SOX2-3′UTR-mut) was also inserted into this reporter system. Then miR-21 wasco-transfected into HEK-293 cells together with luciferase empty vector, SOX2-3′UTR-wt plasmid or SOX2-3′UTR-mut plasmid. Results showed that the fl uorescence intensity in the SOX2-3′UTR-wt group was significantly lower than that in the negative control (miR-con) group. However, there was no signi fi cant difference in in fl orescence intensity between the SOX2-3′UTR-mut group or empty vector group and miR-con group (Figure 4B). These fi ndings suggest that miR-21 regulates SOX2 mRNA translation by binding to the 3′-UTR of SOX2 mRNA.
We also transfected neural crest stem cells with agomir-21 and its negative control (agomir-NC), with antagomir-21 and its negative control (antagomir-NC), and then detected SOX2 expression by western blot assay and SOX2 mRNA expression by quantitative RT-PCR. Results showed that agomir-21 can signi fi cantly decrease intracellular SOX2 expression (P <0.05), while antagomir-21 can signi fi cantly increase intracellular SOX2 expression (P < 0.05; Figure 4C). SOX2 mRNA expression was not in fl uenced by agomir-21 or antagomir-21 (Figure 4D). These fi ndings suggest that miR-21 can regulate SOX2 protein expression at the post-transcriptional level by binding to the 3′-UTR of SOX2 mRNA.
SOX2 over-expression blocked the regulatory effect of miR-21 on Schwann cell differentiation
We silenced SOX2 expression in neural crest stem cells using the lentiviral shRNA approach and compared these SOX2-knocked out neural crest stem cells (SOX2-KD-neural crest stem cells) with those non-interfered neural crest stem cells (control) to investigate the role of SOX2 in miR-21 regulation of neural crest stem cell differentiation. Flow cytometry and quantitative RT-PCR results showed that antagomir-21-transfected neural crest stem cells exhibited a signi fi cantly decreased differentiation capacity than antagomir-NC-transfected cells (P < 0.05). However, there was no signi fi cant difference in the differentiation capacity between antagomir-21-transfected SOX2-KD-neural crest stem cells and antagomiR-NC-transfected SOX2-KD-neural crest stem cells (Figure 5). These results demonstrate that SOX2 knockout can block the effect of antagomir-21 against Schwann cell differentiation, indicating that SOX2 is one downstream pathway by which miR-21 regulates the differentiation of neural crest stem cells.
Discussion
Hair follicle-derived neural crest stem cells are widely accepted as the ideal source of “seed cells” in regenerative medicine owing to their multipotent differentiation capacity, their easy harvesting and wide availability. However, a key problem has been encountered regarding how to acquire highly purified neural crest stem cells (Sieber-Blum and Grim, 2004; Lin et al., 2011). Hair follicle-derived neural crest stem cells are often isolated by culturing hair follicles according to the characteristics of hair follicle-derived neural crest stem cell migration and chemotaxis (Yu et al., 2010). This method is easy to perform and economical, but is time consuming. One issue should be noted that hair follicles contain many stem cell types, including mesenchymal stem cells, which exhibit the characteristics of migration. Neural crest stem cells harvested by culturing hair follicles are often not satisfactory. Flow-cytometric cell sorting employed in this study can help isolate cells expressing only p75 and human natural killer-1, the speci fi c antigens of neural crest stem cells. After culture, the isolated cells form typical lumps of neural crest stem cells.
Under the stimulation of proper inducers, neural crest stem cells can differentiate into Schwann cells successfully. A previous study using immuno fl uorescence staining showed that approximately 78 and 85% of cells express the speci fi c markers of Schwann cells after culture with the medium containing 20 ng/mL neuregulin-1 for 40 days. A long induction period will in fl uence the regulatory effect of miR-21 on the differentiation of neural crest stem cells, so a 4-week induction was designated in this study. Through immunofl uorescence staining, approximately 65% and 73% of cells expressed S100 and glial fi brillary acidic protein, respectively, after a 4-week induction. Results from this study also showed that miR-21 expression was obviously increased at day 10 of induction, approximately 2-fold over that prior to induction, further increased thereafter and tended to be stable at day 30. Together with a previous fi nding that miR-21 expression is up-regulated after myelin sheath injury (Yu et al., 2012a, b), we preliminarily conclude that miR-21 plays a regulatory role in the process of neural crest stem cell differentiation into Schwann cells.
miR-21, one of the early discovered miRNAs, widely exists in animals and plants and is highly conserved across species. It is located at the fragile site FRA17B on chromosome 17q23.2 and contains independent transcriptional units. The transcription of miR-21 is regulated by various factors including STAT3, AP-1 and NFI (Kumarswamy et al., 2011). STAT3 is an important factor that regulates miR-21 transcription and STAT3 activation in some tumors leads to a signi fi cant increase in miR-21 expression (Iliopoulos et al., 2010; Bornachea et al., 2012). A study showed that after binding to cell membrane surface receptor, neuregulin-1 can strengthen STAT3 phosphorylation in the downstream pathway (Liu and Kern, 2002). It is presumed that the increase in miR-21 during induced differentiation of neural crest stem cells into Schwann cells is likely attributable to STAT3 phosphorylation induced by the effect of neuregulin-1 in the downstream pathway.
The function of miR-21 is complex and is an area of interest in diverse fields including embryonic development, tumorigenesis, fibrosis and immune reaction (Chen and Wang, 2013; Smigielska-Czepiel et al., 2013; Zhang et al., 2013). During embryonic development, miR-21 expression is gradually increased and regulates branching morphogenesis via target genes RECK and PDCD4 (Hayashi et al., 2011). In addition, miR-21 expression is up-regulated in many tumors including glioma, lung cancer and liver cancer and miR-21 promotes tumor growth by binding different target genes (Darido et al., 2011; Hermansen et al., 2013; Liu et al., 2013c). Results from this study showed that after transfection with agomir-21, neural crest stem cells had an increased capacity to differentiate into Schwann cells, while after transfection with antagomir-21, the differentiation capacity wasattenuated. This suggests that in addition to aforementioned functions, miR-21 can also promote the differentiation of neural crest stem cells into Schwann cells. Together with the fact that neuregulin-1 promotes miR-21 transcription, it is considered that regulation of miR-21 expression can be used as an important means for neuregulin-1 promotion of stem cell differentiation.

Figure 4 miR-21 down-regulated SOX2 expression by binding to the 3′-UTR of SOX2 mRNA.

Figure 5 SOX2 knockout blocked the effect of antagomir-21 against Schwann cell differentiation.
To investigate the mechanism by which miR-21 regulates the differentiation of neural crest stem cells, we sought to find miR-21 targets via databases. Results showed that a 7-mer-long sequence at the 3′UTR region of SOX2 mRNA was matched to miR-21. This suggests that SOX2 is likely to be a downstream target gene via which miR-21 functions. SOX2 gene encodes a 317 amino acid protein and it exerts the regulatory function of transcriptional factors by binding to target genes through an HMG domain (Gracz and Magness, 2011; Sarkar and Hochedlinger, 2013). SOX2 participates in the embryonic development of vertebrates and maintains the undifferentiated state, multipotent differentiation and self-renewing capabilities of neural stem cells (Liu et al., 2013a; Maucksch et al., 2013). Evidence documents that SOX2 inhibits neural stem cells to differentiate into Schwann cells and subsequent myelination (Le et al., 2005; Adameyko et al., 2012). Therefore, this study selected SOX2 as a potential target of miR-21 and investigated whether miR-21 regulates the differentiation of neural crest stem cells by in fl uencing SOX2 expression. Luciferase assay results showed that after transfection with agomiR-21, the fl uorescence intensity of fl uorescence reporter plasmid containing SOX2-3′UTR-wt was significantly decreased, while the fl uorescence intensity of that containing SOX2-3′UTR-mt was not altered. Western blot assay results showed that when miR-21 was over-expressed, SOX2 protein expression in neural crest stem cells was decreased, while when miR-21 expression was silenced with antagomir-21, SOX2 protein expression was increased. This indicates that miR-21 can inhibit SOX protein expression by binding to the 3′-UTR of miR-21 mRNA.
Although SOX2 is a downstream target of miR-21, whether it participates in miR-21 promotion of neural crest stem cell differentiation needs to be further investigated. For this reason, we transfected antagomiR-21 into SOX2-KD-neural crest stem cells to investigate the relationship of SOX2 and miR-21 in the differentiation of neural crest stem cells. Results showed that antagomiR-21-transfected SOX2-KD-neural crest stem cells exhibited stronger differentiation capacity than non-transfected SOX2-KD-neural crest stem cells and non-interfered neural crest stem cells. These results demonstrate that SOX2 knockout can block the inhibitory effect of antagomiR-21 on the differentiation of neural crest stem cells and directly indicate that SOX2 is an important downstream target of miR-21 in the promotion of neural crest stem cell differentiation.
Taken together, after neuregulin-1 transfection, miR-21 expression was increased and miR-21 promoted the differentiation of neural crest stem cells into Schwann cells through down-regulating SOX protein expression by binding to the 3′-UTR of SOX2 mRNA. This study adds to the findings surrounding miRNAs and Schwann cell differentiation and provides a new insight into the repair of injured peripheral nerve by tissue engineering.
Author contributions:Ni YX and Zhou YM designed and evaluated this study. Graphs were created by Liu XJ. All authors participated in experiment performance and data analysis, and approved the final version of this paper.
Con fl icts of interest:None declared.
Achilleos A, Trainor PA (2012) Neural crest stem cells: discovery, properties and potential for therapy. Cell Res 22:288-304.
Adameyko I, Lallemend F, Furlan A, Zinin N, Aranda S, Kitambi SS, Blanchart A, Favaro R, Nicolis S, Lubke M, Muller T, Birchmeier C, Suter U, Zaitoun I, Takahashi Y, Ernfors P (2012) Sox2 and Mitf cross-regulatory interactions consolidate progenitor and melanocyte lineages in the cranial neural crest. Development 139:397-410.
Amiel J, de Pontual L, Henrion-Caude A (2012) miRNA, development and disease. Adv Genet 80:1-36.
Bhatt S, Diaz R, Trainor PA (2013) Signals and switches in Mammalian neural crest cell differentiation. Cold Spring Harb Perspect Biol. 5.
Bornachea O, Santos M, Martinez-Cruz AB, Garcia-Escudero R, Duenas M, Costa C, Segrelles C, Lorz C, Buitrago A, Saiz-Ladera C, Agirre X, Grande T, Paradela B, Maraver A, Ariza JM, Prosper F, Serrano M, Sanchez-Cespedes M, Paramio JM (2012) EMT and induction of miR-21 mediate metastasis development in Trp53-de fi cient tumours. Sci Rep 2:434.
Bremer J, O’Connor T, Tiberi C, Rehrauer H, Weis J, Aguzzi A (2010) Ablation of Dicer from murine Schwann cells increases their proliferation while blocking myelination. PLoS One 5:e12450.
Chen J, Wang X (2013) MicroRNA-21 in breast cancer: diagnostic and prognostic potential. Clin Transl Oncol 16:225-233.
Cioffi JA, Yue WY, Mendolia-Loffredo S, Hansen KR, Wackym PA, Hansen MR (2010) MicroRNA-21 overexpression contributes to vestibular schwannoma cell proliferation and survival. Otol Neurotol 31: 1455-1462. Darido C, Georgy SR, Wilanowski T, Dworkin S, Auden A, Zhao Q, Rank G, Srivastava S, Finlay MJ, Papenfuss AT, Pandol fi PP, Pearson RB, Jane SM (2011) Targeting of the tumor suppressor GRHL3 by a miR-21-dependent proto-oncogenic network results in PTEN loss and tumorigenesis. Cancer Cell 20:635-648.
Dong D, Jiang M, Xu X, Guan M, Wu J, Chen Q, Xiang L (2012) The effects of NB-UVB on the hair follicle-derived neural crest stem cells differentiating into melanocyte lineage in vitro. J Dermatol Sci 66:20-28.
Dugas JC, Cuellar TL, Scholze A, Ason B, Ibrahim A, Emery B, Zamanian JL, Foo LC, McManus MT, Barres BA (2010) Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron 65:597-611.
Dugas JC, Notterpek L (2011) MicroRNAs in oligodendrocyte and Schwann cell differentiation. Dev Neurosci 33:14-20.
El Seady R, Huisman MA, Lowik CW, Frijns JH (2008) Uncomplicated differentiation of stem cells into bipolar neurons and myelinating glia. Biochem Biophys Res Commun 376:358-362.
Gao X, Wang Y, Chen J, Peng J (2013) The role of peripheral nerve ECM components in the tissue engineering nerve construction. Rev Neurosci 24:443-453.
Garofalo M, Romano G, Di Leva G, Nuovo G, Jeon YJ, Ngankeu A, Sun J, Lovat F, Alder H, Condorelli G, Engelman JA, Ono M, Rho JK, Cascione L, Volinia S, Nephew KP, Croce CM (2012) EGFR and MET receptor tyrosine kinase-altered microRNA expression induces tumorigenesis and ge fi tinib resistance in lung cancers. Nat Med 18:74-82.
Gokey NG, Srinivasan R, Lopez-Anido C, Krueger C, Svaren J (2012) Developmental regulation of microRNA expression in Schwann cells. Mol Cell Biol 32:558-568.
Gracz AD, Magness ST (2011) Sry-box (Sox) transcription factors in gastrointestinal physiology and disease. Am J Physiol Gastrointest Liver Physiol 300:G503-515.
Hall BK (2008) The neural crest and neural crest cells: discovery and signi fi cance for theories of embryonic organization. J Biosci 33:781-793. Hayashi T, Koyama N, Azuma Y, Kashimata M (2011) Mesenchymal miR-21 regulates branching morphogenesis in murine submandibular gland in vitro. Dev Biol 352:299-307.
Hermansen SK, Dahlrot RH, Nielsen BS, Hansen S, Kristensen BW (2013) MiR-21 expression in the tumor cell compartment holds unfavorable prognostic value in gliomas. J Neurooncol 111:71-81.
Huisman MA, Rivolta MN (2012) Neural crest stem cells and their potential application in a therapy for deafness. Front Biosci (Schol Ed) 4: 121-132.
Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K (2010) STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking in fl ammation to cancer. Mol Cell 39: 493-506.
Jiang X, Gwye Y, McKeown SJ, Bronner-Fraser M, Lutzko C, Lawlor ER (2009) Isolation and characterization of neural crest stem cells derived from in vitro-differentiated human embryonic stem cells. Stem cells Dev 18:1059-1070.
Joseph NM, Mukouyama YS, Mosher JT, Jaegle M, Crone SA, Dormand EL, Lee KF, Meijer D, Anderson DJ, Morrison SJ (2004) Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development 131:5599-5612.
Koerner C, Keklikoglou I, Bender C, Woerner A, Muenstermann E, Wiemann S (2013) MicroRNA-31 sensitizes human breast cells to apoptosis by direct targeting of protein kinase C epsilon (PKCepsilon). J Biol Chem 288:8750-8761
Kong YW, Ferland-McCollough D, Jackson TJ, Bushell M (2012) microRNAs in cancer management. Lancet Oncol 13:e249-258.
Krejci E, Grim M (2010) Isolation and characterization of neural crest stem cells from adult human hair follicles. Folia Biol (Praha) 56:149-157.
Kumarswamy R, Volkmann I, Thum T (2011) Regulation and function of miRNA-21 in health and disease. RNA Biol 8:706-713.
Le N, Nagarajan R, Wang JY, Araki T, Schmidt RE, Milbrandt J (2005) Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identi fi es Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc Natl Acad Sci U S A 102:2596-2601.
Lin H, Liu F, Zhang C, Zhang Z, Guo J, Ren C, Kong Z (2009) Pluripotent hair follicle neural crest stem-cell-derived neurons and schwann cells functionally repair sciatic nerves in rats. Mol Neurobiol 40:216-223.
Lin H, Liu F, Zhang C, Zhang Z, Kong Z, Zhang X, Hoffman RM (2011) Characterization of nerve conduits seeded with neurons and Schwann cells derived from hair follicle neural crest stem cells. Tissue Eng Part A 17:1691-1698.
Liu J, Kern JA (2002) Neuregulin-1 activates the JAK-STAT pathway and regulates lung epithelial cell proliferation. Am J Respir Cell Mol Biol 27:306-313.
Liu K, Lin B, Zhao M, Yang X, Chen M, Gao A, Liu F, Que J, Lan X (2013a) The multiple roles for Sox2 in stem cell maintenance and tumorigenesis. Cell Signal 25:1264-1271.
Liu X, Song L, Liu J, Wang S, Tan X, Bai X, Bai T, Wang Y, Li M, Song Y, Li Y (2013b) miR-18b inhibits TGF-beta1-induced differentiation of hair follicle stem cells into smooth muscle cells by targeting SMAD2. Biochem Biophys Res Commun 438:551-556.
Liu ZL, Wang H, Liu J, Wang ZX (2013c) MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo- or radioresistance in non-small cell lung cancer cells by targeting PTEN. Mol Cell Biochem 372:35-45.
Marquardt LM, Sakiyama-Elbert SE (2013) Engineering peripheral nerve repair. Curr Opin Biotechnol 24:887-892.
Maucksch C, Jones KS, Connor B (2013) Concise review: the involvement of SOX2 in direct reprogramming of induced neural stem/ precursor cells. Stem Cells Transl Med 2:579-583.
Nana-Sinkam SP, Croce CM (2013) Clinical applications for microRNAs in cancer. Clin Pharmacol Ther 93:98-104.
Nie X, Deng M, Yang M, Liu L, Zhang Y, Wen X (2013) Axonal regeneration and remyelination evaluation of chitosan/gelatin-based nerve guide combined with transforming growth factor-beta1 and schwann cells. Cell Biochem Biophys 68:163-172.
Pang CJ, Tong L, Ji LL, Wang ZY, Zhang X, Gao H, Jia H, Zhang LX, Tong XJ (2013) Synergistic effects of ultrashort wave and bone marrow stromal cells on nerve regeneration with acellular nerve allografts. Synapse 67:637-647.
Pelaez D, Huang CY, Cheung HS (2013) Isolation of pluripotent neural crest-derived stem cells from adult human tissues by connexin-43 enrichment. Stem Cells Dev 22:2906-2914.
Pereira JA, Baumann R, Norrmen C, Somandin C, Miehe M, Jacob C, Luhmann T, Hall-Bozic H, Mantei N, Meijer D, Suter U (2010) Dicer in Schwann cells is required for myelination and axonal integrity. J Neurosci 30:6763-6775.
Qi L, Hongjuan H, Ning G, Zhengbin H, Yanjiang X, Tiebo Z, Zhijun H, Qiong W (2013) miR-370 is stage-specifically expressed during mouse embryonic development and regulates Dnmt3a. FEBS Lett 587:775-781.
Ren Z, Wang Y, Peng J, Zhao Q, Lu S (2012) Role of stem cells in the regeneration and repair of peripheral nerves. Rev Neurosci 23:135-143.
Rutnam ZJ, Yang BB (2012) The involvement of microRNAs in malignant transformation. Histol Histopathol 27:1263-1270.
Sarkar A, Hochedlinger K (2013) The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell 12:15-30.
Sauka-Spengler T, Bronner-Fraser M (2008) A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol 9: 557-568. Sethi A, Kulkarni N, Sonar S, Lal G (2013) Role of miRNAs in CD4 T cell plasticity during in fl ammation and tolerance. Front Genet 4:8.
Sieber-Blum M, Grim M (2004) The adult hair follicle: cradle for pluripotent neural crest stem cells. Birth Defects Res C Embryo Today 72:162-172.
Smigielska-Czepiel K, van den Berg A, Jellema P, Slezak-Prochazka I, Maat H, van den Bos H, van der Lei RJ, Kluiver J, Brouwer E, Boots AM, Kroesen BJ (2013) Dual role of miR-21 in CD4+T-cells: activation-induced miR-21 supports survival of memory T-cells and regulates CCR7 expression in naive T-cells. PLoS One 8:e76217.
Stefanescu O, Enescu DM, Lascar I (2012) Schwann cell cultures: recent advances and novel approaches to the reconstruction of peripheral nerve defects. Rom J Morphol Embryol 53:467-471.
Strickland IT, Richards L, Holmes FE, Wynick D, Uney JB, Wong LF (2011) Axotomy-induced miR-21 promotes axon growth in adult dorsal root ganglion neurons. PLoS One 6:e23423.
Sviderskaya EV, Easty DJ, Lawrence MA, Sanchez DP, Negulyaev YA, Patel RH, Anand P, Korchev YE, Bennett DC (2009) Functional neurons and melanocytes induced from immortal lines of postnatal neural crest-like stem cells. FASEB J 23:3179-3192.
Verrier JD, Semple-Rowland S, Madorsky I, Papin JE, Notterpek L (2010) Reduction of Dicer impairs Schwann cell differentiation and myelination. J Neurosci Res 88:2558-2568.
Yang R, Xu X (2013) Isolation and culture of neural crest stem cells from human hair follicles. J Vis Exp.
Yu B, Zhou S, Wang Y, Qian T, Ding G, Ding F, Gu X (2012a) miR-221 and miR-222 promote Schwann cell proliferation and migration by targeting LASS2 after sciatic nerve injury. J Cell Sci 125:2675-2683.
Yu B, Qian T, Wang Y, Zhou S, Ding G, Ding F, Gu X (2012b) miR-182 inhibits Schwann cell proliferation and migration by targeting FGF9 and NTM, respectively at an early stage following sciatic nerve injury. Nucleic Acids Res 40:10356-10365.
Yu H, Kumar SM, Kossenkov AV, Showe L, Xu X (2010) Stem cells with neural crest characteristics derived from the bulge region of cultured human hair follicles. J Invest Dermatol 130:1227-1236.
Yun B, Anderegg A, Menichella D, Wrabetz L, Feltri ML, Awatramani R (2010) MicroRNA-de fi cient Schwann cells display congenital hypomyelination. J Neurosci 30:7722-7728.
Zhang Z, Zha Y, Hu W, Huang Z, Gao Z, Zang Y, Chen J, Dong L, Zhang J (2013) The auto-regulatory feedback loop of microRNA-21/Programmed cell death protein 4/activation protein-1 (miR-21/PDCD4/ AP-1) as a driving force for hepatic fibrosis development. J Biol Chem 288:37082-37093.
Copyedited by Aprico K, Haase R, Yu J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.131599
Yanmin Zhou, Ph.D., M.D., Hospital of Stomatology, Jilin University, Changchun 130021, Jilin Province, China,
neurology@live.com.
http://www.nrronline.org/
Accepted: 2014-03-08
杂志排行
中国神经再生研究(英文版)的其它文章
- The synthetic thyroid hormone, levothyroxine, protects cholinergic neurons in the hippocampus of naturally aged mice
- Citalopram increases the differentiation ef fi cacy of bone marrow mesenchymal stem cells into neuronal-like cells
- Fusion protein of single-chain variable domain fragments for treatment of myasthenia gravis
- Similar effects of substance P on learning and memory function between hippocampus and striatal marginal division
- Regulatory effects of anandamide on intracellular Ca2+concentration increase in trigeminal ganglion neurons
- Virtual reality interface devices in the reorganization of neural networks in the brain of patients with neurological diseases
