In vitro antioxidant and cytotoxic activities of essential oil of Feronia elephantum Correa
2014-03-23RamarajThirugnanasampandanDelmaDavid
Ramaraj Thirugnanasampandan, Delma David
In vitro antioxidant and cytotoxic activities of essential oil of Feronia elephantum Correa
Ramaraj Thirugnanasampandan*, Delma David
PG and Research Department of Biotechnology, Kongunadu Arts and Science College, GN Mills, Coimbatore-641029, Tamil Nadu, India
PEER REVIEW ABSTRACT
Peer reviewer
Dr. Jayakumar Rajarajeswaran, Post Doctoral Research Fellow, Department of Molecular Medicine, University of Malaya, Malaysia.
Tel: +60102799286
E-mail: jayakumar7979@gmail.com
Comments
The study focuses on the phytochemical and bioactivities of essential oil isolated from F. elephantum. The breast cancer cell line MCF 7 cells are the suitable cell model for the analysis of cytotoxicity.
Details on Page 293
Objective:To analyse the chemical composition and evaluation of antioxidant, cytotoxic and DNA fragmentation activities of essential oil of Feronia elephantum Correa.
Methods:Chemical composition analysis of hydrodistilled essential oil was determined by gas chromatography-mass spectrometry and in vitro antioxidant activity of oil was determined by DPPH free radical, hydroxyly radical scavenging, metal chelating and prevention of deoxyribose degradation. Cytotoxicity and DNA fragmentation activities against breast cancer cells (MCF-7) were also analyzed.
Results:Gas chromatography-mass spectrometry analysis revealed the presence of 24 compounds with caryophyllene oxide (62.29%) as major compound. A considerable antioxidant, cyotoxic and DNA fragmentation activities of oils was observed.
Conclusions:The result of this study clearly indicates oil could be useful for food preservation and preparation.
Feronia elephantum, Essential oil, Antioxidant, Cytotoxicity, DNA fragmentation
1. Introduction
Feronia elephantumCorrea (F. elephantum) belongs to Rutaceae which is widely distributed throughout India. Leaf, bark, root and fruit pulp are used for the treatment of various ailments.In vitroantioxidant and antibacterial activities of fruit pulp was reported by Nithya and Saraswathi[1]. Essential oil isolated from leaves showed antibacterial and antifungal activities[2]. Bioguided extraction of leaf showed hepatoprotective and cytotoxic activities[3]. Hepatoprotective activity of aqueous extract of leaves was reported[4]. Based on the immense medicinal use, the study was attempted to isolate and analyse the chemical composition, and evaluate the antioxidant and cytotoxic activities of essential oil from the leaves.
2. Materials and methods
2.1. Plant material
Fresh leaves ofF. elephantumwas collected from intact plant of original habitats.
2.2. Hydrodistillation of leaves
Freshly collected leaves (2 kg) were hydrodistilled for 3 h using Clevenger apparatus for essential oil isolation. Isolated oil was dried over anhydrous sodium sulphate and filled in small vials, tightly sealed and stored in a refrigerator at 4 °C until further analysis.
2.3. Chemical composition analysis
2.3.1. Gas chromatography (GC) analysis
GC analysis was carried out using Varian 3800 gas chromatography equipped with mass selective detector coupled to front injector type 1079. The chromatograph was fit with DB 5 column (30 m×0.25 mm). The injector temperature was set at 280 °C, and the oven temperature was initially be at 45 °C then programmed to 300 °C at the rate of 10 °C/min and finally held at 200 °C for 5 min. Helium was used as a carrier gas with the flow rate of 1 mL/min. The percentage of composition of the essential oil wascalculated by the GC peak areas.
2.3.2. Gas chromatography/mass spectrometry (GC-MS) analysis
Gas chromatography coupled with mass spectroscopy was performed using Varian 3800 gas chromatography equipped with Varian 1200 L single quadrupole mass spectrometer. GC conditions were the same as reported for GC analysis and the same column was used. The mass spectrometer operated in the electron impact mode at 70 eV. Ion source and transfer line temperature was maintained 250 °C. The compounds was identified based on the comparison of their retention indices (RI), retention time (RT) and mass spectra. Library search was carried out using the NIST and Wiley GC/ MS spectral database and by co-comparing with the mass spectral data and retention indices in the literature[5].
2.4.DPPHfree radical scavenging activity
Briefly, different concentration (50, 100, 150, 200 and 250 µg/mL) of oil was mixed with 1 mL of methanolic DPPH solution (0.16 mmol/L). Negative control contained methanol instead of a sample solution. The reaction mixture was incubated for 30 min at room temperature and the absorbance was read at 517 nm[6]. The percentage of DPPH free radical scavenging activity was calculated using the following equation:
%Inhibition=[(AB-AA)/AB]×100
Where ABis the absorption of blank sample; AAis the absorption of test sample.
2.5. Hydroxyl radical scavenging activity
Reaction mixture included 7.5 mmol/L FeSO4, 7.5 mmol/ L 1, 10-phenanthroline, 0.2 mol/L phosphate buffer (pH 7.8), 30 mmol/L H2O2and oil at different concentration (50, 75, 100, 125 and 150 µg/mL). The reaction was started by adding H2O2. After incubation at room temperature for 5 min, the absorbance of the mixture was read at 536 nm[7]. The percentage of hydroxyl radical scavenging activity was caculated with the following equation:
%Inhibition=[(AB-AA)/AB]×100
Where ABis the absorption of blank sample; AAis the absorption of test sample.
2.6. Metal chelating activity
Different concentration of oil (125, 150, 175, 200, 225 µg/ mL) was mixed with 50 µL of ferrous chloride and 1.6 mL of 80% methanol. After 5 min, the reaction was initiated by the addition of 5 mmol/L ferrozine and the mixture was shaken on a vortex. The mixture was incubated at room temperature for 10 min. The absorbance of solution was measured at 562 nm on a spectrophotometer[8]. Metal chelating activity was caculated with the equation below:
%Inhibition=[(AB-AA)/AB]×100
Where ABis the absorption of blank sample; AAis the absorption of test sample.
2.7. Prevention of deoxyribose degradation
The different concentration of oil (50, 100, 150, 200 and 250 µg/mL) was mixed with 20 mmol/L deoxyribose, 0.1 mol/ L NaPO4, 20 mmol/L H2O2and 50 mmol/L FeSO4. The reaction mixture was incubated for 60 min at 37 °C. Then 2 mL of 10% ice cold trichloroacetic acid was added, and 1 mL aliquot of the samples was added with 1 mL of 1% TBA. The TBA/sample mixture was heated in a water bath at 95 °C for another 60 min. The absorbance was read at 532 nm[9].
%Inhibition=[(AB-AA)/AB]×100
Where ABis the absorption of blank sample; AAis the absorption of test sample.
2.8. Cell lines and culture
The human breast (MCF-7) cell line was cultured in a T 25 cm2cell culture flask containing Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum, penicillin (100 U/mL) and streptomycin (100 µg/mL). The cell line was incubated in humidified incubator at 37 °C with 5% CO2.
2.8.1.MTTassay
The cell line in T 25 cm2flask was harvested using trypsin and cell number was counted using a hemocytometer. A total of 1×104cells were incubated in 96 well plates containing 100 µL of growth medium for 24 h. Then the cells were treated with various concentration of (25, 50, 75, 100 and 125 µg/mL) essential oil dissolved in medium and further incubated for 48 h. About 20 µL of MTT (5 mg/mL) in phosphate buffered saline was added to each well and the plate was incubated at 37 °C for 4 h. The medium was removed and 100 µL of DMSO was added to each well. After 10 min of incubation at 37 °C, the plate was read at 570 nm using a microplate reader[10]. The percentage of cell viability was calculated as: (absorbance of test/absorption of the control)×100.
2.8.2.DNAfragmentation
Briefly, MCF-7 cells after treatment with different concentration of essential oil for 24 h were centrifuged at 1 500 r/min for 10 min and washed with phosphate buffered saline. The resultant pellet was suspended in lysis buffer (10 mmol/L EDTA, 50 mmol/L Tris-HCL, 0.5% SDS) for 15 min at 55 °C. Lysed cells were digested with proteinase-K (500 µg/mL) at 55 °C for 1 h followed by incubation with 200 µg/mL DNase free RNase at 55 °C for 90 min. The DNA was extracted with twice with 250 µL of phenol: chloroform: isoamyl alcohol (25:24:1) for 1 min and centrifuged at 12 000 r/min for 5 min. The aqueous phase was further extracted with chloroform: isoamyl alcohol (24:1) and centrifuged. DNA was precipitated from aqueous phase with 0.1 volume of 2 mol/L NaCl and 2.5 volumes of chilled ethanol and kept at -20 °C over night. The precipitated DNA was centrifuged at 12 000 r/min for 10 min and dissolved in Tris-EDTA buffer (pH 8.0) and electrophoresed in 1.5% agarose gel at 50 V for 90 min[11]. The gel was photographed using Bio-Rad Gel documentation system.
2.9. Statistical analysis
The data obtained from thein vitroexperiments were analysed using SPSS (10.00) for IC50calculation.
3. Results
3.1. Chemical composition of essential oil
Hydrodistilled leaves ofF. elephantumyielded essential oil with pale yellow in color. Table 1 shows name of the compounds with percentage composition and retention index. A total of 23 compounds were identified and accounted for about 83.87%. The major component of the oil was oxygenated sesquiterpene, caryophyllene oxide which accounted for 62.29% followed by caryophyllene (6.05%), aromadendrene (3.58%), humulene epoxide II (3.00%), phytol (1.21%) and α-copaene (1.03%).
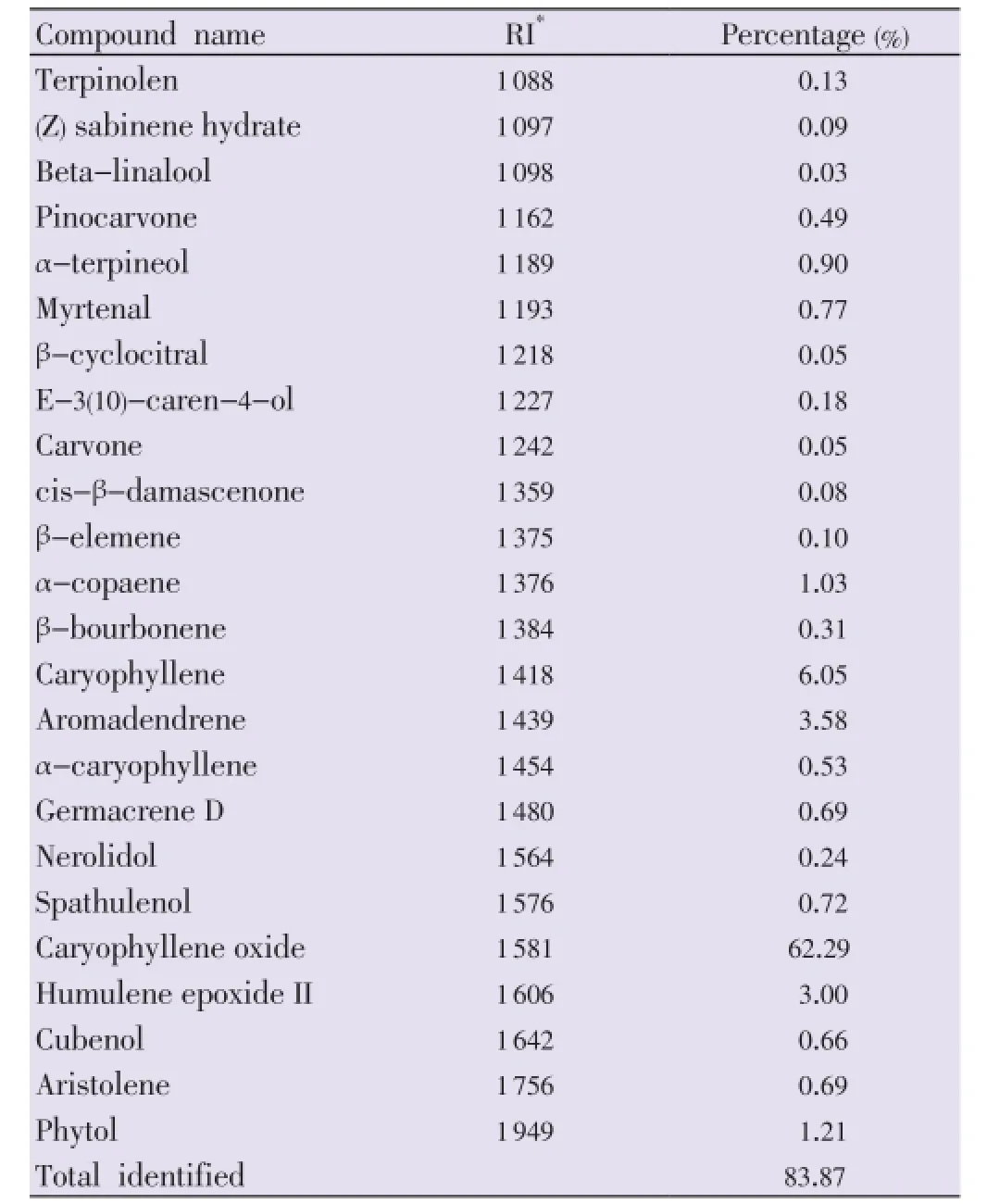
Table 1 Chemical composition of essential oil from the leaves of F. elephantum.
3.2. Antioxidant and cytotoxicity
Antioxidant activity of oil was tested using four different test systems. The oil was observed to significantly scavenge DPPH free radicals with IC50value of (90.18±0.01) µg/mL. Hydroxyl radical scavenging and chelating activity of oil against transition of metal irons were calculated as (79.28± 0.03) and (117.05±0.06) µg/mL respectively. Ability to prevent deoxyribose degradation by inhibiting hydroxyl radicals generated in Fenton reaction was calculated as (55.34±0.03) µg/mL. Concentration of oil needed to kill 50% of human breast cancer cell lines (MCF-7) was observed as (89.19±0.02) µg/mL. Genomic DNA was isolated from treated MCF-7 cells after 24 h and electrophoresed. The result indicated that there was a DNA fragmentation but no clear ladder formation (Figure 1). DNA isolated from untreated cells did not show any DNA fragmentation.

Figure 1. DNA fragmentation of MCF-7 cells treated with essential oil of F. elephantum.Lane 1: Marker 1 000 bp ladder; Lane 2: Control; Lane 3: Con: 312 µg/mL; Lane 4: Con: 625 µg/mL; Lane 5: Con: 1 250 µg/mL.
4. Discussion
Plant essential oils are aromatic oily liquids, volatile, characterized by a strong odour, rarely coloured and generally with a lower density than that of water. They can be synthesized by all plant organs and extracted from these parts, where they are stored in secretory cells, cavities, canals, epidermic cells or glandular trichomes[12]. In this study, caryophyllene oxide has been identified as major compound in oil, but the previous reports have shown betapinene and trans-anethole as the major compounds in theF. elephantumoil[2,13]. This variation may due to geographical distribution, availability of water, climate, stress, temperature,etc.Meanwhile, caryophyllene oxide has been reported as major compound in the essential oil of rutaceae species such asHaplophyllum lissonotum,Haplophyllum buxbaumiiandHaplophyllum megalanthum[14,15].
Essential oils are complex source of numerous small and large bioactive molecules that attract researchers to evaluate their potent antioxidant, anticancer, anticholinesterase and antimicrobial activities. Result of this study clearly indicatesF. elephantumessential oil as a good source of free radical scavenger with considerable cytotoxic activity. The potent biological activities of essential oils depend on their major constituents. It is assumed that observed antioxidant and cytotoxic activities of oil might be due to the presence of major bioactive compound, caryophyllene oxide. Meanwhile,contribution of minor compounds should also be considered.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
We are grateful to PG and Research Department of Biotechnology, Kongunadu Arts and Science College (Grant No. ARF-KASC 5/2012) for financial support and constant encouragement throughout the study.
Comments
Background
The study focuses on the bioactivities such as antioxidant and cytotoxic activities ofF. elephantum. Previous studies showed the presence of active phytochemicals and its diverse activities. Intense investigation on the phytochemicals ofF. elephantummay reveal the identification of a novel phytochemical with pharmacological properties.
Research frontiers
Determination of cytotoxic effects using MTT assay and DNA fragmentation analysis provides vital evidences. Using GC/MS method, the authors have identified 24 active phytochemicals from this plant.
Related reports
Antioxidant and antibacterial activities of fruit pulp of this plant was reported by Nithya and Saraswathi, 2010. Antibaxcterial, cytotoxic and hepatoprotective activities of this has been reported in the literature. Methods used in this study are standard and the result of this study is productive.
Innovations and breakthroughs
The authors have identified 24 phytochemicals from the essential oil of this plant using GC/MS method. It is evident that the high antioxidant and cytotoxic properties comes from these phytochemicals. The results will motivate future researches in identification of new biological activities and purification of active compounds.
Applications
Essential oils possess diverse chemicals with potent bioactivities. They are commonly used for the treatment of various human disorders. Further studies on animal models and cell lines will reveal the mechanism of action of oils. The study identified new phytochemicals and its bioactivities.
Peer review
The study focuses on the phytochemical and bioactivities of essential oil isolated fromF. elephantum. The breast cancer cell line MCF 7 cells are the suitable cell model for the analysis of cytotoxicity. The presence of phytochemicals were analysed using GC/MS method. The results are clear and the identifications from this study will create further research studies in this plant.
[1] Nithya N, Saraswathi U. In vitro antioxidant and antibacterial efficacy of Feronia elephantum Corres fruit. Indian J Nat Prod Resour 2010; 1: 301-305.
[2] Joshi RK, Badakar VM, Kolkute SD, Khatib N. Chemical composition and antimicrobial activity of the essential oil of the leaves of Feronia elephantum (Rutaceae) from north west Karnataka. Nat Prod Commun 2011; 6: 141-143.
[3] Jain M, Kapadia R, Jadeja R, Thounaojam M, Devkar R, Mishra Sh. Cytotoxicity evaluation and hepatoprotective potential of bioassay guided fractions from Feronia limmonia Linn leaf. Asian Pac J Trop Biomed 2011; 1: 443-447.
[4] Sharma P, Bodhankar SL, Thakurdesa PA. Protective effect of aqueous extract of Feronia elephantum correa leaves on thioacetamide induced liver necrosis in diabetic rats. Asian Pac J Trop Biomed 2012; 2: 691-695.
[5] Adams RP. Identification of essential oil components by gas chromatography and mass spectrometry. 3rd ed. USA: Allured Publication Corporation; 1999.
[6] Kremer D, Kosalec I, Locatelli M, Epifano F, Genovese S, Carlucci G. Anthraquinone profiles, antioxidant and antimicrobial properties of Frangula rupestris (Scop.) Schur and Frangula alnus Mill. bark. Food Chem 2012; 131: 1174-1180.
[7] Zhao GR, Xiang ZJ, Ye TX, Yuan YJ, Guo ZX. Antioxidant activities of Salvia miltiorrhiza and Panax notoginseng. Food Chem 2006; 99: 767-774.
[8] Sharma P, Gujral HS. Effect of sand roasting and microwave cooking on antioxidant activity of barley. Food Res Int 2011; 44: 235-240.
[9] Halliwell B, Gutteridge JMC, Aruoma OI. The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions hydroxyl radical. Anal Biochem 1987; 165: 215-219.
[10] Lau CB, Ho CY, Kim CF, Leung KN, Fung KP, Tse TF, et al. Cytotoxic activities of Coriolus versicolor (Yunzhi) extract on human leukemia and lymphoma cells by induction of apoptosis. Life Sci 2004; 75: 797-808.
[11] Tong X, Lin S, Fujii M, Hou DX. Echinocystic acid induces apoptosis in HL-60 cells through mitochondria mediated death path way. Cancer Lett 2004; 212: 21-32.
[12] Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils-a review. Food Chem Toxicol 2008; 46: 446-475.
[13] Pande C, Tewari G, Singh C, Singh S, Padalia RC. Chemical composition of the essential oil of Feronia elephantum Correa. Nat Prod Res 2010; 24: 1807-1810.
[14] Javidnia K, Miri R, Soltani M, Varamini P. Volatile constituents of two species of Haplophyllum A. Juss. from Iran [H. lissonotum C. Town, and H. buxbaumii (Poir.) G. Don. subsp. mesopotamicum (Boiss.) C. Town]. J Essent Oil Res 2009; 21: 48-51.
[15] Ünver-Somer N, Kaya Gİ, Sarikaya B, Önür MA, Özdemir C, Demirci B, et al. Composition of the essential oil of endemic Haplophyllum megalanthum Bornm. from Turkey. Rec Nat Prod 2012; 6: 80-83.
10.12980/APJTB.4.2014B878
*Corresponding author: Dr. Ramaraj Thirugnanasampandan, PG and Research Department of Biotechnology, Kongunadu Arts and Science College, GN Mills, Coimbatore-641029, Tamil Nadu, India.
Tel: +91 9894793074
E-mail: rtsampandan@yahoo.com
Foundation Project: Supported by PG and Research Department of Biotechnology, Kongunadu Arts and Science College (Grant No. ARF- KASC, 5/2012).
Article history:
Received 18 Jan 2014
Received in revised form 25 Jan, 2nd revised form 7 Feb, 3rd revised form 16 Feb 2014
Accepted 27 Mar 2014
Available online 28 Apr 2014
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- The effects of exposure to pesticides on the fecundity status of farm workers resident in a rural region of Fars province, southern Iran
- A cross sectional study on antibiotic resistance pattern of Salmonella typhi clinical isolates from Bangladesh
- Comparative susceptibility to permethrin of two Anopheles gambiae s.l. populations from Southern Benin, regarding mosquito sex, physiological status, and mosquito age
- Effects of melatonin on changes in cognitive performances and brain malondialdehyde concentration induced by sub-chronic coadministration of chlorpyrifos and cypermethrin in male Wister rats
- Phytochemical and in vitro biological investigations of methanolic extracts of Enhydra fluctuans Lour.
- Theoretical and experimental study on lipophilicity and wound healing activity of ginger compounds
