New flavonoids from bioactive extract of Algerian medicinal plant Launeae arborescens
2014-03-23KhaledSekkoumNasserBelboukhariAbdelkrimCheriti
Khaled Sekkoum, Nasser Belboukhari,*, Abdelkrim Cheriti
1Bioactive Molecule & Chiral separation Laboratory, University of Bechar, Bechar, 08000, Algeria
2Phytochemistry & Organic Synthesis Laboratory, University of Bechar, Bechar, 08000, Algeria
New flavonoids from bioactive extract of Algerian medicinal plant Launeae arborescens
Khaled Sekkoum1, Nasser Belboukhari1,2*, Abdelkrim Cheriti2
1Bioactive Molecule & Chiral separation Laboratory, University of Bechar, Bechar, 08000, Algeria
2Phytochemistry & Organic Synthesis Laboratory, University of Bechar, Bechar, 08000, Algeria
PEER REVIEW
Peer reviewer
Prof. Salih Hacini, Laboratory of Fine Chemistry, Department of Chemistry, Faculty of Exacts and Applied Sciences, University of Oran, Algeria. Tel: +213-662330812; +21341519229
E-mail: hacini.salih@univ-oran.dz
Comments
The research presented by the authors complements previous work on the phytochemical study of this plant. In fact, after studying the methanol fraction of the extraction of this plant, the authors are interested in the butanol fraction by isolating and identifying new flavonoids using efficient chromatographic and spectroscopic methods. The description of these novel compounds of quite complex structures is a very interesting scientific result to use to search for active substances of this medicinal plant used by the people of these regions.
Details on Page 271
Objective:To investigate the butanol fraction of the water/acetone extract and isolate of the new flavonoids from Launeae arboescens.
Launeae arborescens, Asteraceae, Flavanone, Isoflavanone, Glycosid flavanone
1. Introduction
Launeae arborescens(L. arborescens) is a medicinal plant having capacities of important propagation. Following its biotope, associate to different species, it is frequently notably in the whole region of Algerian southwest of Wadi-Namous until the region of Karzaz. According to our ethnopharmacological survey[1,2],L. arborescensis used for treatment of the illnesses gastric. Following our phytochemical works achieved on the polyphenols of the methanolic extract of aerial part ofL. arborescens, we are also interested to investigate the butanol fraction of the water/acetone extract and isolate of the new flavonoids from this plant[3].
2. Materials and methods
2.1. General experimental procedure
UV spectra were obtained in MeOH solvent with Unicam UV 300 spectrophotometer. IR spectra were obtained with a Avatar 320 FT-IR spectrophotometer. The NMR spectra were taken on a Bruker GP 250 (1H, 300 MHz;13C, 125 MHz) Spectrometer. EIMS spectra were obtained on a VG Trio-2 spectrometer. TLC was carried out on silica gel 60 F254plates (Merck, Germany). Column chromatography was performed over silica gel 60 (Merck, particle size 230-400 mesh).
2.2. Plant materials
The aerial part ofL. arborescenswere collected in March 2000 from Bechar (hammada, Oued saoura, Bechar, Algeria). The botanical identification and a voucher specimen was conserved at the phytochemical herbarium of Phytochemistry and Organic Synthesis Laboratory of University Center of Bechar under to accession number CA99/25[2,4].
2.3. Extraction and isolation
The dried aerial part of plants (200 g) ofL. arborescenswere extracted with acetone-water (70:30) using soxhlet apparatus; reflux for 6 h was performed. The residue was evaporated in vacum apparatus until two third, then the third of aqueous residue was partitioned sequentially withn-hexan, ethyl ether, EtOAc andn-BuOH[5]. To purify and to identify the constituents of butanol fraction (2.36 g), one achieved some separations by liquid chromatography on column, one using a column in glass of type 20 mm/300 mm (29/39) filled with a stationary phase of silica gel (0,20 mm) and the mobile phase chosen for this separation is acetone/toluene/formic acid (60:80:10)[6].
3. Results
The separation has been done previously on a mass of 533 mg of butanol extract in the same conditions. We regrouped the final results after several separations and analysis chromatographic (Table 1).TLC analysis of the samples separated by liquid chromatography on column revealed the existence of eight products to differentRfof which one recovered them after spraying of the solvents for determination of the structures using spectroscopic methods (Table 1).
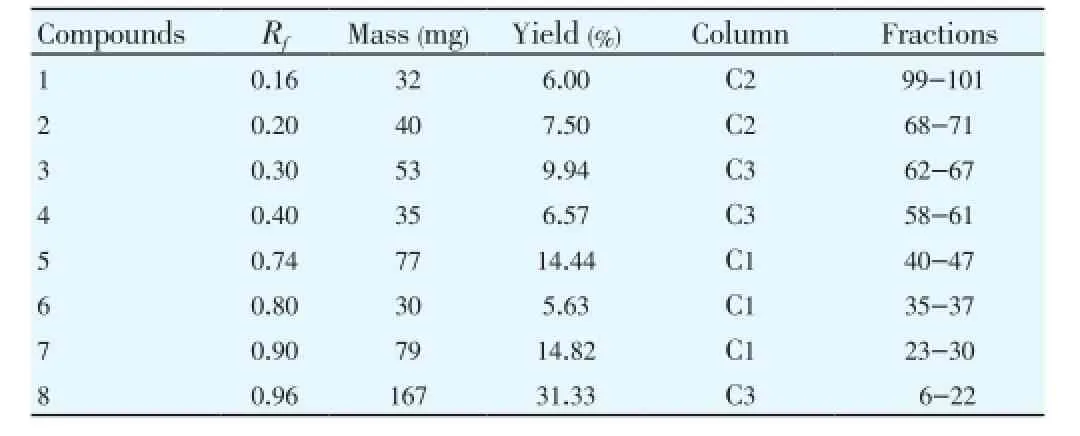
Table 1 Results of liquid column chromatography (C1,C2,C3: first, second and third column).
We have successfully separated three products in the first column (5, 6, 7), two other in the second column (C1, C2) and three in third column (3, 4, 8) by this protocol (Figure 1).
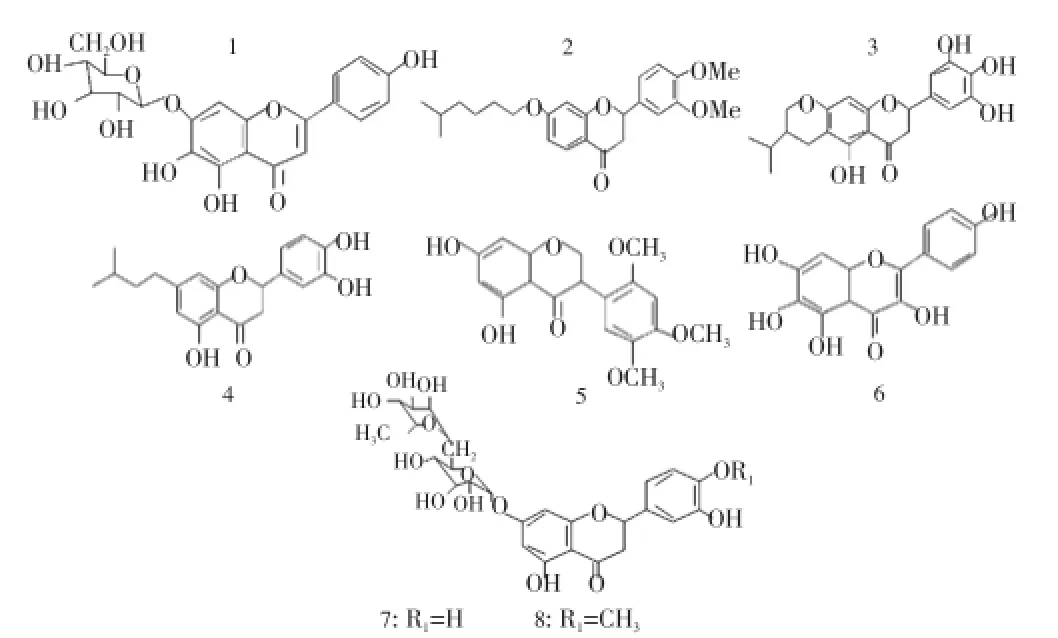
Figure 1. Structures of flavonoids isolated from aerial part of L. arborescens.
7-O-[α-rhamnopyranosyl 4’,5,6-Trihydroxy flavone. (1): Tf=169 °C. UVmax(MeOH): 237, 275, 331 nm. [35] IR(KBr): 3 415, 2 924, 1 613, 1 503, 1 388, 1 257, 1 071, 815, 755 cm-1. 1H NMR: 6.59 (H-3 , br s ), 6.98 (H-8, s), 7.84 (H-2’, d,J= 6.8 Hz), 6.91 (H-3’, d,J=7.3 Hz), 6.91 (H-5’, d,J=7.3 Hz), 7.84 (H-6’, d,J=6.8 Hz), 5.09 (H-1”, d,J=5.9 Hz), 3.59 (H-2”, m), 3.56 (H-3”, m), 3.43 (H-4”, t,J=8.3 Hz ), 3.59 (H-5”, m), 3.74 (H-6”a, m), 3.98 (H-6”b, d,J=11.7 Hz). RMN13C: 166.77 (C-2), 103.49 (C-3), 184.39 (C-4), 147.92 (C-5), 131.86 (C-6), 152.75 (C-7) 95.82 (C-8), 151.75 (C-9), 107.47 (C-10), 123.29 (C-1’), 129.56 (C-2’), 117.00 (C-3’), 162.78 (C-4’), 117.00 (C-5’), 129.56 (C-6’), 102.66 (C-1’’), 74.73 (C-2”), 77.52 (C-3”), 71.42 (C-4”), 78.58 (C-5”), 62.55 (C-6”).
4’,5’-Di-Methoxy 7-(5’’-Me Hexan)1-oyl flavanone (2) molecular formula C24H30O5, 72.34 (C), 7.59 (H), 20.07(O), MW=398.50, UV spectra: 254, 276, 336, IR (KBr): 3 405, 2 924, 2 853, 1 771, 1 738, 1 509, 1 684, 1 613, 1 252, 1 127, 1 061, 1 383, 755, 815 cm-1, 1H NMR (CDCl3, 300 MHz): 5.36 (t, H-2, 3 Hz, 1.8 Hz), 2.95 (d, H-3a, 1.8), 2.88 (d, H-3b, 3 Hz), 7.392 (d, H-5, 6.8 Hz), 7.325 (d, H-6, 6.8 Hz), 7.28 (s, H-8), 7.455 (d, H-6’, 7.2 Hz), 7.303 (d, H-5’, 7.2 Hz), 7.37 (s, H-2’), 3.738 (t, 7 Hz, H-1”, 2H), 3.668 (s, OCH3, 6H), 1.67 (m, 1.6 Hz, 2H-2, 2H-3, 2H-4, H-5, 7H), 0.86 (d, 6.6 Hz, H-6, 6H),13C NMR (CDCl3, 300 MHz):
3”-isopropyl pyrano [1”:7,4”:6] 3’,4’,5’,5-Tetrahydroxy flavanone (3): molecular formula C21H22O7: 65.28 (C), 5.74 (H), 28.98 (O), MW=386.41, UV spectra: 238, 271, 335 IR (KBr): 3 404, 2 923, 2 847, 1 738, 1 459, 1 607, 1 170, 1 121, 1 377, 618, 1H NMR (CDCl3, 300 MHz): 5.32 (H-2), 2.37 (H-3), 7.21 (s, H-8), 7.28 (s, H-2’), 7.28 (s, H-6’), 3.68 (d, H-1”a, 6 Hz), 3.7 (d, H-1”b, 6Hz) , 2.13 (H-3”a, 1.2 Hz) , 2.12 (H-3”b, 1.2 Hz) , 0.75 (H-4”), 0.90 (H-5”) , 1.63 (m, H-2”, 1H),13C NMR (CDCl3, 300 MHz):
5,4’,5’-Tri-Hydroxy 7-(3’’-Me butan) -yl flavanone (4): molecular formula C20H22O5, 70.16 (C), 6.48 (H), 23.36 (O), MW=342.40, UV spectra: 249, 270, 327, IR (KBr): 3 475, 2 923, 2 847, 1 650, 1 536, 1 612, 1 383, 1 252, 1 033, 760, 618,1H NMR (CDCl3, 300 MHz): 5.32 (H-2, 1H), 2.8 (H-3, 2H), 7.058 (H-6, 1H), 7.019 (H-8, 1H), 7.70 (H-2’, 1H), 7.487 (H-5’, 1H), 7.675 (H-6’, 1H), 2.20 (H-1”, 2H), 1.85 (H-2”, 2H), 1.27 (H-3”, 1H), 0.87 (H-4”, 6H),13C NMR (CDCl3, 300 MHz): 76 (C2), 63 (C3), 184.9 (C4), 100.57 (C5), 116.44 (C6), 110.33 (C7), 111.366 (C8), 130.65 (C9), 87.00 (C10), 39 (C1”), 38 (C2”), 30.25 (C3”), 27.25 (C4”a, C4”b).
5,7-Dihydroxy-2’,4’,5’-trimethoxy-isoflavanone (5), MW=346.34, molecular formula, C18H18O7, 62.42% (C), 5.24% (H), 32.34% (O), Tf=192 °C. UV max (MeOH): 253, 279, 325.[31] IR (KBr): 3 470, 2 951, 2 847, 1 733, 1 613, 1 536, 1 383, 1 258, 1 132, 1 000, 815, 755 cm-1.1H NMR: 4.57 (H2a, dd, 10.8, 17.0), 4.44 (H2b, dd, 10.7, 5.5), 4.33 (H3a, dd, 11.5, 5.5), 5.99 (H6, d, 2.1), 5.97 (H8, d, 2.1), 6.77 (H3’, s), 6.86 (H6’, s), 12.32 (5’-OH, s), 3.8 (2’-OCH3, s) , 3.86 (4’-OCH3, s), 3.74 (5’-OCH3, s).13C NMR: 70.6 (C-2), 47.2 (C-3), 197.5 (C-4), 165.2 (C-5), 96.4 (C-6), 166.5 (C-7), 95.1 (C-8), 164.0 (C-9), 103.1 (C-10), 115.3 (C-1’), 152.8 (C-2’), 99.7 (C-3’), 150.7 (C-4’), 144.1 (C-5’), 116.5 (C-6’), 56.4 (2’-OCH3), 56.0 (4’-OCH3), 56.8 (5’-OCH3).
5,6,7,4’-tetrahydroxy flavonol (6): Tf=196 °C. MW=302.24, C15H10O7, 59.61% (C), 3.33% (H), 37.05% (O), UV max (MeOH): 258, 277, 354. IR(KBr): 3 399, 1 635, 1 514, 1 376, 1 143, 1 405, 777 cm-1.1H NMR: 8.05 (2H, d, 9, H-2’, 6’), 6.91 (2H, d, 9, H-3’, 5’), 6.60 (1H, s, H-8).13C NMR: 146.8 (C-2), 135.6 (C-3), 175.9 (C-4), 160.7 (C-5), 127.3 (C-6), 162.5 (C-7), 94.5 (C-8), 156.2 (C-9), 103.1 (C-10), 121.7 (C-1’), 129.5 (C-2’), 115.4 (C-3’), 159.2 (C-4’), 115.4 (C-5’), 129.5 (C-6’).
7-O-[α-rhamnopyranosyl-(1->6)-β-glucopyranosyl]-4’, 5, 7-tri-hydroxy-flavanone (7), molecular formula: C27H32O14, 55.86 (C), 5.56 (H), 38.58 (O), MW=580.55. UV max (MeOH): 248, 274 , 333. IR(KBr): 3 383, 2 913, 1 613, 1 488, 1 389, 1 252, 1 193, 1 072 cm-1.1H NMR: 5.42 (H-2, dd,J=12.2; 2.9 Hz ), 2.48 (H-3a dd,J=17.1; 2.9 Hz ), 3.62 (H-3b, dd,J=17.1; 2.9 Hz), 6.05 (H-6, t,J=2.4 Hz), 6.07 (H-8, m), 6.76 (H-2’, m), 6.74 (H-3’, H-5’, d), 7.31 (H-2’, H-6’, d), 5.09 (H-1’’, t,J=7.3 Hz), 3.62 (H-2”), 3.22 (H-3”, m), 3.13 (H-4”, dd,J=9; 5 Hz), 3.53 (H-5”, m), 3.42 (H-6”a), 3.80 (H-6”, d,J=10.3 Hz ), 4.71 (H-1’’’, s), 4.47 (H-2’’’, m), 4.45 (H-3’’’), 3.64 (H-4’’’, d,J=10.2 Hz), 4.45 (H-5’’’), 1.09 (H-6’’’, d,J=6.3 Hz).13C NMR: 79.88 (C-2), 43.36 (C-3), 198.46 (C-4), 163.99 (C-5), 96.35 (C-6), 166.10 (C-7), 97.50 (C-8), 164.17 (C-9), 104.55 (C-10), 129.80 (C-1’), 116.45 (C-2’, C-6’), 129.70 (C-3’, C-5’), 159.07 (C-4’), 98.52 (C-1’’), 77.44 (C-2”), 73.06 (C-3”), 70.82 (C-4”), 77.32 (C-5”), 61.69 (C-6”), 101.63 (C-1’’’), 71.63 (C-2’’’), 71.71 (C-3’’’), 72.63 (C-4’’’), 69.53 (C-5’’’), 19.30 (C-6’’’).
7-O-[α-rhamnopyranosyl-(1->6)-βglucopyranosyl]3’,5-Dihydroxy 4’-Methoxy flavanone (8). [26,33] UV max (MeOH): 245, 286, 330 nm. IR (KBr): 3 384, 2 929, 1 613, 1 503, 1 263, 1 176, 1 383, 810, 761 cm-1,1H NMR: 5.50 (H-2, dd,J=12.2; 2.9 Hz), 2.77 (H-3a, dd,J=17.1; 2.9 Hz), 3.28 (H-3b, m), 6.12 (H-6, d,J=2.0 Hz), 6.12 (H-8, d,J=2.0 Hz), 6.93 (H-2’, m), 6.95 (H-5’, d,J=8.3 Hz), 6.90 (H-6’, t,J=8.3 Hz ), 4.97 (H-1”, d,J=7.3 Hz), 3.27 (H-2”, m), 3.22 (H-3”, m), 3.12 (H-4”, t,J=9.3 Hz), 3.53 (H-5”, m), 3.42 (H-6”a, m), 3.80 (H-6”b, m), 4.52 (H-1’’’, s), 3.64 (H-2’’’, m), 3.43 (H-3’’’, m), 3.14 (H-4’’’, t,J=9.3 Hz), 3.40 (H-5’’’, m), 1.08 (H-6’’’, d,J=6.3 Hz).13C NMR: 78.5 (C-2), 2.21 (C-3), 198.4 (C-4), 162.64 (C-5), 95.58 (C-6), 165.28 (C-7), 96.52 (C-8), 163.50 (C-9), 103.40 (C-10), 131.06 (C-1’, 114.23 (C-2’), 146.51 (C-3’), 148.11 (C-4’), 112.23 (C-5’), 118.05 (C-6’), 55.85 (MeOH), 99.61 (C-1’’), 76.42 (C-2”) , 73.13 (C-3”), 69.73 (C-4”), 75.58 (C-5”), 66.18 (C-6”), 100.75 (C-1’’’), 70.40 (C-2’’’), 70.84 (C-3’’’), 72.21 (C-4’’’), 68.45 (C-5’’’), 17.96 (C-6’’’).
4. Discussion
For all compounds isolated, the absorption bands in UV characterized by the lengths of maximal waves of basis situated between 203-254 nm (band I) correspond to double link C=C link. The band II situated between 260-284 nm corresponding to the carbonyle groups (ketone function) have associated to the transition of strong energy (π→π*) (bande II)[7,8]. Who do the bands of absorption have maximal languor waves more elevated located toward 306-345 nm (band III) associated to the transition of weak energy
The UV specter of the compound (8) present a maximum has 286 nm (band II) and another has 330 nm (band III), that indicates the presence of a flavanone. The use of the displacement reagents permits to determine the positions of the substituting. One displacement of + 22 nm of the band II is observed after the addition of AlCl3, indicating the presence of hydroxyl group in the C-5 position. The addition of a basis as: (NaOAc or NaOMe) doesn’t have any effect, showing the absence of hydroxyl group in C-7 position[9].
In infra red spectra, the bands matched toward (3 383-3 415 cm-1) correspond to the elongation vibration of(valence vibration), the aliphatic links C-H is presented in the IR specter by fine and intense bands toward 2 934 cm-1(asymmetric valence vibration of the CH3). The frequencies of vibrations situated between (2 913-2 929 cm-1) correspond to the asymmetric valence vibrations of CH2, the absorption bands to 2 853 cm-1associate has vibrations of symmetrical valence of the CH2[6]. The frequencies of vibration 990 cm-1corresponds to the vibrations of distortion out plan of the unsaturated hydrocarbons. The vibrations of valence of the ketone cyclic to six linkages or more, or aliphatic ketone (C=O) to be located toward 1 717 cm-1, in the same way 1 738 cm-1corresponds to the carbonyl functions for a saturated ester. The vibrations have 1 766 cm-1and 1 771 cm-1for thevinyl esters of alcohol that possess the fragment of structure following:this group entails an increasing importance of the frequency of vibration carbonyl (acetate of vinyl absorbs 1 776 cm-1and the phenyl acetate absorbs to 1 770 cm-1. The vibration of distortion outside of the plan of C-H aromatic depends mainly of the position of the various substituting fixed on the benzene core and not of their nature[9].
We note the existence of the bands toward 1 150-1 200 cm-1in all separated products, what confirms the presence of an aromatic alcohol corresponds to the vibration (OH aroma), and also the strips toward 1 033 to 1 170 cm-1made us think about a primary, secondary alcohol or even tertiary. One noticed that all these separated compounds represent an intense band toward 1 600-1 700 cm-1, this band indicates the existence of ketone function this grouping (C=O) is conjugated probably[10].
The specters of these products indicate the presence of an aromatic cycle substituted confirmed by the vibrations of elongation (C=C) situated toward 1 500 cm-1and 1 600 cm-1, although the vibrations (=C-H aromatic) don’t appear well on the IR specter due to the masking by the large bands of the function-OH in all compounds safe for: compound 2. This last link is identified well by the vibration of distortions (= C-H aromatic) that absorbs toward 700 cm-1. The vibrations of elongation of -CH3, -CH2- appears between 2 847 cm-1to 2 951 cm-1. The vibrations that are in the domain of absorption between 1 104 cm-1to 1 275 cm-1extra in all IR specters of the products, representing the ether oxide group. The band of absorption toward 1 250 cm-1characterizes an ether-oxide aliphatic arylealiphatic. A band of absorption in IR situates to 924, 984 cm-1characterize a link ethylic trans, and the strip to 1 601 cm-1can be probably assigns to the vibration of valence of the C=C group conjugated. From these primary results of UV spectra and IR spectra, the al compounds can possess the structure general of a flavanone, flavonol and flavone[9].
The compound 1 is a yellow strong product isolated in second column with 6% yield identified as 7-O-[αrhamnopyranosyl 4’,5,6-Trihydroxy flavone. In H1NMR spectra, we observe 14 signals, two singular signals appears toward 6.59 and 6.98 corresponds to benzenic protons respectively 3 and 4 positions, four other signals appears in weak fields as the unarmored doublets toward 6.91 and 7.84 ppm, the rest of signals represent the protons of sugar moiety or one has two diastereoisotopic protons of the methylene group. These results are confirmed by13C NMR spectra, that presents 21 signals, six signals appears between 60.2-103 ppm corresponds to rhamnopyranosyl carbons, substituted by an oxygen in 7 position, proved by the unarmored carbons 7 and 1”, the more unarmored carbon appears to 184 ppm correspond to carbonyl group (C=O) in position 4[6]. The compound 2 isolated in second column with 7.5% yield, identified as 4’,5’-Di-Methoxy 7-(5’’-Me Hexan)1-oyl flavanone, the caracterestic signals of diasteroisotopic protons appears as two doublets toward 2.95 and 2.88 ppm, so the carbonyle group appears in unarmord zone to 190 ppm, these results proved that this compound is a flavanone substituted with an alkoyl of seven carbon in the unarmored position. The compound 3 and 4 isolated in third column in yield respectively to 9.94% and 6.57%, compound 3 possessed two asymmetric carbons, therefore it presents six diastereoisotopic protons assigned to three methylene groups in 3, 2”, 4” positions. This proton is chemically non equivalent and appears in different chemical shifts. Compound 4 identified as 5,4’,5’-Tri-Hydroxy 7-(3’’-Me butan) -yl flavanone, NMR spectra proved the centesimal analysis, the alkyl substituted in 7 position,1H NMR spectra present a signal to 0.87 correspond a two methyl equivalent integrated to 6H, these remarks show that the substituted alkyl correspond to the (3’’-Me butanyl). Compound 5 isolated in first column identified as isoflavanone, it is a white powder. In1H NMR spectra, we notice two unarmored diastereoisotopic protons of methylene goup in position 2, it appears under shape two doublet respectively toward to 4.57 and 4.44 ppm[11,12]. In the first column, we arrived to isolate a flavonol (compound 6) in 5.56%, this compound is identified as 5,6,7,4’-tetrahydroxy flavonol,1H NMR spectra present three unarmored signals for five protons, one corespond to a protons in position 8, the other signals correspond respectively to tow protons chemicaly equivalent. Glycoside 7 and 8 isolated respectively in first and third column. The compounds have already been isolated fromCitrusspp. (Rutaceae),Mentha piperita(Lamiaceae) andMyoporum tenuifolium(Myoporaceae)[13], it was identified by analysis of its spectroscopic data1H NMR and13C NMR experiments and comparison with those previously reported for the hesperidin (hesperetin-7-rutinoside). The hydrolysis and comparison with authentic samples using the same procedure described in the literature were used to identify the carbohydrates Lrhamnose and D-glucose of the rutinoside unit. The same spectrum shows a cross peak of H-2’ [δH 7.12 (s)] with δC 121.0 (C-2’,1JCH) and 161.2 (C-3’,2JCH) which were used to confirm the location of the hydroxyl group at C-3’.
The presence of different types of bioactive flavonoids inL. arborescensextract can explain the large ethnopharmacological uses and the potential activity of this medicinal plant.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
This work was financially supported by MESRS-Algeria (Grant No. E03820100011).
Comments
Background
Phytochemical study of medicinal plants is an important area of research for a good understanding of the biological properties of these plant species. Ethnopharmacological studies onL. arborescensin Algeria have shown medicinal properties from which the interest to know the different bioactives components of this plant.
Research frontiers
In this work, the research aims to isolate and determine the structures of flavonoids present in the butanol fraction of the extract of the plant. The separation and identification of the target compounds were performed respectively by liquid chromatography and by spectroscopic methods.
Related reports
According to ethnopharmacological survey authors,L. arborescensis used for the gastric illnesses. These works have been developed to study the phytochemical constituents that are behind the pharmacological properties of this plant.
Innovations and breakthroughs
Polyphenols of the methanolic extract of aerial part ofL. arborescenswere isolated precedently.
Following these initial phytochemical studies, the authors’work in this part relates to the study of the butanol fraction of the water/acetone extract and isolating new flavonoids from this plant.
Applications
The new flavonoids extracted and isolated from this plantL. arborescenscould be tested to determine which of these flavonoids are the most biologically actives in the pharmacological action of this plant.
Peer review
The research presented by the authors complements previous work on the phytochemical study of this plant. In fact, after studying the methanol fraction of the extraction of this plant, the authors are interested in the butanol fraction by isolating and identifying new flavonoids using efficient chromatographic and spectroscopic methods. The description of these novel compounds of quite complex structures is a very interesting scientific result to use to search for active substances of this medicinal plant used by the people of these regions.
[1] Ozenda P. [Flora of the Sahara]. Paris: French National Center for Scientific Research; 1983, p. 270. French.
[2] Sekkoum K, Cheriti A, Taleb S, Bourmita Y, Belboukhari N. Traditional phytotherapy for urinary diseases in Bechar district (south west of Algeria). Electron J Environ Agric Food Chem 2011; 10(8): 2616-2622.
[3] Belboukhari N, Cheriti A. Flavonoids of Limoniastrum feei. Res J Phytochem 2007; 1(2): 74-78.
[4] Belboukhari N, Cheriti A. Antibacterial and antifungal activities of crude extract of Launeae arborescens. Pak J Biol Sci 2006; 9(15): 2930-2932.
[5] Shin HJ, Nam JW, Yoon UJ, Han A, Seo E. Identification of three new flavonoids from the peels of Citrus unshiu. Helvetica Chimica Acta 2012; 95(2): 240-245.
[6] Wagner H, Bauer R, Melchart D, Staudinger A, Xiao P. Chromatographic fingerprint analysis of herbal medicines thinlayer and high performance liquid chromatography of chinese drugs. New York: Springer; 2011, p. 1063.
[7] Zellagui A, Gherraf N, Rhouati S. Chemical composition and antibacterial activity of the essential oils from Launeae resedifolia L. Org Med Chem Lett 2012; 2: 31.
[8] Belboukhari N, Cheriti A, Roussel C, Vanthuyne N. Chiral separation of hesperidin and naringin and its analysis in a butanol extract of Launeae arborescens. Nat Prod Res 2010; 24(7): 669-681.
[9] Calderon LA. Chromatography-the most versatile method of chemical analysis. Rijeka: Intech; 2012, p. 428.
[10] Bitam F, Letizia Ciavatta M, Manzo E, Dibi A, Gavagnin M. Chemical characterisation of the terpenoid constituents of the Algerian plant Launeae arborescens. Phytochemistry 2008; 69(17): 2984-2992.
[11] Zhang D, Surapaneni S, Jian W, Edom RW, Lin ZJ, Weng N. Chromatographic separation methods. Hoboken: John Wiley & Sons; 2012, p. 340.
[12] Kim MJ, Lee HH, Seo MJ, Kang BW, Park JU, Kim KS, et al. Identification of 5-hydroxy-3,6,7,8,3´,4´-hexamethoxyflavone from Hizikia fusiforme involved in the induction of the apoptosis mediators in human AGS carcinoma cells. J Microbiol Biotechnol 2012; 22(12): 1665-1672.
[13] Fattahi M, Nazeri V, Torras-Claveria L, Sefidkon F, Cusido RM, Zamani Z, et al. Identification and quantification of leaf surface flavonoids in wild-growing populations of Dracocephalum kotschyi by LC-DAD-ESI-MS. Food Chem 2013; 141(1): 139-146.
10.12980/APJTB.4.2014C708
*Corresponding author: Dr. Nasser Belboukhari, Professor, PhD, Bioactive Molecule & Chiral separation Laboratory, University of Bechar, Bechar, 08000, Algeria.
Tel: 00213670156261
Fax: 0021349815244
E-mail: belboukhari.nasser@yahoo.com
Foundation Project: Supported by MESRS-Algeria (Grant No. E03820100011).
Article history:
Received 28 Feb 2014
Received in revised form 10 Mar, 2nd revised form 19 Mar, 3rd revised form 14 Mar 2014
Accepted 22 Mar 2014
Available online 28 April 2014
Methods:The compounds were isolated by liquid chromatographic methods and their structures were identified by using spectroscopic analysis.
Results:The isolated compounds were identified as: 7-O-[α-rhamnopyranosyl 4',5,6-Trihydroxy flavone 1,4’,5’-Di-Methoxy 7-(5’’-Me Hexan)1-oyl flavanone 2, 3”-isopropyl pyrano [1”:7,4”:6] 3’,4’,5’,5-Tetrahydroxy flavanone 3,5,4’,5’-Tri-Hydroxy 7-(3’’-Me butan) -yl flavanone 4, 5,7-Dihydroxy-2',4',5' -trimethoxy-isoflavanone 5,5,6,7,4'-tetrahydroxy flavonol 6,7-O-[α-rhamnopyranosyl-(1->6)-β-glucopyranosyl]- 4',5,7-tri-hydroxy-flavanone 7,7-O-[αrhamnopyranosyl-(1->6)-β-glucopyranosyl] 3',5-Dihydroxy 4’-Methoxy flavanone 8.
Conclusions:The presence of different types of bioactive flavonoids in Launeae arboescens extract can explain the large ethnopharmacological uses and the potential activity of this medicinal plant.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- The effects of exposure to pesticides on the fecundity status of farm workers resident in a rural region of Fars province, southern Iran
- A cross sectional study on antibiotic resistance pattern of Salmonella typhi clinical isolates from Bangladesh
- Comparative susceptibility to permethrin of two Anopheles gambiae s.l. populations from Southern Benin, regarding mosquito sex, physiological status, and mosquito age
- Effects of melatonin on changes in cognitive performances and brain malondialdehyde concentration induced by sub-chronic coadministration of chlorpyrifos and cypermethrin in male Wister rats
- Phytochemical and in vitro biological investigations of methanolic extracts of Enhydra fluctuans Lour.
- Theoretical and experimental study on lipophilicity and wound healing activity of ginger compounds
