In vitro anti oxidant activity and acute oral toxicity of Terminalia paniculata bark ethanolic extract on Sprague Dawley rats
2014-03-23RamgopalMopuriBalajiMeriga
Ramgopal Mopuri, Balaji Meriga
Department of Biochemistry, Sri Venkateswara University, Tirupati-517502, Andhra Pradesh, India
In vitro anti oxidant activity and acute oral toxicity of Terminalia paniculata bark ethanolic extract on Sprague Dawley rats
Ramgopal Mopuri, Balaji Meriga*
Department of Biochemistry, Sri Venkateswara University, Tirupati-517502, Andhra Pradesh, India
PEER REVIEW
Peer reviewer
Dr. Santosh Kumar, Associate Professor, Department of Plant Science, J.N.V. University, Jodhpur-3420011, India.
Tel: 9466152416
E-mail: santoshkumar.1@rediffmail.comComments
Results presented by the authors are well understandable and also useful for further reference to explain the safety of T. paniculata extracts. With regard to anti oxidant activity, ethanolic extract showed very good activity, so it can also play a useful role to detoxify the toxins in the body and improve the antioxidant system.
Details on Page 297
Objective:To ensure the safety and evaluate the anti oxidant activity of Terminalia paniculata (T. paniculata) ethanolic extract in Sprague Dawley rats.
Terminalia paniculata, Acute toxicity, 2, 2-Diphenyl-1-picrylhydrazyl, Biochemical activity, Hematological parameters
1. Introduction
The usage of medicinal plants has great importance from ancient times[1], because plants produce a wide range of drugs to widen the therapeutic arsenal[2,3]. However, during the past few decades, traditional system of medicine has drawn tremendous attention forin vivostudies[4] and for this reason, more researches are carried out in order to determine the toxicity of medicinal plants and their products. Toxicity is an expression of being poisonous, indicating the state of adverse effects led by the interaction between toxicants and cells. This mechanism of action may vary depending on the cell membrane and chemical properties of the toxicants. It may occur within the cell membrane or on the cell surface or tissue beneath as well as at the extracellular matrix. In most of the cases vital organs such as liver and kidney are affected by the toxicants[5].
Terminalia paniculata(T. paniculata) is a tropical tree belonging to Combretaceae family, with a large natural distribution in Southern and Western parts of India. Variousparts of the plant possess good medicinal values. Flower and bark extracts have been reported to treat cholera, inflammation and menstrual disorders[6]. Some of the phytochemicals like tannins and flavonoids have been isolated from the heartwood ofT. paniculata. Some of the isolated compounds including ellagic acid, dimethylellagic acid, pentamethyl flavellagic acid, trimethyl flavellagic acid and β sitosterol have been isolated[7]. Nearly 14% of the tannins along with gallic acid are isolated from the bark ofT. paniculata[8]. It is also used to treat cough, bronchitis, cardiac debility, hepatitis and diabetes and has spermicidal activity too[8,9]. The anti oxidant activity with 2, 2 diphenyl-1-picrylhydrazyl (DPPH) has been evaluated by Shaluet al[10]. The present study aims to evaluate the toxicity ofT. paniculatabark ethanolic extract on Sprague Dawley rats for ensuring safety and exploring the beneficial role of this plant to treat various ailments.
2. Materials and methods
2.1. Collection and extraction of plant material
The bark ofT. paniculatawas collected from Tirumala forest hills in Tirupati, Andhra Pradesh, India. The plant was authenticated by Dr. Madhavachetty, Department of Botany, S.V. University, Tirupati, voucher number 136, and a specimen has been preserved at the departmental herbarium. The bark ofT. paniculatawas dried under shade, pulverized to coarse powder and extracted with 99% ethanol. The filtrate obtained was evaporated to dryness at 50-65 °C in a rotary vacuum evaporator to obtain a dark colored molten mass.
2.2. Phytochemical analysis and in vitro antioxidant assay
Preliminary phytochemical analysis ofT. paniculatawas carried out by standard methods[11]. Phytochemical constituents such as saponins, steroids, carbohydrates, anthroquinones, polyphenols, tannins, triterpenoids, flavonoids and alkaloids were qualitatively analyzed in this study.
2.3. In vitro antioxidant activity
The 2, 2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity ofT. paniculataextracts (hexane, ethyl acetate and ethanolic) were determined by an assay method reported earlier[12]. Solution of DPPH in 95% ethanol (0.1 mmol/L) was prepared and 1 mL of this solution was added to 3 mL of various concentrations of plant extract as well as to standard compound (ascorbic acid 1-5 mg). After 30 min, absorbance was measured at 517 nm. The percentage of inhibition was calculated by comparing the absorbance values of control and samples. The percentage of inhibition was calculated using the following equation:
% Inhibition=[(AB-AA)/AB]×100
Where, AB=Absorbance of blank DPPH solution, AA=
Absorbance of tested extract.
2.4. Animals
Male Sprague Dawley rats were obtained from National Institute of Nutrition, Hyderabad, India. All animals were housed under (22±2) °C temperature, 40%-60% humidity and 12-12 h light-dark cycle. Rats weighing 150-160 g were taken for the study and they were broadly divided in to two groups. Experimental protocols were followed as per ethical guidelines of our institute (Resolution No: 36/2012-2013/ (i)/a/ CPCSEA/IAEC/SVU/MB-MRG).
2.5. Experimental design
Rats were divided into two groups, normal control group and treated group (n=6). Group 1 served as normal control group (NC). Group 2 wasT. paniculataethanolic extract treated group (1 000 mg/kg body weight).
2.6. Collection of serum
For biochemical analysis, all rats were fasted overnight on the 14th day, blood samples were collected from retro orbital puncturing and serum was separated by centrifugation at 3 000 r/min for 10 min, and then stored at -80 °C for further analysis.
2.7. Biochemical parameters
2.7.1. Hematological tests
Blood samples were collected in heparinized tubes and used for estimating blood cell counts and hemoglobin percent.
2.7.2. Liver and renal function tests
The activities of serum glutamate pyruvate transaminase (SGPT), serum glutamate oxaloacetate transaminase (SGOT) and alkaline phosphatase (ALP), albumin and bilirubin levels were estimated using the assay kits (Lab Care Diagnostics, India). Total protein was estimated by the method of Lowryet al[13]. Serum creatinine, urea and uric acid were measured by Jaffe’s method and duacetyl monoxime methods[14].
2.8. Statistical analysis
Results are expressed as mean±SD. The statistical analyses was carried out by using One-way analysis (ANOVA) andPvalue is significant atP<0.05.
3. Results
3.1. Phytochemical screening of T. paniculata
In the present study, the solvent extracts were prepared based on their polarity. Qualitative phytochemical analysis was carried out in all the extracts (hexane, ethyl acetate and ethanol) and results were presented in Table 1. Among the three solvent extracts, ethanol extract was found to contain high levels of polyphenols and tannins, moderate levels of steroids, triterpenoids and alkaloids.

Table 1 Phytochemical analysis of T. paniculata bark extracts.
3.2.DPPHradical scavenging activity
DPPH assay evaluates the ability of antioxidants to scavenge free radicals. The reduction in DPPH absorption indicates the capacity of the extract to scavenge free radicals. In the present study, the antioxidative activity of various extracts fromT. paniculatawas determined and compared with the standard, ascorbic acid (Figure 1). The concentration responses of the extracts were shown in Figure 1. Among all the tested extracts with various concentrations (1 to 5 mg/mL), ethanolic extract (5 mg/mL) possessed highest radical scavenging activity (80%-87%) and results were very near to ascorbic acid.

Figure 1. DPPH radical scavenging activity of T. paniculata extracts.
3.3. Toxic effect of T. paniculata ethanolic extract
The toxic effect ofT. paniculataethanolic extract on the appearance and behavioral observations of rats were shown in Table 2. In our study even at a dose of 1 000 mg/kg body weight, we observed no toxic symptoms or mortality rate in treated group. All the animals in treated group were alive up to 14 d after administration of the plant extract. The behavioural changes were observed from first day to 14th day in control and treated groups. Both the groups were normal and no changes were observed in behavior, sleep, eyes, salivation, diarrhea like problems, skin and hair loss, lethargy and food consumption and water intake. Moreover, body weights were (not shown) also very similar in normal control and test groups.
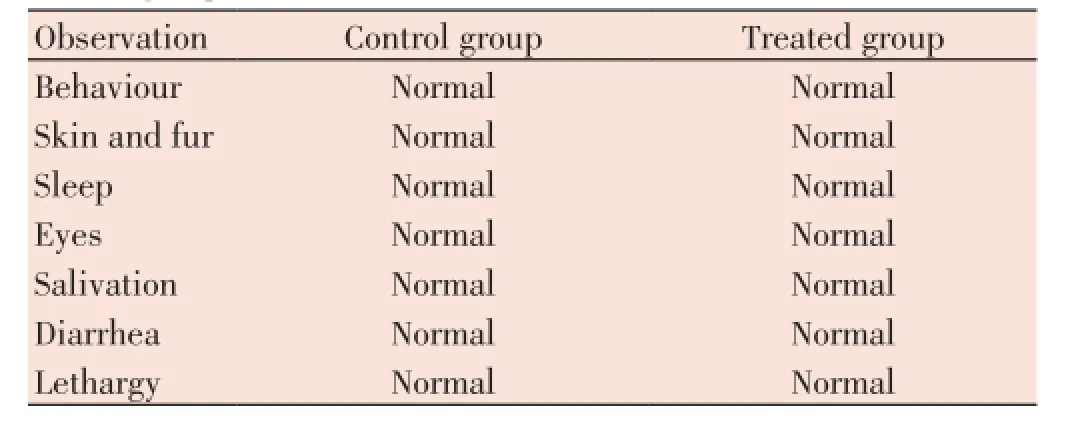
Table 2 General appearance and behavioral observations in control and treated groups.
3.4. Hematological parameters
Blood samples were collected from all animals on 15th day and hematological parameters were observed and results are shown in Table 3. Hemoglobin concentration was increased in treated group compared to control group, remaining parameters were similar in both treated and normal control groups (Table 3).
3.5. Liver and renal function parameters
Biochemical parameters such as SGOT, SGPT and ALP, total proteins, albumins, total bilirubin, serum creatinine, urea and uric acid levels were studied in control and treated groups (Tables 4 and 5). Liver markers SGPT, SGOT, ALP, creatinine, urea and uric acid levels were found to be little lower in treated groups compared to the control group, where as total proteins, albumin levels and total bilrubins were almost same in both the groups. Administration ofT. paniculataethanolic extract had very effective antioxidant activity and protective character, which is supported by theimprovements of biochemical parameters.

Table 3 Oral administration of T. paniculata ethanolic extract on hematological changes in Sprague Dawley rats.

Table 4 Effect of T. paniculata ethanolic extract on liver function tests.

Table 5 Effect of TPEE on renal function tests.
4. Discussion
Due to negligible adverse effects, natural compounds take an important role in therapeutic applications. However, there is a lack of scientific validation on the toxicity and adverse effects of these natural compounds. Therefore, scientific knowledge towards acute oral toxicity study is much needed, which will not only help identify the range and concentration of dose that could be used subsequently, but also to reveal the possible clinical signs elicited by the substances under investigation. In addition, it is also a useful parameter to investigate the therapeutic index of drugs and xenobiotics[15]. In our work, the phytochemical analysis of different solvent extracts (hexane, ethyl acetate and ethanol) fromT. paniculatawas carried out. Among the three solvent extracts, ethanolic extract contained high levels of polyphenols, tannins, alkaloids, and triterphenoids. Similar results were reported in heart wood ofT. paniculata[8].In vitroDPPH radical scavenging activity ofT. paniculataethanolic extract showed that, ethanolic extract ofT. paniculatapossessed scavenging activity very near to ascorbic acid, the standard. Previously Shaluet al.[10] reported the DPPH radical scavenging activity in methanolic extract ofT. paniculataandMentha longifolia.
Acute toxicity study was carried out with a dose of 1 000 mg/kg body weight. Under this we have considered parameters such as skin color, hair loss, eating and sleeping patterns and other behavioural observations. Potential toxicity effect, hematological and biochemical parameters (liver and renal function tests) were studied. Generally elevation of SGOT, SGPT, ALP, creatinine, bilrubins and uric acid are found in liver and kidney damage by any toxic substance or under disease condition, but very healthy and protective results were observed in the present work whenT. paniculataethanolic extract was administered. Some of the species ofTerminaliagenus such asTerminalia chebula, Terminalia belerica, Terminalia arjuna, Terminalia mollisandTerminalia avicennioideshave been previously reported to exhibit similar activities and have been used as good therapeutic agents[16-20]. Based on the results found in our study, we conclude that,T. paniculataethanol extract was safer and non toxic and could be well used for pharmacological and therapeutic purposes.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The Authors express thanks to University Grants Commission (F.No.42-666/2013) and Council of Scientific and Industrial Research (09/152 (292)/2013, EMR-I), New Delhi, India, for providing financial support.
Comments
Background
Medicinal plants are versatile sources for a variety of phytochemicals. Few of them show toxic effects. So, it is necessary to carry out cytotoxic studies before proceeding to further studies. This will ensure a plant product to be used as therapeutic agent.
Research frontiers
In the present research work authors reported thein vitroantioxidant activity by DPPH method and evaluated the acute toxicity ofT. paniculatabark ethanolic extract on Sprague Dawley rats.
Related reports
Behavior, hematological and biochemical activities are the most important experiments to assess the toxicity. SGOT, SGPT, and ALP are the most commonly used indicators of liver functioning. Normally in acute injury condition thelevels of SGOT, SGPT and ALP increases. In other cytotoxic studies the said observations were reported.
Innovations and breakthroughs
Liver and renal function tests are primary objectives to evaluate toxicity. Authors in the present study explained about acute oral toxicity with good evident biochemical parameters.
Applications
In medication, safety and mode of the use of medicine is very important. When compared to pharmaceutical drugs, natural plant compounds usually show negligible side effects. However, to ensure this and safety, cytotoxic effect of bark extract ofT. paniculatawas evaluated. Based on the results and existed literature, it can be concluded thatT. paniculatais an excellent medicinal plant and further studies can be continued to reveal its therapeutic efficiency.
Peer review
Results presented by the authors are well understandable and also useful for further reference to explain the safety ofT. paniculataextracts. With regard to anti oxidant activity, ethanolic extract showed very good activity, so it can also play a useful role to detoxify the toxins in the body and improve the antioxidant system.
[1] Parveen, Upadhyay B, Roy S, Kumar A. Traditional uses of medicinal plants among the communities of Churu district in the Thar Desert, India. J Ethanopharmacol 2007; 113(3): 387-399.
[2] Patwardhan B, Mashelkar RA. Traditional medicine-inspired approches to drug discovery: can ayurveda show the way forward. Drug Discov Today 2009; 14(15-16): 804-811.
[3] Patil UH, Gaikwad DK. Phytochemical profile and antibacterial activity of stem bark of Anogeissus latifolia. Pharmacogn J 2010; 2(17): 72-73.
[4] Mazid M, Khan TA, Mohammad F. Medicinal plants of rural India. A review of use by indian folks. Indo Global J Pharm Sci 2012; 2(3): 286-304.
[5] Asante-Duah K. Public health risk assessment for human exposure to chemicals. USA: Springer; 2002, p. 6.
[6] Talwar S, Nandakumar K, Nayak PG, Bansal P, Mudgal J, Mor V, et al. Anti-inflammatory activity of Terminalia paniculata bark extract against acute and chronic inflammation in rats. J Ethnopharmacol 2011; 134(2): 323-328.
[7] Talwar S, Jagani HV, Nayak PG, Kumar N, Kishore A, Bansal P, et al. Toxicological evalution of Terminalia paniculata bark extract and its protective effect against CCl4induced liver injury in rodents. BMC Complement Altern Med 2013; 13: 127.
[8] Eesha BR, Mohanbabu Amberkar V, Meena Kumari K, Sarath B, Vijay M, Lalit M, et al. Hepatoprotective activity of Terminalia paniculata against paracetamol induced hepatocellular damage in Wistar albino rats. Asian Pac J Trop Med 2011; 4: 466-469.
[9] Ramachandran S, Rajasekaran A, Manisenthilkumar KT. Investigation of hyperglycemic, hyperlipidemic and antioxidant activity of aqueous extract of Terminalia paniculata bark in diabetic rats. Asian Pac J Trop Biomed 2012; 2(4): 262-268.
[10] Agrawa S, Kulkarni GT, Sharma VN. A comparative study on the antioxidant activity of methanolic extracts of Terminalia panicualta and Madhuca longifolia. Free Radicals and Antioxidents 2011; 1(4): 62-68.
[11] Williams CA, Grayer RJ. Anthocyanins and other flavonoids. Nat Prod Rep 2004; 21(4): 539-573.
[12] Sangilimuthu AY, Anitha JR, Sathishkumar R. In vitro antioxident activity of Barleria noctiflora L.f. Asian Pac J Trop Biomed 2012; 2(Suppl 2): S716-S722.
[13] Lowry OH, Rosenbrough NJ, Farr Al, Randall RJ. Protein measurement with the folin phenol reagent. J Boil Chem 1951; 193: 265-275.
[14] Sirasanagandla S, Kasetti RB, Shaik AN, Natava R, Surtineni VP, Cirradur SR, et al. Anti hyperglycemic and anti hyperlipidemic activity of 2-(4-[(2-hydroxybenzyl) amino]-phenyl aminomethyl)-phenol in STZ induced diabetic rats. Eur J Med Chem 2013; 66: 400-406.
[15] Adaramoye OA, Osaimoje DO, Akinsanya AM, Nneji CM, Fafunso MA, Ademowo OG. Changes in anti oxidant status and biochemical indices after acute administration of artemether, artemether-lumefantrine and halofantrine in rats. Basic Clin Pharmacol Toxicol 2008; 102(4): 412-418.
[16] Panunto W, Jaijoy K, Lerdvuthisopon N, Lertprasertsuke N, Jiruntanat N, Soonthorchareonnon N, et al. Acute and chronic toxicity studies of the water extract from dried fruits of Terminalia chebula in rats. Int J Appl Res in Nat Products 2010; 3(4): 36-43.
[17] Thanabhorn S, Jaijoy K, Thamaree S, Ingkaninan K. Acute and subacute toxicity of the ethanolic extract from the fruits of Terminalia belerica. Mahidol Univ J Pharm Sci 2006; 33(1-4): 23-30.
[18] Biswas M, Karan TK, Bhattacharya S, Suresh kumar RB, Ghosh AK, Haldar PK. Acute and sub chronic toxicity study of Terminalia arjuna leaf in Swiss Albino mice. Pharmacologyonline 2011; 1: 366-371.
[19] Bulus T, Atawodi SE, Mamman M. Acute toxicity effect of the aqueous extract of Terminalia avicennioides on white albino rats. Sci World J 2011; 6(2): 1-4.
[20] Bulus T, Atawodi SE, Mamman M. Acute toxicity evolution of aqueous extract of Terminalia mollis on rats. ChemClass J 2007; 4: 57-60.
10.12980/APJTB.4.2014C1068
*Corresponding author: Balaji Meriga, Assistant Professor, Department of Biochemistry, Sri Venkateswara University, Tirupati-517502, Andhra Pradesh, India.
Tel: 91-98490-86856
E-mail: Balaji.merega@gmail.com
Foundation Project: Supported by University Grants Commission (F.No.42-666/2013) and Council of Scientific and Industrial Research (09/152 (292)/2013, EMR-I), New Delhi, India.
Article history:
Received 2 Feb 2014
Received in revised form 12 Feb, 2nd revised form 16 Feb, 3rd revised form 22 Feb 2014
Accepted 15 Mar 2014
Available online 28 Apr 2014
Methods:The solvent extracts (hexane, ethyl acetate and ethanol) of T. paniculata were subjected to phytochemical analysis and their DPPH radical scavenging activity was assayed. The oral acute toxicity was evaluated using ethanolic extract of T. paniculata.
Results:Ethyl acetate and ethanolic extracts showed more phytochemicals, whereas highest DPPH scavenging activity was found in ethanolic extract. In an acute toxicity study, T. paniculata ethanolic extract was orally administered (1 000 mg/kg body weight) to rats and observed for 72 h for any toxic symptoms and the dose was continued up to 14 d. On the 15th day rats were sacrificed and blood samples were collected from control and test animals and analyzed for some biochemical parameters. We did not observe any behavioral changes in test groups in comparison with their controls. Also, there were no significant alterations in biochemical, hematological (hemoglobin content and blood cells count) and liver function parameters such as serum glutamate pyruvate transaminase, serum glutamate oxaloacetate transaminase, alkaline phosphatase, total proteins, albumin and bilirubin levels between T. paniculata ethanolic extract treated and normal control groups.
Conclusions:Together our results demonstrated that T. paniculata ethanolic possessed potent antioxidant activity and it was safer and non toxic to rats even at higher doses and therefore could be well considered for further investigation for its medicinal and therapeutic efficacy.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Extreme human annoyance caused by Ctenocephalides felis felis (cat flea)
- Theoretical and experimental study on lipophilicity and wound healing activity of ginger compounds
- The effects of exposure to pesticides on the fecundity status of farm workers resident in a rural region of Fars province, southern Iran
- Effects of melatonin on changes in cognitive performances and brain malondialdehyde concentration induced by sub-chronic coadministration of chlorpyrifos and cypermethrin in male Wister rats
- Comparative susceptibility to permethrin of two Anopheles gambiae s.l. populations from Southern Benin, regarding mosquito sex, physiological status, and mosquito age
- A cross sectional study on antibiotic resistance pattern of Salmonella typhi clinical isolates from Bangladesh
