Second time around: Corticospinal responses following repeated sportsrelated concussions within the same season. A stimulation study.
2014-03-22AlanPearceDanielCorpCharlotteDaviesBrendanMajorJeromeMaller
Alan J Pearce, Daniel T Corp, Charlotte B Davies, Brendan P Major, Jerome J Maller
1Cognitive Neuroscience Laboratory, School of Psychology, Deakin University, Melbourne, Victoria, Australia
2Monash-Alfred Psychiatric Research Centre, The Alfred and Central Clinical School, Monash University, Melbourne, Victoria, Australia
Second time around: Corticospinal responses following repeated sportsrelated concussions within the same season. A stimulation study.
Alan J Pearce1*, Daniel T Corp1, Charlotte B Davies1, Brendan P Major1, Jerome J Maller2
1Cognitive Neuroscience Laboratory, School of Psychology, Deakin University, Melbourne, Victoria, Australia
2Monash-Alfred Psychiatric Research Centre, The Alfred and Central Clinical School, Monash University, Melbourne, Victoria, Australia
Objective: To investigate the degree of neurophysiological and cognitive performance changes resulting from repeat concussions sustained in a single season of Australian Rules football.
Methods: Three amateur football players were recruited after sustainingtwo concussions during a single season of playing. Each player was assessed at multiple time points by transcranial magnetic stimulation (TMS) and electromyography, as well as tested for fine motor and cognitive performance after each concussion. Results: In all three cases, concussions resulted in reduction in fine dexterity and visuomotor reaction time, cognitive attention performance and increase in intracortical inhibition from TMS. No changes in performance or TMS outcomes were found as a result of the order of the concussions. However, changes observed were dependent on the severity of the concussion. Conclusions: This multiple-case study has demonstrated that concussion result in increased intracortical inhibition and reduction in cognitive and motor performance. Further, TMS, in conjunction with tests of cognitive and motor performance, can be useful as a prognostic technique in assessing recovery from acute concussion injury.
ARTICLE INFO
Article history:
Received 8 August 2013
Received in revised form 15 September 2013
Accepted 24 September 2013
Available online 20 June 2014
Brain concussion
1. Introduction
Concussions sustained during sports are an international growing public health concern. Most frequently reported in contact sports, sports-related concussions are defined as a neurological disturbance following an external force applied to the skull causing trauma to the brain[1]. Clinical symptoms typically emerge rapidly after a sports concussion[2], and include headaches, nauseas, visual disturbances, fatigue, transient changes in mental states (disorientation or amnesia), and loss in motor control (balance and unsteadiness)[3]. Loss of consciousness may also occur following a concussion injury, however these occur in only 10%-20% of cases[3,4].
Neuroimaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) are usually normal following a concussion[3], and may only present in CT/MRI following a more severe traumatic brain injury that induces visible lesions, cortical contusions and intracranial hemorrhage[5]. Although it has been suggested that multiple concussions result in long-termneuropathological changes in the brain[6-8], acute symptoms are reflective of functional disturbance[5] from neuronal membrane disruption, axonal stretching triggering undiscerning release of neurotrasmitters and ionic flux[4]. With no obviousstructural trauma in the brain, CT and MRI do not usually show abnormalities with these techniques. Advanced neuroimaging techniques, such as diffuse tensor imaging (DTI). Susceptibility weighted imaging (SWI) and magnetic resonance spectroscopy (MRS), show promise in detecting subtle changes. For further discussion on the potential of DTI and SWI/MRS, please see Dimou and Lagopoulos[5], and Haackeet alrespectively[9].
Further to diagnostic methods of evaluating concussion, the prognostic aspect of measuring recovery following a concussion injury should also be considered. Concussion recoveryis disparate, with outcomes reflecting individual responses, as well as the severity of the injury. It has been suggested that the majority of concussion symptoms resolve within a period between five days to approximately one week[10,11], however recent studies have suggested that despite dissipation of symptoms, and neurocognitive performance returning to pre-concussion levels, neuronal function may remain abnormal, for up to several weeks to years post-injury[12-16].
Transient disruption of neural activity can be measured using electrophysiology techniques. Using electroencephalography (EEG) to measure event-related potentials (ERPs), De Beaumont and colleagues[14,17] have demonstrated slowing in latency and amplitude ERPs during mental task performance demonstrating subclinical cognitive attention and working-memory deficits in otherwise asymptomatic but concussed athletes. Prichepet al[13] demonstrated changes in EEG brain activity (expressed as the EEG-based TBI index) 45 days after a concussion, even though clinical symptoms, cognitive tests and postural performance had resolved by five days.
Transcranial magnetic stimulation (TMS) has also been used to observe acute and long-term changes of corticospinal excitability and intracortical inhibition ina number of neurological conditions including brain injury[18]. Corticospinal excitability is measured, using a single pulse TMS technique, by the amplitude of the motor evoked potential (MEP). Intracortical inhibition is quantified via the length or duration of the cortical silent period (cSP), reflecting γ-aminobutyric acid (GABAB) receptor activity. In addition, paired-pulse TMS paradigms, such as short-interval intracortical inhibition (SICI), can measure intracortical inhibition mediated by GABAA receptor activity[18,19].
Specifically with TMS and concussion, recent studies have demonstrated the prognostic potential of TMS in measuring recovery following concussion[20]. For example, Pearceet al[12] recently showed abnormal changes in motor cortex inhibition 10 days following a concussion despite resolution of motor symptoms within 2-5 days. Livingstonet al[21], despite not measuring motor cortex inhibition, did observe changes in motor cortex excitability following concussion for 10 days. Milleret al[22] found inhibition changes following concussion at five days and two months post concussion.
To date, there have been no studies presenting data on electrophysiological changes following a recurrent concussion injury within the same season. A previous study by De Beaumontet al[14], investigating the pervasive electrophysiological changes following a concussion injury nine months or longer previously, presented data in a sub-group of collegiate athletes who had sustained a further concussion during the study between 6 and 15 months following the second concussion, observing further pronouncement of intracortical inhibition compared to their pervious measure. With concerns of increased risk of a repeat concussion or a musculoskeletal injury following a concussion[23-25], it is important to investigate the effect ofa recurrent concussion in order to quantify if further cortical inhibition occurs to those suffering a second concussion within the same season.
This study presents motor, neurocognitive, and TMS responses in three participants who had sustained two concussions within the same competitive season of Australian Rules football. Using a multiple-case study design with repeated measures, testing involved measurements at three time points up to 10 days post concussion. We hypothesized that greater alterations in inhibition, as shown by increased cSP and lower SICI measures, would be reflected following the subsequent concussion, compared to baseline prior to the first concussion.
2. Materials and Methods
Participants in the current study were drawn from a larger sample of sample of 43 male football players (mean age 25.1±4.5 years) participatingin a larger study on timecourse recovery following concussion[12]. Three participants from this group(mean age 25.0±2.6 years) sustained a second concussion in the same season and agreed to be re-tested. Consent from individual participants and the football club was obtained prior to data collection, with all methods approved by the University Human Research Ethics Committee.
Following the same research design previously described[12], athletes visited the laboratory for pretesting prior to the start of the season, and then three times following their concussion at 48 hours, 5 days, and 10 days post injury. All testing sessions were completed within 60 min.
Functional and TMS testing methods have been previously described[12,16] involving three elements. Firstly, fine motor control using the O’Connor Finger dexterity test (Lafayette Instruments, USA). This well-established test[26], requiring the individual to manipulate and place three small pins into each hole, has been previously demonstrated goodto-excellent predictive validity[27]. Participants were fully familiarised prior to actual assessments, to reduce learning effects[26-28].
Secondly, cognitive testing was completed using the Cambridge Neuropsychological Test Automated Battery (CANTAB; Cambridge Cognition, UK). The CANTAB tests are non-linguistic, culturally neutral and previously validated in a number of population groups, including concussion[29]. Three tests were employed, visuomotor reaction time, paired-associative learning (PAL), and intraextra dimensional (IED) set shift. Visuomotor reaction time assessed the participant’s ability to respond, by releasing the press-pad key, and move to touch the stimulus (yellow dot) displayed on the computer monitor. PAL required participants to learn the location of coloured-patterned shapes hidden behind white boxes revealed in randomised order. IED tests visual discrimination and shifting and flexibility of attention by displaying two simple color-filled shapes, with participants learning which one is correct and maintaining the correct response for six consecutive trials[29].
Finally, corticospinal excitability and inhibition was measured via TMS. Adhering to the TMS checklist for methodological quality[30] and recommendations for surface electromyography (EMG) for non-invasive assessment of muscles (SENIAM)[31], TMS using a D702 figure of eight coil (Magstim, UK) was applied over the contralateral motor cortex projecting to the participant’s first dorsal interosseous (FDI) muscle. Surface electrodes (bipolar Ag-AgCl) with an inter-electrode distance of 20 mm where placed over the FDI muscle. EMG (PowerLab 4/35, ADInstruments Australia) signals were amplified (1000x), filtered (10-1000 Hz) and sampled at 2 kHz for 500 ms recordings.
Determination of optimal site for TMS, and identification of motor thresholds were undertaken using previously published protocols[12,16,32-34]. Briefly, using single pulse TMS, active motor threshold (aMT) was measured during a controlled, low-level voluntary contraction of 10% of maximal voluntary contraction, identified as a MEP waveform of 200 µV amplitude[35]. Resting motor threshold (rMT) was quantified in the relaxed muscle and identified as a MEP waveform of 50 µV amplitude[35]. For both identification of aMT and rMT, the stimulation was delivered at an intensity below the estimated participant’s threshold and increasing in 5% then 1% steps of stimulator output with respective waveforms observed in 5 of 10 stimuli[36]. For main TMS studies, 15 x 500 ms sweeps were recorded at 125% of the participant’s aMT or rTMS.
Evaluating the inhibitory elements of the motor cortex, paired-pulse TMS was employed using the short latency intracortical inhibition (SICI) paradigm. SICI involves a sub-motor threshold (conditioning) stimulus prior to the subsequent test stimulus between 1 and 5 ms[37]. Fifteen 500 ms SICI sweeps were recorded with the FDI at rest using an interstimulus interval of 2 ms with the conditioning stimulus at 80% of rMT and a test stimulus of 125% of rMT[12].
Fine motor control was quantified in the time taken to complete three rows (30 holes) of the O’Connor test[12,16,28]. Visuomotor reaction time was split between the time taken to react (taking finger off the button) to the stimulus on the screen, and the movement time from the button release to touching the stimulus on the monitor. PAL recorded total number of errors made and total errors at the 6-pattern stage. IED recorded the stage level completed successfully and the number of errors made.
Single pulse MEP latency was measured from the TMS stimulus signal to the first deflection of the MEP waveform (Figure 1A)[38]. The MEP was quantified by measuring the peak-to-peak amplitude of the waveform[35], and cSP duration was measured from the onset of the MEP waveform (during a tonic contraction of the FDI) to the return of uninterrupted EMG activity (Figure 1A)[39]. SICI (Figure 1B) was expressed as a ratio of the paired pulse SICI waveform to the unconditioned single pulse resting MEP at 125%.
Data measured from each individual player at baseline and then following concussion one and concussion two at 48 hours, five days and 10 days are presented as individual cases. Fine motor control and cognitive testing were compared to mean control data from the larger sample measured at each time point[12]. Similarly, TMS data were compared to mean control data. To illustrate changes over time, mean (±SD) MEP latency, MEP amplitude and cSPduration from 15 sweeps, were compared within each player post-concussion to that player’s baseline TMS data using Cohen’s d effect sizes for small (<0.2), moderate (0.21–0.79) and large effects (>0.8)[40].

Figure 1. (A) Example of a 10 overlaied motor evoked potential (MEP) during tonic contraction of the first dorsal inter osseous muscle. Latency duration is measured from stimulus artifact to MEP onset and is shown at (i), peak to peak MEP amplitude is shown at (ii). Silent Period duration is measured from onset of MEP to return of EMG at (iii). Return of EMG activity is shown at (iv).(B) Example of paired pulse TMS measures. (v) Short intracortical inhibition (SICI) waveform (right) next to a single pulse MEP (left). Following a paired pulse (in this illustration with an interval of 3 ms, on right) the inhibited smaller SICI waveform (on right) is calculated to a single pulse MEP taken separately (on left, as indicated by broken double line) and expressed as a ratio. (vi) Long intracortical inhibition (LICI) occurs after a paired pulse with an interval of 100 ms with the second inhibited waveform on right, calculated as a ratio of the first waveform on left. Figures from the authors’ personal collection.
3. Results
Data from the three players following concussion one and two are presented in Table 1 (fine dexterity and cognitive testing) and Table 2 (TMS). Player 1 sustained his second concussion 4 weeks after his first concussion, Player 2 sustained his second concussion 8 weeks later, and Player 3 sustained his concussion at 6 weeksafter his first. Players 1 and 3 reported that their second concussion resulted in loss of consciousness; Player 1 for less than 30 seconds, Player 3 for over 5 minutes. Player 2 reported no loss of consciousness for either concussion, but did self-report that concussion one was the more severe of the two.
Fine dexterity (O’Connor test) performance in all three players was found to have slowed in testing at 48 hours following a concussion. Compared to non-concussed group, Player 1 was outside one standard deviation (1SD) at 48 hours and 5 days post concussion two. Player 2 was outside 1SD at 48 hours post concussion one. Player 3 was outside 1SD at 48 hours after both concussions, with performance reduced at 5 days following concussion two. All players’ fine dexterity was returned to baseline by 10 days.
Players 1 and 3 were found to have slowed reaction time after concussion two, which did not return to baseline until day 10 (Table 1). As seen in Table 1, reaction time for Player 2 was found to be 1SD faster than the non-concussed group. However following the first concussion, reaction time was measured to have slowedat 48 hours falling within the SD of the non-concussed group. Slowing in movement time was observed in Player 2 and 3, falling outside of 1SD of the non-concussed group at 48 hours, returning to baseline by 5 days.

Table 1 Fine motor control (O’Connor), and cognitive tests (CANTAB) results for the three concussed players as compared to group data.
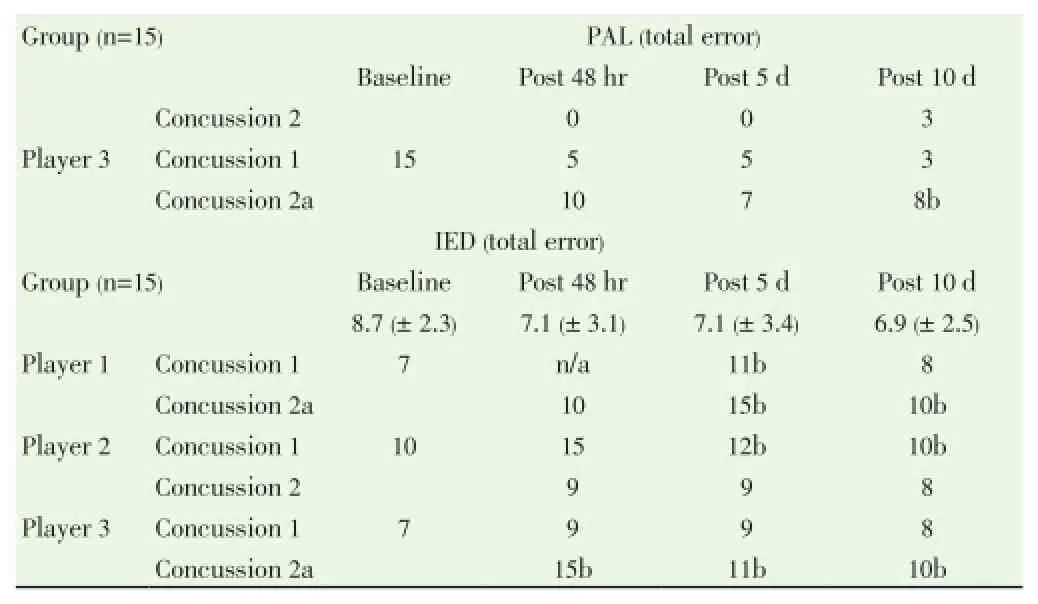
Table 1, continued Fine motor control (O’Connor), and cognitive tests (CANTAB) results for the three concussed players as compared to group data.
No changes were found in the three concussed players for the paired-associated learning task. However, all three players demonstrated increased error with the intra-extra dimensional shifting test. Following concussion one, Player 1 was 1SD outside of the group at 5 days, returning to baseline by 10 days. After concussion two, Player 1 demonstrated increased errors that remained 1SD outside of the control group at 10 days. Player 3 showed no change in total errors after concussion one, but following concussion two he performed 1SD outside of the group for all time points. Conversely, Player 2 demonstrated increased errors after concussion one which remained 1SD outside of the group at 10 days post injury, but showed no change in performance after concussion two.
All TMS dataare illustrated in Table 2. Active and resting motor thresholds showed no change after concussion.

Table 2. Transcranial magnetic stimulation results for motor thresholds, latency, motor evoked potential amplitude, cortical silent period and short intracortical inhibition.

Table 2., continued Transcranial magnetic stimulation results for motor thresholds, latency, motor evoked potential amplitude, cortical silent period and short intracortical inhibition.
Similarly, MEP latency varied little post concussion, with no data falling outside of the control group 1SD. Compared to baseline, observed effect sizes for latency were overall found to be small, ranging between 0 to 0.1, 0.2 to 0.4, and 0 to 0.2 for both concussion in Players 1 to 3 respectively. MEP amplitude similarly did not fall outside of the control group 1SD. Amplitude changes also varied noticeably. Player 1 showed a decrease in MEP amplitude following both concussion one (range d between 1.3 to 1.5) and concussion two (range d between 1.0 to 2.1). Players 2 and 3 however showed varied MEP responses following both concussions (Player 2 range d from 0.4 to -1.6; Player 3 range d from -0.2 to 1.6).
Examples of single pulse TMS sweeps from the three players are illustrated in Figure 2. cSP was found to have increased in duration following both concussions. Player 1 demonstrated moderate changes in cSP at five days (2.5 ms difference;d=0.3) and at 10 days (3.2 ms difference;d=0.4). Following concussion two, greater cSP durationwas observed at 48 hours (12.8 ms increase;d=1.1), with moderate changes at 5 days (-4.6 ms;d=0.5), returning to baseline by 10 days (4 ms difference). Following concussion one, Player 2 showed large increases in cSP duration at all time points (22.8 ms, 22.2 ms, 23.3 ms;d=1.1, 1.0, and 1.2 for 48 hours, 5 days and 10 days respectively). A large increase in cSP duration (16.3 ms and 18.3 ms;d=1.0 and 0.9) at 48 hours and 5 days respectively was observed following concussion two, with cSP returning to baseline by day 10 (4.7 ms difference; Table 2). Player 3 revealed large increases in cSP following concussion one at 48 hours (13.6 ms;d=1.3) and 5 days (15.8 ms;d=1.8), returning to baseline by day 10. Following the second concussion, Player 3 showed large increases in cSP duration at 48 hours (13.2 ms;d=2.0), 5 days (14.2 ms;d=1.2) and also at 10 days (12.0 ms;d=1.3).
SICI data showed for Player 1 variable responses with large increases in SICI ratio at 5 days (d=-1.4) and at 10 days (d=-0.8). However following the second concussion SICI was observed to have large decreases in SICI ratio at 48 hours (d =1.1) and 5 days (d=1.2), continuing to be moderately reduced (d=0.6) at 10 days. Player 2 showed large decreases in SICI ratio at 48 hours (d=1.0), five days (d=1.6) and at 10 days post concussion (d=2.1). However after the second concussion, small to moderate changes in SICI ratio were observed (d=0.04, 0.0, and 0.04 at 48 hours, 5 days and 10 days respectively). Player 3 revealed moderate reductions in SICI ratio at 48 hours (d=0.55) and at 5 days (d=0.44), returning to baseline by 10 days (d=0.21). Following the second concussion, SICI ratio showed large decreases at 48 hours (d=0.8), 5 days (d=1.0) and 10 days (d=1.1).
4. Discussion
This is the first study to present motor, cognitive and neurophysiological data following repeated concussions within individuals concussed in the same season. Contrary to our hypothesis, we did not find greater performance decrements or TMS alterations as a result of the order of concussions (in other words, the second concussion was hypothesized to produce more noticeable results), but changes were reflected dependent on the severity of the concussion, being that the concussions resulting in loss of consciousness showed in increased effect in cSP duration. However, with only three repeat-concussion players reported in this study, we advise caution in generalizing these findings to the wider concussion research.
Currently, TMS is under-utilized in concussion research. However, a recent systematic review by Majoret al[20] highlighted that TMS has the capacity to measure electrophysiological changes in the motor cortex following an acute concussion injury.Nevertheless, TMS measures are not condition-specific and therefore should be interpreted along with other testing data[18]. For example, Livingstonet al[21] showed significant difference in the Head Injury Scale scores and processing and reaction time speed in neurocognitive testing along with slowing in MEP latency, alterations in motor threshold between groups and differences in MEP amplitude, over time, up to 10 days. Christyakovet al[41] reported increased motor thresholds in those with mild and moderate head injuries, along with subjective complaints of fatigue, dizziness, headaches, and memory and concentration disturbances, compared to those with minor injury and controls. Although the recent study by Pearceet al[12] did not quantify symptom severity, TMS changes were found to be associated with slowing in loss in attentional ability and visuomotor reaction time.
The present study did not observe changes in latency or motor thresholds, but did observe cSP duration lengthening supporting previous findings[12,22,41]. Christyakovet al[41] demonstrated lengthened cSP duration in moderately head injured patients two weeks post injury compared to minor or non-injured controls. More recently Pearceet al[12] and Milleret al[22] showed lengthening in cSP duration following concussion at five days and two months respectively,demonstrating that TMS has potentially useful prognostic technique when measuring an individual’s recovery following a concussive injury.
Collectively, data from this study, along with previous acute concussion studies showing increased cSP duration[12,22,1]may reflect, the complex neurometabolic and ionic cascade of events that occur immediately following a concussion lasting up to several weeks to months[4]. As well as ionic and metabolic changes, post-concussion neurotransmitter alterations have also been observed in excitatory neurotransmitters (glutamate), and also in inhibitory neurotransmitters, in particular GABA that may affectcortical inhibition. The increased corticomotor inhibition observed in this study, as well as previous studies[12,22,41], may provide a plausible explanation for the suggestion of increased risk of subsequent concussion injury[23,24], as well as recent findings of a 50% risk in asubsequent injury of any nature[25].
It should be noted that a limitation of this study was the inability for quantification of concussion severity at time of the injury. At non-elite levels of sport, it is recognised that healthcare professionals with expertise in assessing and managing concussions is limited[42]. The amateur team, of which the three players in this study were a part of, did not have access to professional healthcare personnel, with the concussion observed by a sports trainer or by the individual players themselves, making evaluation less than ideal. However, as suggested by Livingstonet al[21], at the sub-elite level self-reported symptoms form an integral component of concussion assessment and management.
In conclusion, this multiple-case study has demonstrated that concussions sustained in the same season affected intracortical inhibition, as measured by TMS, along with tests of attentional switching and motor performance. Therefore, in conjunction with tests of cognitive and motor performance, TMS can be useful as a prognostic technique in assessing recovery from acute concussion injury.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This study was supported, in part, by a seeding grant from Smart Head Play. JJM is supported by an Acute Care Postdoctoral Fellowship Centre for Traumatic Brain Injury Research, and NHMRC Industry Career Development Fellowship. The authors sincerely thank Mr. Matthew Gray and the Hampton Rovers amateur football club for assistance with participant recruitment.
[1] Shaw NA. The neurophysiology of concussion. Progress Neurobiol 2002; 67: 281-344.
[2] McCrory P, Meeuwisse WH, Aubry M, Cantu B, Dvořák J, Echemendia RJ, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med 2013; 47: 250-258.
[3] Ropper AH. Concussion and other head injuries. In: Harrison’s principles of internal medicine. New York: McGraw-Hill Medical 2008; p. 2596-2600.
[4] Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Ath Train 2001; 36: 228.
[5] Dimou S, Lagopoulos J. Toward objective markers of concussion in sport: a review of white matter and neurometabolic changes in the brain after sports-related concussion. J Neurotrauma 2014; 31: 413-24.
[6] Stein TD, Alvarez VE, McKee AC. Chronic traumatic encephalopathy: a spectrum of neuropathological changes following repetitive brain trauma in athletes and military personnel. Alzheimers Res Ther 2014; 6.
[7] Stern RA, Daneshvar DH, Baugh CM, Seichepine DR, Montenigro PH, Riley DO, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology 2013; 81: 1122-1129.
[8] McKee AC, Daneshvar DH, Alvarez VE, Stein TD. The neuropathology of sport. Acta Neuropathol 2014; 127: 29-51.
[9] Haacke EM, Xu Y, Cheng YCN, Reichenbach JR. Susceptibility weighted imaging (SWI). Magn Reson Med 2004; 52: 612-618.
[10] Ellemberg D, Henry LC, Macciocchi SN, Guskiewicz KM, Broglio SP. Advances in sport concussion assessment: from behavioral to brain imaging measures. J Neurotrauma 2009; 26: 2365-2382.
[11] McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA 2003; 290: 2556-2563.
[12] Pearce AJ, Hoy K, Rogers MA, Corp DT, Davies CB, Maller JJ, et al. Acute motor, neurocognitive and neurophysiological change following concussion injury in Australian amateur football. A prospective multimodal investigation. J Sci Med Sport. In Press;
[13] Prichep LS, McCrea M, Barr W, Powell M, Chabot RJ. Time course of clinical and electrophysiological recovery after sport-related concussion. J Head Trauma Rehab 2013; 28: 266-273.
[14] De Beaumont L, Lassonde M, Leclerc S, Théoret H. Long-Term and Cumulative Effects of Sports Concussion on Motor Cortex Inhibition. Neurosurgery 2007; 61: 329-37.
[15] De Beaumont L, Théoret H, Mongeon D, Messier J, Leclerc S, Tremblay S, et al. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain 2009; 132: 695-708.
[16] Pearce AJ, Hoy K, Rogers MA, Corp DT, Maller JJ, Drury HG, et al. The long-term effects of sports concussion on retired Australian football players: A study using Transcranial Magnetic Stimulation. J Neurotrauma 2014; 31: 1-7.
[17] De Beaumont L, Mongeon D, Tremblay S, Messier J, Prince F, Leclerc S, et al. Persistent motor system abnormalities in formerly concussed athletes. J Athl Train 2011; 46: 234-40.
[18] Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol 2003; 2: 145-156.
[19] Hallett M. Transcranial magnetic stimulation and the human brain. Nature 2000; 406: 147-50.
[20] Major BP, Rogers MA, Pearce AJ. Using transcranial magnetic stimulation to quantify electrophysiological changes following concussive brain injury: A systematic review. Clin Exp Pharmacol Physiol. In Press;
[21] Livingston SC, Goodkin HP, Hertel JN, Saliba EN, Barth JT, Ingersoll CD. Differential rates of recovery after acute sport-related concussion: electrophysiologic, symptomatic, and neurocognitive indices. J Clin Neurophysiol 2012; 29: 23-32.
[22] Miller NR, Yasen AL, Maynard LF, Chou L-S, Howell DR, Christie AD. Acute and longitudinal changes in motor cortex function following mild traumatic brain injury. Brain Inj 2014; 1-7.
[23] Guskiewicz KM, McCrea M, Marshall SW, Cantu RC, Randolph C, Barr W, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA 2003; 290: 2549-2555.
[24] Abrahams S, Mc Fie S, Patricios J, Posthumus M, September AV. Risk factors for sports concussion: an evidence-based systematic review. Br J Sports Med 2014; 48: 91-97.
[25] Nordström A, Nordström P, Ekstrand J. Sports-related concussion increases the risk of subsequent injury by about 50% in elite male football players. Br J Sports Med 2014; 48: 1447-1450.
[26] Corlett E, Salvendy G, Seymour W. Selecting operators for fine manual tasks: A study of the O’Connor Finger Dexterity Test and the Purdue Pegboard. Occup Psychol 1971; 45: 57-65.
[27] Yancosek KE, Howell D. A narrative review of dexterity assessments. J Hand Ther 2009; 22: 258-270.
[28] Berger MAM, Krul AJ, Daanen HAM. Task specificity of finger dexterity tests. Appl Ergo 2009; 40: 145-147.
[29] Sandberg MA. Cambridge Neuropsychological Testing Automated Battery. In: Encyclopedia of Clinical Neuropsychology. Springer, 2011; p. 480-482.
[30] Chipchase L, Schabrun S, Cohen L, Hodges P, Ridding M, Rothwell J, et al. A checklist for assessing the methodological quality of studies using transcranial magnetic stimulation to study the motor system: an international consensus study. Clin Neurophysiol 2012; 123: 1698-1704.
[31] Hermens HJ, Freriks B, Merletti R, Stegeman D, Blok J, Rau G, et al. European recommendations for surface electromyography: Roessingh Research and Development The Netherlands, 1999.
[32] Pearce AJ, Kidgell DJ. Comparison of corticomotor excitability during visuomotor dynamic and static tasks. J Sci Med Sport 2010; 13: 167-171.
[33] Wilson SA, Thickbroom GW, Mastaglia FL. Transcranial magnetic stimulation mapping of the motor cortex in normal subjects: The representation of two intrinsic hand muscles. J Neurol Sci 1993; 118: 134-144.
[34] Pearce AJ, Thickbroom GW, Byrnes ML, Mastaglia FL. The corticomotor representation of elite racquet sport athletes. Exp Brain Res 2000; 130: 238-43.
[35] Wilson S, Thickbroom G, Mastaglia F. Topography of excitatory and inhibitory muscle responses evoked by transcranial magnetic stimulation in the human motor cortex. Neurosci Lett 1993; 154: 52-56.
[36] Pearce AJ, Clark RA, Kidgell DJ. A comparison of two methods in acquiring stimulus-response curves with transcranial magnetic stimulation. Brain Stimul 2013; 6: 306-309.
[37] Kujirai T, Caramia M, Rothwell J, Day B, Thompson P, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol 1993; 471: 501-519.
[38] Pearce AJ, Rowe GS, Whyte DG. Neural conduction and excitability following a simple warm up. J Sci Med Sport 2012; 15: 164-168.
[39] Wilson SA, Lockwood RJ, Thickbroom GW, Mastaglia FL. The muscle silent period following transcranial magnetic cortical stimulation. J Neurol Sci 1993; 114: 216-22.
[40] Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum, 1988.
[41] Chistyakov A, Soustiel J, Hafner H, Trubnik M, Levy G, Feinsod M. Excitatory and inhibitory corticospinal responses to transcranial magnetic stimulation in patients with minor to moderate head injury. J Neurol Neurosurg Psychiatry 2001; 70: 580-587.
[42] Putukian M, Aubry M, McCrory P. Return to play after sports concussion in elite and non-elite athletes? Br J Sports Med 2009; 43: i28-i31.
ment heading
10.1016/S2221-6189(14)60042-1
*Corresponding author: Dr. Alan Pearce, Deakin University, 221 BurwoodHighway, Burwood, Melbourne, Victoria, 3125, Australia.
Phone: (+61) 3 9251 7224
Fax: (+61) 3 9244 6858
E-mail: alan.pearce@deakin.edu.au
Funding project: This study was supported, in part, by a seeding grant from Smart Head Play. JJM is supported by an Acute Care Postdoctoral Fellowship Centre for Traumatic Brain Injury Research, and NHMRC Industry Career Development Fellowship.
Sports
Transcranial magnetic stimulation
Recovery of function
杂志排行
Journal of Acute Disease的其它文章
- Posterior reversible leukoencephalopathy syndrome presenting in a post-partum, 25-year-old-female with concomitant subarachnoid hemorrhage
- Cardioprotective potential of hydro-alcoholic fruit extract of Ananas comosus against isoproterenol induced myocardial infraction in Wistar Albino rats.
- Patients with the tako-tsubo cardiomyopathy-clinical evaluation and outcome
- Acute brain hemorrhage in dengue
- The epidemiology of tick-borne relapsing fever in Bijar County, North-Western Iran
- Heart, tracheo-bronchial and thoracic spine trauma. Succesful multidisciplinary management: a challenging thoracic politrauma
