Adipose-derived mesenchymal stem cell transplantation promotes adult neurogenesis in the brains of Alzheimer’s disease mice
2014-03-24YufangYanTuoMaKaiGongQiangAoXiufangZhangYandaoGong
Yufang Yan, Tuo Ma, Kai Gong, Qiang Ao, Xiufang Zhang, Yandao Gong
1 State Key Laboratory of Biomembrane and Membrane Biotechnology, School of Life Sciences, Tsinghua University, Beijing, China
2 Institute of Neurological Disorders, Yuquan Hospital, Tsinghua University, Beijing, China
Adipose-derived mesenchymal stem cell transplantation promotes adult neurogenesis in the brains of Alzheimer’s disease mice
Yufang Yan1, Tuo Ma1, Kai Gong1, Qiang Ao2, Xiufang Zhang1, Yandao Gong1
1 State Key Laboratory of Biomembrane and Membrane Biotechnology, School of Life Sciences, Tsinghua University, Beijing, China
2 Institute of Neurological Disorders, Yuquan Hospital, Tsinghua University, Beijing, China
In the present study, we transplanted adipose-derived mesenchymal stem cells into the hippocampi of APP/PS1 transgenic Alzheimer’s disease model mice. Immunofluorescence staining revealed that the number of newly generated (BrdU+) cells in the subgranular zone of the dentate gyrus in the hippocampus was signi fi cantly higher in Alzheimer’s disease mice after adipose-derived mesenchymal stem cell transplantation, and there was also a significant increase in the number of BrdU+/DCX+neuroblasts in these animals. Adipose-derived mesenchymal stem cell transplantation enhanced neurogenic activity in the subventricular zone as well. Furthermore, adipose-derived mesenchymal stem cell transplantation reduced oxidative stress and alleviated cognitive impairment in the mice. Based on these fi ndings, we propose that adipose-derived mesenchymal stem cell transplantation enhances endogenous neurogenesis in both the subgranular and subventricular zones in APP/PS1 transgenic Alzheimer’s disease mice, thereby facilitating functional recovery.
nerve regeneration; stem cells; Alzheimer’s disease; adipose-derived mesenchymal stem cells; cell transplantation; cognitive impairment; oxidative stress; neurogenesis; 863 Program; neural regeneration
Funding: This work was supported by the National High-Tech Research and Development Program of China (863 Program), No. 2012AA020905; Tsinghua-Yue-Yuen Medical Sciences Fund, No. 20240000514.
Yan YF, Ma T, Gong K, Ao Q, Zhang XF, Gong YD. Adipose-derived mesenchymal stem cell transplantation promotes adult neurogenesis in the brains of Alzheimer’s disease mice. Neural Regen Res. 2014;9(8):798-805.
Introduction
Alzheimer’s disease, one of the most common dementias among the elderly, is pathologically characterized by extracellular senile plaques (composed of amyloid-β peptide aggregates), intracellular neuro fi brillary tangles, synaptic dysfunction, and the loss of neurons in the brain[1-2]. Although the pathogenesis of Alzheimer’s disease remains unclear, mutations in amyloid precursor protein and presenilins, which underlie familial forms of Alzheimer’s disease, and tau hyperphosphorylation induce neuronal cell death[3-4]. The neuronal cell death leads to a reduction in size of the temporal and frontal lobes of the brain, which play critical roles in learning and memory processes and other mental functions, resulting in progressive memory and learning dysfunction and cognitive impairment in Alzheimer’s disease patients[5-6]. It was previously thought that the adult mammalian brain was devoid of stem cells that could regenerate after injury. However, recent studies have shown that neurogenesis occurs throughout the lifespan of adult mammals[7-8]. Indeed, studies in the adult rodent brain[2-4]have advanced the development of therapeutic strategies to replace the lost neurons[9-11].
It is widely accepted that neurogenesis primarily occurs within two restricted regions in the adult central nervous system: the subgranular zone of the dentate gyrus in the hippocampus and the subventricular zone adjacent to the lateral ventricles[12-13]. Newborn cells in the subventricular zone migrate along the rostral migratory stream and become periglomerular neurons in the olfactory bulb, and newly generated cells in the subgranular zone migrate and integrate into existing neural circuits in the granule cell layer of the dentate gyrus and function as mature granule neurons[14-15]. Accumulating evidence indicates that these newborn neurons actively participate in cognitive functions involving olfaction- and hippocampus-dependent learning and memory processes[16-17]. Neurogenesis is thought to be impaired in the subgranular and subventricular zones in some animal models of Alzheimer’s disease[18-20].
Neurogenesis can be readily modulated by physiological and/or pathological factors at all stages, including the proliferation of neural stem cells, the differentiation of these newborn cells, and their survival and maturation[21-23]. For example, physical exercise and enriched environment can increase the proliferation of neural stem cells in the dentate gyrus and lead to better cognitive performance[16,24]. Con-versely, depression and stress can reduce neurogenesis[25-26]. Many other factors modulate neurogenesis in the brain. Recent studies have shown that neurogenic rates in the subgranular and subventricular zones decrease with age[27-28]. Under pathological conditions, such as neurodegenerative diseases and other central nervous system disorders, there are also signi fi cant declines in neurogenesis[29-30]. Accordingly, numerous recent studies have aimed at fi nding drugs, agents and factors with neurogenic activity that could increase the number of newborn neurons to replace the lost neural cells. For example, Tchantchou et al.[31]found that EGb 761, the standardized Ginkgo biloba extract, can enhance neurogenesis, reduce Aβ oligomers, and alleviate cognitive defects in transgenic Alzheimer’s disease model mice by restoring phosphorylation of the cAMP response element binding protein. A study by Garza and co-workers[32]showed that leptin, an adipose-derived hormone, could regulate neurogenesis in cultured adult neural stem cells as well as in the dentate gyrus of adult mice, and that the Akt and STAT3 signaling pathways are involved in mediating this neurogenic activity of leptin. Furthermore, Pérez-González et al.[33]demonstrated that chronic leptin treatment significantly increases the amount of newly generated cells in the subgranular zone in transgenic Alzheimer’s disease mice. In addition, reactive oxygen species have been recently found to modulate the neurogenic process[34-35]. Le Belle et al.[34]showed that a change in cellular reactive oxygen species levels affects the self-renewal capacity of neural stem cells. However, excessive production and accumulation of reactive oxygen species cause oxidative stress, leading to the death of newly generated cells via apoptosis or necrosis, attenuating neurogenesis[34,36].
Recent studies suggest that the transplantation of mesenchymal stem cells can stimulate neurogenesis in the brains of adult rodents[37-38]. These cells can secrete growth factors and enhance the proliferation of endogenous neural stem cells in the subgranular zone of the dentate gyrus[37]. Moreover, mesenchymal stem cell transplantation also increases the proliferation and neural differentiation of newly generated cells in the subventricular zone[38]. Transplantation of mesenchymal stem cells could facilitate functional recovery in animal models of neurological disorders by promoting neurogenesis. In a rat stroke model, Yoo et al.[39]showed that mesenchymal stem cell transplantation could promote neurogenesis in the subventricular zone by enhancing endogenous neural stem cell proliferation while suppressing the death of these newborn cells. In a similar animal model, Bao and co-workers[39-40]demonstrated that the expression of brain-derived neurotrophic factor, vascular endothelial growth factor and neurotrophin-3 were dramatically higher in cell-transplanted animals, and more neuroblasts were generated within the subventricular zone to form mature functional neurons.
Adipose-derived mesenchymal stem cells (ADSCs) have been demonstrated to promote neurogenesis in vitro. Kang et al.[41]showed that ADSCs can interact with cultured neural stem cells and significantly support their proliferation and survival, and that direct physical contact between these two cell types is necessary to induce neuronal differentiation. However, the neurogenic potential and the therapeutic effects of ADSCs in the rodent Alzheimer’s disease model is unknown. The present study aimed to investigate whether intrahippocampal ADSC transplantation could exert beneficial effects in APP/PS1 transgenic Alzheimer’s disease model mice, and whether neurogenesis is affected by ADSC transplantation.
Results
Quantitative analysis of experimental animals
A total of 10 APP/PS1 transgenic Alzheimer’s disease model mice were included in this study. Mice were equally and randomly divided into two groups: Hank’s balanced salt solution (HBSS) group and ADSC group. ADSCs were suspended in HBSS and delivered into the hippocampi of APP/ PS1 transgenic Alzheimer’s disease model mice at 8 months, and HBSS-infused model mice were used as controls.
ADSC transplantation alleviated cognitive impairment in APP/PS1 transgenic mice
To examine whether ADSC transplantation can alleviate cognitive de fi cits in APP/PS1 transgenic mice, the novel object recognition test was performed with ADSC-transplanted mice and HBSS-infused controls 4 weeks after transplantation. The APP/PS1 transgenic mice treated with HBSS failed to discriminate between the novel and the familiar objects, while ADSC-transplanted APP/PS1 transgenic mice signi ficantly interacted more with the novel object than with the familiar one (P < 0.05; Figure 1). This fi nding suggests that ADSC transplantation can alleviate cognitive impairment in APP/PS1 transgenic Alzheimer’s disease model mice.
ADSC transplantation reduced oxidative stress in the
hippocampus of APP/PS1 transgenic mice
Oxidative stress has been observed in Alzheimer’s disease brains and is considered an important pathological feature of the disease[42]. Therefore, we next investigated whether the transplantation of ADSCs impacts oxidative stress levels in APP/PS1 transgenic mice. Dihydroethidium staining showed that oxidative stress in the hippocampus was dramatically lower in APP/PS1 transgenic mice transplanted with ADSCs than in HBSS-infused control animals (P < 0.01; Figure 2).
ADSC transplantation promoted neurogenesis in the subgranular zone of the hippocampus in APP/PS1 transgenic mice
The cognitive decline in animal models of Alzheimer’s disease has been linked to synaptic dysfunction and neuronal loss, particularly in the hippocampus. In addition, a strong interaction between oxidative status and neurogenesis was recently found[34-35]. Therefore, we next examined if neurogenesis is the underlying mechanism by which cell transplantation induces the generation of new neuronal cells that facilitate functional recovery. Immunofluorescence staining showed that the newly generated (BrdU+) cells were dispersed throughout the dentate gyrus in ADSC-transplanted Alzheimer’s disease mice, while they were restricted to the subgranular zone in HBSS-infused controls (Figure 3A). We found that the number of BrdU+newborn cells in the dentate gyrus was significantly higher inADSC-transplanted APP/PS1 transgenic mice than in HBSS-treated controls (P < 0.01; Figure 3B).
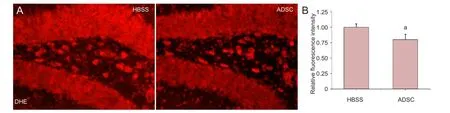
Figure 2 Oxidative stress levels in the hippocampus of APP/PS1 transgenic mice.

Figure 3 Newborn cells in the dentate gyrus of APP/PS1 transgenic mice.

Figure 1 Adipose-derived mesenchymal stem cell (ADSC) transplantation alleviates cognitive impairment in APP/PS1 transgenic mice.
Some newborn cells differentiate into neurons, mature and integrate into the existing neural network to carry out their functions. Although many more BrdU+cells were found in the dentate gyrus of ADSC-implanted APP/PS1 transgenic mice compared with HBSS-treated controls, we did not know how many would differentiate into neuronal cells. Co-labeling showed that the number of cells positive for both BrdU and DCX in the subgranular zone of the dentate gyrus was dramatically higher in the ADSC-transplanted APP/PS1 transgenic mice than in the HBSS-treated controls (P < 0.01; Figure 4). These results indicate that ADSC transplantation enhances neurogenesis in the subgranular zone of APP/PS1 transgenic Alzheimer’s disease mice.
ADSC transplantation increased the number of neuroblasts in the subventricular zone of APP/PS1 transgenic mice
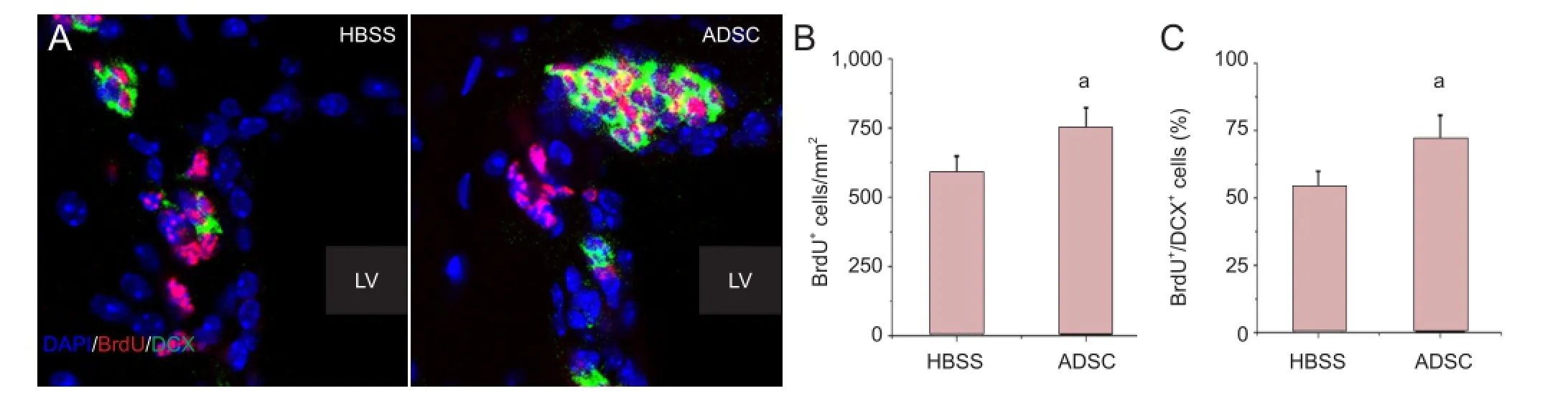
Figure 5 Neuronal progenitors in the subventricular zone of APP/PS1 transgenic mice.

Figure 4 Newly generated neuronal cells in the subgranular zone of APP/PS1 transgenic mice.
To further evaluate the neurogenesis-promoting effect of ADSC transplantation, we next examined another active neurogenic region, the subventricular zone (Figure 5A). Immuno fl uorescence analysis indicated that the number of newly generated BrdU+cells in the subventricular zone was signi fi cantly increased by ADSC transplantation (P < 0.01; Figure 5B), and an increase in the proportion of newborn neuroblasts (BrdU+/DCX+cells) out of the total pool of proliferating BrdU+cells was also observed in ADSC-transplanted APP/PS1 transgenic mice compared with HBSS-treated animals (P < 0.01; Figure 5C). These results provide clear evidence that ADSC transplantation signi fi cantly promotes neurogenesis in the brains of APP/PS1 transgenic Alzheimer’s disease mice.
Discussion
In the present study, we evaluated the therapeutic potential of intrahippocampal transplantation of ADSCs in APP/PS1 transgenic Alzheimer’s disease model mice. ADSC transplantation significantly alleviated cognitive impairment, substantially reduced oxidative stress in the hippocampus, and enhanced neurogenesis in both the subgranular and subventricular zones in the brains of APP/PS1 transgenic mice. Given that oxidative stress can impair neurogenesis, and that reduced neurogenesis is an early pathological event in Alzheimer’s disease[36,43], we propose that transplanted ADSCs alleviate cognitive impairment in Alzheimer’s disease mice by stimulating neurogenesis. Our fi ndings provide clear evidence that ADSC transplantation may be a promising therapeutic strategy for Alzheimer’s disease.
Recent studies on mesenchymal stem cells have provided promising new avenues for tissue repair in central nervous system diseases[44-45]. Our results indicate that ADSC transplantation dramatically restores cognitive decline in APP/PS1 transgenic Alzheimer’s disease model mice, although the underlying mechanisms are likely complex. This study focused on changes in the endogenous environment following ADSC transplantation. We first examined oxidative stress levels in the brain of these APP/PS1 transgenic mice. Dihydroethidium staining was used to assess levels of the superoxide anion (•O2
-), a highly reactive oxygen species, in the hippocampus. In most cell types, mitochondria are the major source of reactive oxygen species, which are generated mainly because of electron leakage from the mitochondrial electron transport chain located in the mitochondrial inner membrane[46-47]. Superoxide anions are generated by the interaction of oxygen with unpaired electrons, and can be converted into more reactive species, i.e., hydrogen peroxide (H2O2) and hydroxyl radical (•OH). Reactive oxygen species participate in cellular metabolism and signaling by oxidizing cellular components such as membrane lipids, proteins and nucleic acids[48-49]. A sophisticated defense system consisting of antioxidants, both enzymatic and nonenzymatic, exist within cells to achieve redox balance[42,50]. A disruption of this balance induces oxidative stress, in which the generation of reactive oxygen species exceeds antioxidant defense capacity. Oxidative stress leads to cellular dysfunction and plays a signi fi cant role in numerous pathological processes, including neurodegenerative diseases such as Alzheimer’s disease[51-52].
In our previous study, we found increased basal levels ofreactive oxygen species in a cellular model of Alzheimer’s disease. This oxidative stress, induced by elevated levels of Aβ peptide, resulted in impairment of mitochondrial function and cellular damage[42,53]. These cellular insults accelerate the generation of reactive oxygen species in the mitochondria to form a deleterious feedback loop exacerbating the disease[54-55]. In the present study, ADSC transplantation significantly decreased oxidative stress levels in the hippocampus of APP/PS1 transgenic mice. This neuroprotective effect could result from a decrease in reactive oxygen species generation, the enhancement of reactive oxygen species scavenging capability, or an elevated capacity to repair oxidized molecules. Further study is required to clarify the mechanisms underlying the neuroprotective effect of ADSC transplantation. Given that oxidative stress is an early event in Alzheimer’s disease pathogenesis[56], the improvements in the tissue microenvironment produced by ADSC transplantation could inhibit the pathological process and help alleviate the de fi cit in neurogenesis.
Recent studies have suggested a modulatory role of reactive oxygen species in neurogenesis[34-35,57]. Le Belle et al.[34]demonstrated that reactive oxygen species level influences the self-renewal capacity of neural stem cells and neurogenesis through the NADPH oxidase and PI3K/Akt pathway. An appropriate reactive oxygen species level is necessary for neural stem cells to maintain their self-renewal capacity, but excessive levels of reactive oxygen species can result in toxicity and cell death, implying that regulation of reactive oxygen species level is important for neural stem cell proliferation. Furthermore, antioxidants such as curcumin and EGCG have also been found to increase neurogenesis in the dentate gyrus in adult rodents[58-59], providing additional evidence that reactive oxygen species downregulate neurogenesis. Numerous studies have investigated the use of antioxidants for Alzheimer’s disease treatment, and some have focused on their effect on neurogenesis. In this study, we found that the reduced oxidative stress level was accompanied with enhanced neurogenesis in the subgranular and subventricular zones in APP/PS1 transgenic mice. Considering that oxidative stress is closely linked with cell death and the neurogenic process, it is reasonable to propose that by reducing oxidative stress, ADSC transplantation promotes the survival of newly generated cells, thereby promoting neurogenesis.
The neurogenic potential of mesenchymal stem cells has been demonstrated by several recent studies. Munoz et al.[37]found that the transplantation of bone marrow-derived mesenchymal stem cells promoted the proliferation of endogenous neural stem cells and affected their differentiation in the hippocampus of adult mice, likely by secreting various cytokines. Furthermore, mesenchymal stem cell transplantation has been reported to improve neurological function under pathological conditions. For example, studies by Yoo et al.[39]and Bao et al.[40]suggest that bone marrow-derived mesenchymal stem cell transplantation promotes endogenous neurogenesis and behavioral recovery in animal models of cerebral ischemia. In addition, T fi lin and co-workers[60]found that bone marrow-derived mesenchymal stem cell transplantation enhances hippocampal neurogenesis and improves behavioral performance in animal models of depressive-like behavior. Compared with bone marrow-derived mesenchymal stem cells, ADSCs are also capable of expansion in vitro, and these cells are more plentiful and much easier to obtain. Therefore, ADSCs could serve as an attractive alternative source of stem cells for tissue repair. Moreover, ADSCs can modulate and interact with cultured neural stem cells, affecting their proliferation and increasing neural differentiation and migration[41]. In line with these fi ndings, our results show that ADSCs can also effectively promote the proliferation and differentiation of neural stem cells in vivo in a model of Alzheimer’s disease. ADSC transplantation significantly decreased oxidative stress, which might result in a cellular microenvironment more suitable for neurogenesis. However, the mechanisms by which ADSCs reduce oxidative stress and promote neurogenesis remain unclear. The transdifferentiation and cellular fusion of implanted mesenchymal stem cells occur at very low frequency in vivo and can likely not contribute signi fi cantly to the alleviation of cognitive impairment. The cognitive improvement more likely results from the release of cytokines from these cells[37]. Accordingly, we hypothesize that the oxidative stress reducing and neurogenesis promoting activities of transplanted ADSCs might be mediated by their paracrine activities. Of course, further investigations are required to clarify the mechanisms underlying the therapeutic effects of ADSCs, particularly as these cells have been reported to exert multiple neurotrophic effects in several central nervous system disorders[39-40,60-61].
The efficacy and feasibility of ADSC transplantation in APP/PS1 transgenic mice and the mechanisms mediating the neurogenic effects of these cells are the main focus of the present work. Other important issues, such as the fate of the transplanted ADSCs, are unresolved and require further study. Recent studies indicate that the majority of implanted mesenchymal stem cells disappear within days after transplantation[37,62-63]. Munoz et al.[37]found that although BMSC transplantation enhanced hippocampal neurogenesis in adult mice, the cells failed to proliferate in vivo, and only one-quarter of these cells remained 3 days after implantation. The researchers proposed that the neurogenic potential of bone marrow-derived mesenchymal stem cells might be conferred by their ability to secrete neurotrophins that enhance the proliferation of endogenous neural stem cells. Therefore, the bene fi cial effects of these mesenchymal stem cells are not necessarily dependent upon their persistence in the brain tissue.
Materials and Methods
Design
A controlled, comparative, in vivo experiment.
Time and setting
Experiments were performed in the School of Life Sciences, Tsinghua University, China from October 2011 to April 2012.
Materials
Sprague-Dawley rats, aged 6-8 weeks, weighing 280-300 g, were purchased from Vital River Corporation, Beijing, China(license No. SCXK (Jing) 2007-0001). Transgenic APP/PS1 mice expressing the human APPswe (K595N/M596L) and presenilin 1 (PS1ΔE9) mutants were purchased from Jackson Laboratories (Bar Harbor, ME, USA). These transgenic mice were maintained on their original genetic background until the age of 8 months, when they developed Aβ deposits and exhibited signi fi cant cognitive impairment[64]. The mice were raised at 22 ± 1°C, under a 12-hour light/dark cycle, with free access to standard food and water. All procedures were in strict accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[65].
Methods
Isolation and culture of ADSCs
Adipose tissue was obtained from the testicular fat pads of 6-8-week-old healthy rats. ADSC isolation and culture were performed according to published protocols[66-67]. Brie fl y, the adipose tissue was mechanically dissociated with surgical scissors, then bathed in ice-cold HBSS (KCl 2.7 mmol/L, NaCl 138 mmol/L, KH2PO41.5 mmol/L, Na2HPO48.1 mmol/L, glucose 10 mmol/L, pH 7.2-7.4). After digestion with collagenase type I (Gibco, Grand Island, NY, USA) at 37°C for 45 minutes, these tissues were gently dissociated with pipettes. The cell suspension was fi ltered with a cell strainer (70 μm; BD Biosciences, San Jose, CA, USA) and then subjected to centrifugation at 800 × g for 5 minutes, separating the stromal vascular fraction from the fl oating adipocytes. Then, the resuspended stromal vascular fraction was plated onto cell culture dishes (approximately 105cells/mL) with DMEM/ F12 medium (Invitrogen, Grand Island, NY, USA) containing penicillin/streptomycin (Sigma, St. Louis, MO, USA) and fetal bovine serum (Hyclone, Logan, UT, USA). Cell cultures were kept in a humidi fi ed incubator at 37°C, with 5% CO2. 24 hours later, non-adherent cells were removed by changing the culture medium. The cells were expanded in vitro for serial passaging, and passages 3-5 were used for transplantation. These cells were positive for CD44 and CD90, and negative for CD11b, CD31 and CD45[66]. They were also capable of differentiating into multiple lineages, including osteocytes and adipocytes[67].
ADSC transplantation
After anesthetization with 5% chloral hydrate (8 mL/kg, i.p.), the mice were fixed on a stereotaxic apparatus (RWD Life Science, Shanghai, China). Approximately 105cells suspended in 3 μL HBSS were injected into the bilateral hippocampi with an automated infusion pump (1 μL/min; PHD 2000, Harvard Apparatus, Holliston, MA, USA). The stereotaxic coordinates were as follows: 2 mm posterior to the bregma, 2 mm bilateral from the midline, and 2 mm ventral to the skull surface[68]. In control groups, HBSS vehicle alone was injected into the hippocampi of Alzheimer’s disease model mice at the same time. After surgery, the mice were kept warm until recovery from anesthesia, and then returned to their home cages.
Novel object recognition test for cognitive function
Mice have greater curiosity towards novel objects than familiar ones. This intrinsic behavior has been widely used to study the learning/memory function of these animals[69]. The mouse was put into a 40 cm × 25 cm × 25 cm black box, with an open top. A black curtain was used to surround the open box with dim illumination to achieve an uniform environment. For the training session, each mouse was allowed a 10-minute exposure to the box, with two identical objects placed symmetrically and equidistantly from the side walls and the corners. After the training session, the mouse was put back into the home cage for 30 minutes. Then, the test session started with the mouse lowered into the box where one of the familiar objects used in the training session was replaced with a novel one. The novel object had a similar texture and size to the familiar one, but with a different shape. The mouse was permitted to explore the objects for 5 minutes. Exploration took place when the mouse touched or sniffed the objects with the forepaws and/or the nose. The times spent by the mouse exploring the novel and familiar objects were manually recorded. The recognition index was expressed by the ratio TN/(TF+ TN), where TNrepresents the time interacting with the novel object, and TFrepresents the time exploring the familiar object. The box and objects were cleaned with 75% ethanol solution between tests.
Tissue preparation
When the novel object recognition test was completed, mice were anesthetized with 5% chloral hydrate (8 mL/kg, i.p.). They were perfused intracardially with PBS and then fi xed with 4% paraformaldehyde. Then, the brain was removed from the cranium. After postfixation in the same medium at 4 °C overnight, the brain was equilibrated in 30% PBS-buffered sucrose solution for another 48 hours. The brains were then cut into serial coronal sections with a freezing microtome (CM 1900, Leica, Nussloch, Germany) at a thickness of 40 μm. The sections were stored at 4°C until further processing.
Detection of oxidative stress levels in the brain
Dihydroethidium (Beyotime Institute of Biotechnology, Haimen, Jiangsu Province, China) was used to measure superoxide anion levels in the brain sections. Brie fl y, the sections were immersed in 10 μmol/L dihydroethidium (in PBS solution) in a humidi fi ed environment at room temperature for 30 minutes. Dihydroethidium is oxidized by superoxide to form ethidium, which binds to DNA in the nucleus and emits red fl uorescence. After washing with PBS three times, the sections were cover-slipped and then visualized under a fl uorescence microscope (BX41, Olympus, Tokyo, Japan).
Immunofluorescence staining for BrdU incorporation and
DCX expression in the brains of APP/PS1 transgenic mice
BrdU, a thymidine analog, can incorporate into DNA during the S-phase when cells are undergoing division. Doublecortin is a widely used marker for neuroblasts and often used to track migrating cells. To quantify newborn cells in the brains of these mice, they were given 5-bromo-2′-deoxyuridine (BrdU; 50 mg/kg; Sigma) daily via intraperitoneal injection in the last week before sacri fi ce. The mice were killed on the second day of the last BrdU administration. The brain sec-tions were immersed in PBS (pH 7.4) for 15 minutes, and then heated in citric acid buffer (0.01 mol/L, pH 6.0) for another 15 minutes to denature DNA. The sections were then blocked with goat serum (5% goat serum in 0.1 mol/L PBS with 0.3% Triton X-100) in a humidi fi ed chamber at room temperature for 1 hour, and then incubated with mouse anti-BrdU antibody (1:100; Millipore, Billerica, MA, USA) in TBST [NaCl 0.15 mol/L, Tris-HCl 50 mmol/L, Tween 20 0.05% (w/v), pH 7.6] overnight at 4°C. After washing, they were immersed in TRITC-conjugated goat anti-mouse antibody (1:100; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) at room temperature for 2 hours. For double staining with BrdU and doublecortin, the mouse anti-BrdU antibody, together with rabbit anti-doublecortin polyclonal antibody (1:200; Cell Signaling, Danvers, MA, USA), were used to incubate the sections overnight at 4°C. Then, the sections were incubated with TRITC-conjugated goat anti-mouse and FITC-conjugated goat anti-rabbit secondary antibodies (1:100; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd). DAPI (1:1,000; Dojindo, Kumamoto, Kyushu, Japan) was used to stain the nucleus. Sections were observed with a laser scanning confocal microscope (Olympus).
Statistical analysis
Data were expressed as mean ± SD. Statistical analysis was conducted using Origin 7.0 software (OriginLab Corporation, Northampton, MA, USA), and one-way analysis of variance with Bonferroni correction was performed for comparison between groups. Values of P < 0.05 were considered statistically signi fi cant.
Author contributions:Yan YF designed and performed the experiments, analyzed the data, and wrote the manuscript. Gong YD was responsible for the experimental design, authorization and instruction of the study. Gong K, Ma T, and Ao Q helped with the experimental procedures and the statistical processing. Zhang XF revised the manuscript. All authors approved the final version of the manuscript.
Con fl icts of interest:None declared.
Peer review:The present study investigated the effect of adipose-derived mesenchymal stem cells transplantation on oxidative stress and adult neurogenesis in the brains (subgranular zone of dentate gyrus and subventricular zone of lateral ventricle) of transgenic mice by using histological and immunofluorescence staining.
[1] Lacor PN, Buniel MC, Chang L, et al. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24(45): 10191-10200.
[2] Wu L, Rosa-Neto P, Hsiung GY, et al. Early-onset familial Alzheimer’s disease (EOFAD). Can J Neurol Sci. 2012;39(4):436-445.
[3] Um HS, Kang EB, Koo JH, et al. Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer’s disease. Neurosci Res. 2011;69(2):161-173.
[4] Antequera D, Bolos M, Spuch C, et al. Effects of a tacrine-8-hydroxyquinoline hybrid (IQM-622) on Aβ accumulation and cell death: involvement in hippocampal neuronal loss in Alzheimer’s disease. Neurobiol Dis. 2012;46(3):682-691.
[5] Scheff SW, Price DA, Schmitt FA, et al. Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2011;24(3):547-557.
[6] Lemmens MA, Sierksma AS, Rutten BP, et al. Age-related changes of neuron numbers in the frontal cortex of a transgenic mouse model of Alzheimer’s disease. Brain Struct Funct. 2011;216(3):227-237.
[7] Czaja K, Fornaro M, Geuna S. Neurogenesis in the adult peripheral nervous system. Neural Regen Res. 2012;7(14):1047-1054.
[8] Kim HJ, Sun W. Adult neurogenesis in the central and peripheral nervous systems. Int Neurourol J. 2012;16(2):57-61.
[9] Miles DK, Kernie SG. Hypoxic-ischemic brain injury activates early hippocampal stem/progenitor cells to replace vulnerable neuroblasts. Hippocampus. 2008;18(8):793-806.
[10] Brinton RD, Wang JM. Therapeutic potential of neurogenesis for prevention and recovery from Alzheimer’s disease: allopregnanolone as a proof of concept neurogenic agent. Curr Alzheimer Res. 2006;3(3): 185-190.
[11] Jiang JD, Bu XY, Liu M, et al. Transplantation of autologous bone marrow-derived mesenchymal stem cells for traumatic brain injury. Neural Regen Res. 2012;7(1):46-53.
[12] Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645-660.
[13] Li ZN, Lü WL, Dong HY, et al. Subcellular distribution of N-methyl-D-aspartic acid receptor subunit 1 in neural stem cells within subventricular zone of adult rats. Neural Regen Res. 2011; 6(28):2188-2192.
[14] Luikart BW, Perederiy JV, Westbrook GL. Dentate gyrus neurogenesis, integration and microRNAs. Behav Brain Res. 2012;227(2): 348-355.
[15] Arisi GM, Foresti ML, Mukherjee S, et al. The role of olfactory stimulus in adult mammalian neurogenesis. Behav Brain Res. 2012;227(2):356-362.
[16] Lazarov O, Marr RA. Neurogenesis and Alzheimer’s disease: at the crossroads. Exp Neurol. 2010;223(2):267-281.
[17] Kageyama R, Imayoshi I, Sakamoto M. The role of neurogenesis in olfaction-dependent behaviors. Behav Brain Res. 2012;227(2):459-463.
[18] Donovan MH, Yazdani U, Norris RD, et al. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J Comp Neurol. 2006;495(1):70-83.
[19] Wang JM, Singh C, Liu L, et al. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2010;107(14):6498-6503.
[20] Faure A, Verret L, Bozon B, et al. Impaired neurogenesis, neuronal loss, and brain functional de fi cits in the APPxPS1-Ki mouse model of Alzheimer’s disease. Neurobiol Aging. 2011;32(3):407-418.
[21] Kempermann G. Seven principles in the regulation of adult neurogenesis. Eur J Neurosci. 2011;33(6):1018-1024.
[22] Balu DT, Lucki I. Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev. 2009;33(3):232-252.
[23] Tanti A, Rainer Q, Minier F, et al. Differential environmental regulation of neurogenesis along the septo-temporal axis of the hippocampus. Neuropharmacology. 2012;63(3):374-384.
[24] Speisman RB, Kumar A, Rani A, et al. Environmental enrichment restores neurogenesis and rapid acquisition in aged rats. Neurobiol Aging. 2013;34(1):263-274.
[25] Kubera M, Obuchowicz E, Goehler L, et al. In animal models, psychosocial stress-induced (neuro)in fl ammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):744-759.
[26] Torner L, Karg S, Blume A, et al. Prolactin prevents chronic stressinduced decrease of adult hippocampal neurogenesis and promotes neuronal fate. J Neurosci. 2009;29(6):1826-1833.
[27] Walter J, Keiner S, Witte OW, et al. Age-related effects on hippocampal precursor cell subpopulations and neurogenesis. Neurobiol Aging. 2011;32(10):1906-1914.
[28] Ma DK, Kim WR, Ming GL, et al. Activity-dependent extrinsic regulation of adult olfactory bulb and hippocampal neurogenesis. Ann N Y Acad Sci. 2009;1170:664-673.
[29] Kaneko N, Sawamoto K. Adult neurogenesis and its alteration under pathological conditions. Neurosci Res. 2009;63(3):155-164.
[30] Zhao N, Zhong C, Wang Y, et al. Impaired hippocampal neurogenesis is involved in cognitive dysfunction induced by thiamine de ficiency at early pre-pathological lesion stage. Neurobiol Dis. 2008; 29(2):176-185.
[31] Tchantchou F, Xu Y, Wu Y, et al. EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer’s disease. FASEB J. 2007;21(10):2400-2408.
[32] Garza JC, Guo M, Zhang W, et al. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem. 2008; 283(26):18238-18247.
[33] Pérez-González R, Antequera D, Vargas T, et al. Leptin induces proliferation of neuronal progenitors and neuroprotection in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2011;24 Suppl 2:17-25.
[34] Le Belle JE, Orozco NM, Paucar AA, et al. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8(1):59-71.
[35] Taupin P. A dual activity of ROS and oxidative stress on adult neurogenesis and Alzheimer’s disease. Cent Nerv Syst Agents Med Chem. 2010;10(1):16-21.
[36] Hamilton A, Holscher C. The effect of ageing on neurogenesis and oxidative stress in the APP(swe)/PS1(deltaE9) mouse model of Alzheimer’s disease. Brain Res. 2012;1449:83-93.
[37] Munoz JR, Stoutenger BR, Robinson AP, et al. Human stem/ progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci U S A. 2005;102(50):18171-18176.
[38] Kan I, Barhum Y, Melamed E, et al. Mesenchymal stem cells stimulate endogenous neurogenesis in the subventricular zone of adult mice. Stem Cell Rev. 2011;7(2):404-412.
[39] Yoo SW, Kim SS, Lee SY, et al. Mesenchymal stem cells promote proliferation of endogenous neural stem cells and survival of newborn cells in a rat stroke model. Exp Mol Med. 2008;40(4):387-397.
[40] Bao X, Wei J, Feng M, et al. Transplantation of human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats. Brain Res. 2011;1367:103-113.
[41] Kang SK, Jun ES, Bae YC, et al. Interactions between human adipose stromal cells and mouse neural stem cells in vitro. Brain Res Dev Brain Res. 2003;145(1):141-149.
[42] Sheng B, Gong K, Niu Y, et al. Inhibition of gamma-secretase activity reduces Abeta production, reduces oxidative stress, increases mitochondrial activity and leads to reduced vulnerability to apoptosis: Implications for the treatment of Alzheimer’s disease. Free Radic Biol Med. 2009;46(10):1362-1375.
[43] Demars M, Hu YS, Gadadhar A, et al. Impaired neurogenesis is an early event in the etiology of familial Alzheimer’s disease in transgenic mice. J Neurosci Res. 2010;88(10):2103-2117.
[44] Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011;10(7):649-656.
[45] Lunn JS, Sakowski SA, Hur J, et al. Stem cell technology for neurodegenerative diseases. Ann Neurol. 2011;70(3):353-361.
[46] Ten VS, Starkov A. Hypoxic-ischemic injury in the developing brain: the role of reactive oxygen species originating in mitochondria. Neurol Res Int. 2012;2012:542976.
[47] Lenaz G. Mitochondria and reactive oxygen species. Which role in physiology and pathology? Adv Exp Med Biol. 2012;942:93-136.
[48] Murphy MP, Holmgren A, Larsson NG, et al. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011;13(4): 361-366.
[49] Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol. 2011; 7(8):504-511.
[50] Carr WJ, Oberley-Deegan RE, Zhang Y, et al. Antioxidant proteins and reactive oxygen species are decreased in a murine epidermal side population with stem cell-like characteristics. Histochem Cell Biol. 2011;135(3):293-304.
[51] Jomova K, Vondrakova D, Lawson M, et al. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. 2010;345(1-2):91-104.
[52] Mohsenzadegan M, Mirsha fi ey A. The immunopathogenic role of reactive oxygen species in Alzheimer disease. Iran J Allergy Asthma Immunol. 2012;11(3):203-216.
[53] Aslan M, Ozben T. Reactive oxygen and nitrogen species in Alzheimer’s disease. Curr Alzheimer Res. 2004;1(2):111-119.
[54] Leuner K, Schütt T, Kurz C, et al. Mitochondrion-derived reactive oxygen species lead to enhanced amyloid beta formation. Antioxid Redox Signal. 2012;16(12):1421-1433.
[55] Axelsen PH, Komatsu H, Murray IV. Oxidative stress and cell membranes in the pathogenesis of Alzheimer’s disease. Physiology (Bethesda). 2011;26(1):54-69.
[56] Verri M, Pastoris O, Dossena M, et al. Mitochondrial alterations, oxidative stress and neuroinflammation in Alzheimer's disease. Int J Immunopathol Pharmacol. 2012;25(2):345-353.
[57] Kennedy KA, Sandiford SD, Skerjanc IS, et al. Reactive oxygen species and the neuronal fate. Cell Mol Life Sci. 2012;69(2):215-221.
[58] Kim SJ, Son TG, Park HR, et al. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol Chem. 2008;283(21):14497-14505.
[59] Yoo KY, Choi JH, Hwang IK, et al. (-)-Epigallocatechin-3-gallate increases cell proliferation and neuroblasts in the subgranular zone of the dentate gyrus in adult mice. Phytother Res. 2010;24(7): 1065-1070.
[60] T fi lin M, Sudai E, Merenlender A, et al. Mesenchymal stem cells increase hippocampal neurogenesis and counteract depressive-like behavior. Mol Psychiatry. 2010;15(12):1164-1175.
[61] Maltman DJ, Hardy SA, Przyborski SA. Role of mesenchymal stem cells in neurogenesis and nervous system repair. Neurochem Int. 2011;59(3):347-356.
[62] Lee JK, Jin HK, Bae JS. Bone marrow-derived mesenchymal stem cells reduce brain amyloid-beta deposition and accelerate the activation of microglia in an acutely induced Alzheimer’s disease mouse model. Neurosci Lett. 2009;450(2):136-141.
[63] Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40(7):609-619.
[64] Reiserer RS, Harrison FE, Syverud DC, et al. Impaired spatial learning in the APPSwe + PSEN1DeltaE9 bigenic mouse model of Alzheimer’s disease. Genes Brain Behav. 2007;6(1):54-65.
[65] The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
[66] Wei Y, Gong K, Zheng Z, et al. Chitosan/silk fi broin-based tissueengineered graft seeded with adipose-derived stem cells enhances nerve regeneration in a rat model. J Mater Sci Mater Med. 2011; 22(8):1947-1964.
[67] Wei Y, Gong K, Zheng Z, et al. Schwann-like cell differentiation of rat adipose-derived stem cells by indirect co-culture with Schwann cells in vitro. Cell Prolif. 2010;43(6):606-616.
[68] Blurton-Jones M, Kitazawa M, Martinez-Coria H, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106(32):13594-13599.
[69] Bevins RA, Besheer J. Object recognition in rats and mice: a onetrial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006;1(3):1306-1311.
Copyedited by Norman C, Patel B, Yu J, Yang Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.131596
Yandao Gong, State Key Laboratory of Biomembrane and Membrane
Biotechnology, School of Life Sciences, Tsinghua University, Beijing 100084, China, gongyd@tsinghua.edu.cn.
http://www.nrronline.org/
Accepted: 2014-03-24
杂志排行
中国神经再生研究(英文版)的其它文章
- The synthetic thyroid hormone, levothyroxine, protects cholinergic neurons in the hippocampus of naturally aged mice
- Citalopram increases the differentiation ef fi cacy of bone marrow mesenchymal stem cells into neuronal-like cells
- Fusion protein of single-chain variable domain fragments for treatment of myasthenia gravis
- Similar effects of substance P on learning and memory function between hippocampus and striatal marginal division
- Regulatory effects of anandamide on intracellular Ca2+concentration increase in trigeminal ganglion neurons
- Virtual reality interface devices in the reorganization of neural networks in the brain of patients with neurological diseases
