GC/GCMS analysis of the petroleum ether and dichloromethane extracts of Moringa oleifera roots
2014-03-22ShaheenFaiziSaimaSumbulMuhammedAliVersianiRubeenaSaleemAishaSanaHiraSiddiqui
Shaheen Faizi, Saima Sumbul, Muhammed Ali Versiani, Rubeena Saleem, Aisha Sana, Hira Siddiqui
1HEJ Research Institute of Chemistry, International Center for Chemical and Biological Sciences, University of Karachi, Karachi-75270, Pakistan
2Department of Chemistry, Federal Urdu University of Arts, Sciences and Technology, Gulshan-e-Iqbal, Science Campus, Karachi-75300, Pakistan
3Pharmaceutical Chemistry, Faculty of Pharmacy, Hamdard University, Karachi-74600, Pakistan
4Dr. HMI Institute of Pharmacology and Herbal Sciences, Hamdard University, Karachi-74600, Pakistan
GC/GCMS analysis of the petroleum ether and dichloromethane extracts of Moringa oleifera roots
Shaheen Faizi1, Saima Sumbul1, Muhammed Ali Versiani2*, Rubeena Saleem3,4, Aisha Sana3, Hira Siddiqui2
1HEJ Research Institute of Chemistry, International Center for Chemical and Biological Sciences, University of Karachi, Karachi-75270, Pakistan
2Department of Chemistry, Federal Urdu University of Arts, Sciences and Technology, Gulshan-e-Iqbal, Science Campus, Karachi-75300, Pakistan
3Pharmaceutical Chemistry, Faculty of Pharmacy, Hamdard University, Karachi-74600, Pakistan
4Dr. HMI Institute of Pharmacology and Herbal Sciences, Hamdard University, Karachi-74600, Pakistan
PEER REVIEW
Peer reviewer
Dr. Shah Alam Khan, Associate Professor of Pharmaceutical Chemistry, Department of Pharmacy, Oman Medical College, PO Box 620, Postal code 130, Muscat, Oman. Tel: 00968-24504608 (165)
E-mail: sakhan@omc.edu.om
Comments
I reckon this is a pilot study aimed to identify the fatty/volatile constituents in the petroleum ether and dichloromethane extracts. This plant has been extensively explored phytochemically well as for different pharmacological activitires due to its wide spread use in traditional medicines throughout the world.
Details on Page 653
Objective:To explore the phytochemical constituents from petroleum ether and dichloromethane extracts of Moringa oleifera (M. oleifera) roots using GC/GC-MS.
Moringa oleifera, Moringaceae, Roots, Petroleum ether and dichloromethane extracts, Cyclooctasulfur S8, GC/GCMS
1. Introduction
Moringa oleifera(M. oleifera) Lam., commonly known as sajana, drumstick or horseraddish tree, is a member of the Moringaceae family that grows throughout most of the tropics including Pakistan, Afghanistan and Northwest India[1]. It is one of those multipurpose “Miracle” plants which have received attention as “Natural nutrition of the tropics”[2]. The tree is highly valued as every part of the plant is edible and has established medicinal and folkloric significance in treating different diseases[3]. Various parts of the plant are highly reputed in traditional medicine for the treatmentof a variety of ailments[4]. Also, as a traditionally important food commodity, the leaves, fruits, flowers and roots are locally esteemed as vegetable[2]. The pharmacological studies showed that its various parts possess hypotensive, antioxidant, anti-inflammatory, antinociceptive, wound healing, anthelmintic, hypolipidaemic, antiatherosclerotic, antiurolithic, antiulcerogenic, analgesic, anesthetic, anti-HIV and antimicrobial activities[5-7]. Phytochemical investigation ofM. oleiferahas resulted in the isolation of a number of constituents belonging to different classes of compounds[5-7]. It has also been incorporated in various marketed formulations[8]. It is a part of medicine “Metrafduabete”which completely removes the signs of diabetes and possesses hypoglycemic activity[9]. In pursuance of this chemical and biological studies onMoringapods and leaves[5], the present work was undertaken on the roots to explore chemistry of its non polar extracts.
2. Materials and methods
2.1. Collection of plant material
The fresh roots ofM. oleiferawere collected from HEJICCBS garden, University of Karachi, in June 2007. A voucher specimen (No. 66250 KUH) had been deposited in the herbarium of the department of Botany, University of Karachi, where it was authenticated by Mr. Abrar Hussein.
2.2. Extraction of the roots of M. oleifera
Fresh and undried crushed root (5.11 kg) ofM. oleiferawere cut into small pieces and successively extracted twice with petroleum ether and dichloromethane (20 L each) at room temperature for 2 d. The first and second extracts were combined on the basis of thin layer chromatography profile and evaporated under reduced pressure to furnish their respective residues marked as petroleum ether extract ofM. oleiferaroots (MRP) (1.94 g) and dichloromethane extract ofM. oleiferaroots (MRDC) (189 g).
2.3. Petroleum ether extract of M. oleifera roots (MRP)
MRP was actually a two phase extract, found to contain some insoluble crystals. These were separated from brownish petroleum ether soluble part by decantation. As a result of this process, crystals embedded in brown matrix were separated from petroleum ether soluble part marked as (MRPSJ-7, 1.60 g). Crystals were washed with methanol, affording methanol soluble fraction (MRPXMSJ-7) and methanol insoluble crystals embedded in brown gum marked as (MRPXMISJ-7). In order to separate the crystals from brown gummy material, petroleum ether was added into it, as a result of which brown gum CX-1 (0.07 g) get separated from pure pale colored crystals marked as CX-2 (0.30 g). As a result, four fractions marked as petroleum ether soluble (MRPSJ-7), methanol soluble (MRPXMSJ-7), brown gum (CXI) and pure crystals (CX-2) were obtained. These fractions were subjected to gas chromatography/gas chromatography-mass spectrometer (GC/GC-MS) analysis along with parent petroleum ether extract (MRP), while CX-2 was subjected to EI-MS analysis, leading to its identification as cyclooctasulfur S8. The compounds identified through GC/ GC-MS analysis are given in Table 1.

Table 1 Chemical analysis of MRP and it fractions, petroleum ether soluble (MRPSJ-7), methanol soluble (MRPXMSJ-7) and brown gum (CXI) through GC/GC-MS studies.
2.4. The dichloromethane extract of M. oleifera roots (MRDC)
MRDC was fractionated employing solvent-solventseparation by using different ratios of petroleum ether : ethyl acetate and ethyl acetate : MeOH (PE: EA and EA: MeOH), increasing 10% polarity affording twenty five eluents, which were marked as MRDCSS-1-25 and subjected to GC, GC-MS studies.
The chemical composition of some of the fractions of dichloromethane extract of root obtained through solventsolvent separation was examined through GC/GC-MS analysis and summarized in Table 2.
2.5. Gas chromatography
GC spectra were run on a 17. A. Shimadzu [FID Mode; column, fused silica capillary column OV-1, DB-1 (30 m× 0.53 mm, 0.5 μm film thickness)], at 75 °C and programmed to 75 °C at 240 °C/min and 3-5 min hold. Injector and detector were at 240 and 250 °C respectively. About 2 μL of each sample were injected triplicate split/split less and quantities represented as relative area (%) as derived from integrator.
2.6. Gas chromatography-mass spectrometry (GC-MS)
For GC-MS, a 6890 N Agilent gas chromatograph coupled with a JMS 600 H JEOL mass spectrometer was used. The compound mixture was separated on a fused silica capillary SPBI column, 30 m×0.32 mm, 0.25 μm film thickness in a temperature program from 50 to 256 °C with a rate of 4 °C/ min with 2 min hold. The injector was at 260 °C and the flow rate of the carrier gas helium, was 1 mL/min. The EI mode JMS 600 H JEOL mass spectrometer had ionization volt of 70 eV, electron emission of 100 μA, ion source temperature of 250 °C and analyzer temperature of 250 °C. Sample was injected manually in split mode. Ratio of sample in split mode was1:45.
2.7.GC/GC-MSidentification of components
GC/GC-MS identification of components of each extract and fractions were based on the computer evaluation of mass spectra of samples through NIST based AMDIS V 2.69 (Automated mass spectral deconvolution and identification software), direct comparison of peaks and retention time with those for standard compounds, with eight peak index[10] and computer matching with the NIST as well as by following the characteristic fragmentation patterns of the mass spectra of particular class of compounds.
2.8. Chemical characterization of isolated compound Cyclooctasulfur (S8)
State: crystalline solid; thin layer chromatography solvent system; [Hexane,Rf=1]; M.P.=119.1 °C [13c]. EI-MS m/z (%): 257.7 (M++2, 13.6), 256.7 (M++1, 2.18), 255.7 (M+, 36.13), 159.9 (19.94), 127.9 (33.87), 95.9 (20.48), 63.9 (100)[11].
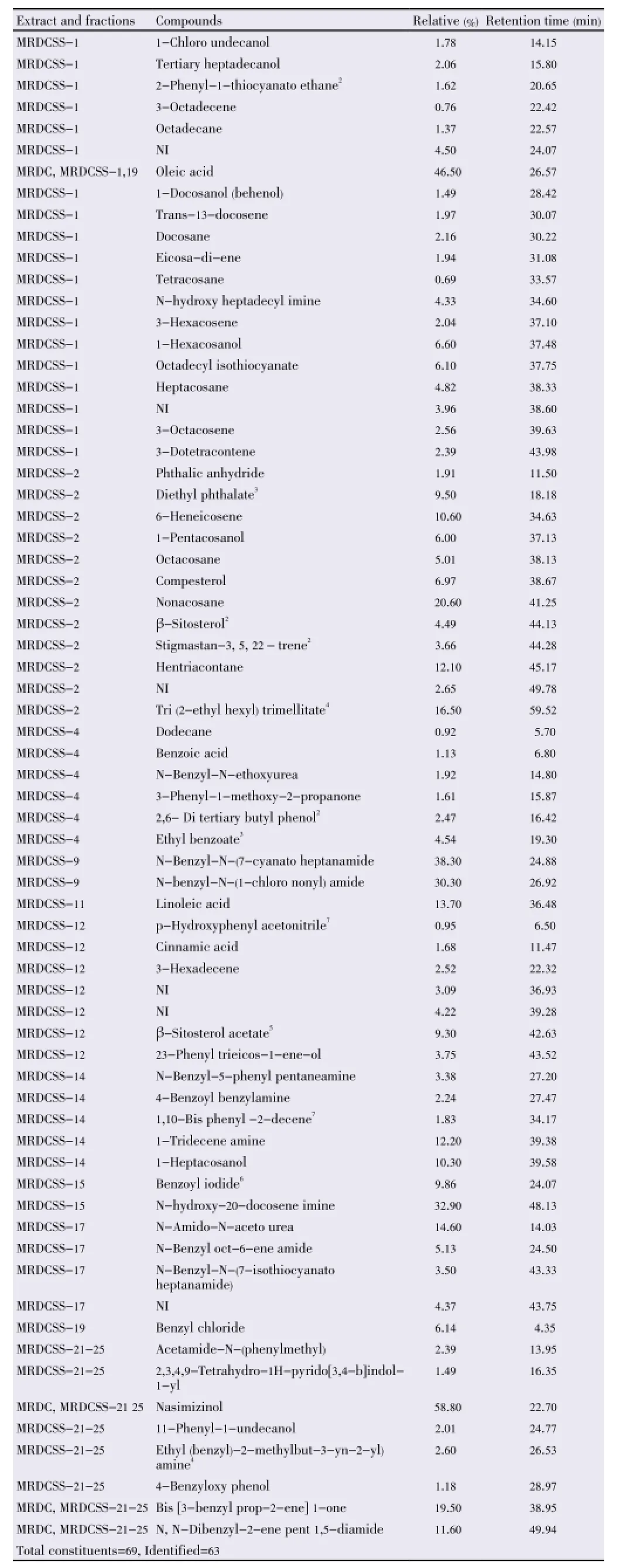
Table 2 Chemical compositions of MRDC and its solvent- solvent separated fractions (MRDCSS-1, 2, 4, 9, 11, 12, 14, 15, 17, 19 and combined 21-25)1.
3. Results
The present communication describes a detailed analysis of the petroleum ether and dichloromethane extracts of root ofM. oleiferaas they showed good antimicrobial activities against a number of microbes. It is for the first time that the composition of the two extracts ofM. oleiferaroots has been investigated through GC/GC-MS analysis. A total of 102 identified through GC/GC-MS analysis of the extracts MRP and MRDC as well as their fractions are mentioned in Tables 1 and 2 respectively.
MRP contained crystals and gummy material, which on classical separation through successive treatment with petroleum ether and methanol afforded four fractions, petroleum ether soluble, methanol soluble, brown gum and pure crystals. The latter was identified as cyclooctasulfur S8, which has been earlier identified through GC/GC-MS analysis from the same source[5], however, it has been isolated previously from various plants and bacteria[12-14]. MRP and all its fractions were subjected to GC/GC-MS analysis which helped in identification of 39 compounds, classified into nine groups including hydrocarbons, acids, esters, alcohols, aromatics and alkamides along with a sulfur, cyanide and steroidal compound (Table 1). As whole petroleum ether extract was found to be rich in odd carbon metabolites. The major compounds identified were trans-13-docosene (37.9%), nonacosane (32.6%), cycloartenol (28.6%) nonadecanoic acid (13.9%) and cyclooctasulfur S8 (13.9%).
MRDC was found to contain two prominent compounds identified as oleic acid and 5-ethyl-3-fluoro-2-pyrazinamine (nasimizinol). The chemical composition of some of the fractions of MRDC obtained through solventsolvent separation (experimental) was studied through GC/GC-MS analysis and is summarized in Table 2. It was found to be constitutive of 63 compounds. The major compounds identified were nasimizinol (58.8%), oleic acid (46.5%), N-benzyl-N-(7-cyanato heptanamide (38.3%), N-benzyl-N-(1-chlorononyl) amide (30.3%), bis [3-benzyl prop-2-ene]-1-one (19.5%) and N, N-dibenzyl-2-ene pent 1, 5-diamide (11.6%). MRDC was found to contain two prominent compounds identified as oleic acid and 5-ethyl-3-fluoro-2-pyrazinamine (nasimizinol).
4. Discussion
The GC-MS analysis was based on the computer evaluation of mass spectra of samples through NIST based AMDIS V 2.69 (automated mass spectral deconvolution and identification software), direct comparison of peaks and retention time with those for standard compounds, with eight peak index[10] and computer matching with the NIST. Besides that, the characteristic fragmentation patterns greatly helped in the identification of a particular class of compounds[10]. However, position of the double bond in the unsaturated hydrocarbons is tentative. Fatty acids and esters showed their corresponding McLafferty fragmentation peaks and the characteristic hydrocarbon profile. On the other hand, benzyl amine also called moringine (a well-known constituent from root ofM. oleifera) derivatives showed their characteristics peaks at m/z 77, 91 and 106 for benzenebenzyland benzyl aminecations. Their long chain amide derivatives, alkamides which give rise to characteristics mass peak at m/z 149 (along with 77, 91 and 106) arising due to McLafferty rearrangement with an intensity of a base peak[15]. In addition to this, thiocyanates and isothiocyanates were identified on the basis of their respective -SCN and -NCS losses from the molecular ion peak. N-hydroxylimines give rise to a peak at those mass to charge ratios that corresponds to (M+-44) indicating the loss of N-hydroxylimine group from the molecule.
This study helps to predict the formula and structure of active molecules which can be used as drugs. This result also enhances the traditional usage ofM. oleiferawhich possesses a number of bioactive compounds.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
This work is dedicated to the fond memory of Prof. Salimuzzman Siddiqui FRS (1897-1994), the founding director of H.E.J. Research Institute of Chemistry, University of Karachi. One of the authors Saima Sumbul wants to thank the Government of Pakistan, Ministry of Youth Affairs for an Internship award, under National Internship Programme.
Comments
Background
Various parts of Moringa oleifera or drum stick tree are used in traditional medicine for the treatment of a variety of ailments. Authors have analyzed the petroleum ether and dichloromethane extracts of Moringa oleifera roots by GC-MS to identify the phytoconstituents present in theroot. GC- MS analysis resulted in the identification of one hundred and two (102) compounds which could play an important role in exhibiting biological activity.
Research frontiers
It is a pilot study that identified only volatile constituents in Petroleum and Dichloromethane extract of Moringa roots, however there could be other constituents present, responsible for their biological activity. Efforts should be made to isolate and identify those non fatty acid components of the roots by other suitable chromatographic techniques.
Related reports
Previous Phytochemical studies conducted on roots of Moringa reported it to have high concentration of 4-(α-L-rhamnopyranosyloxy) benzyl glucosinolates and benzylglucosinolate (J Agri food chem. 2003;51(12)3546-3553) which are partly responsible for their antimicrobial activity. Another study concluded that Moringa roots possess potent antimicrobial activity that was due to the presence of pterygospermin (Indian J Pharm Sci 1998; 60: 33-35.). The aglycone of deoxy-niazimicine (N-benzyl, S-ethyl thioformate) isolated from the chloroform fraction of an ethanol extract of the root bark was also found to be responsible for the antibacterial and antifungal activities (Pak J Biol Sci 2003;22:1888-1890.). The roots also contain alkaloids (0.2% of the total) [Rev Nutr Campinas 2008; 21(4):431-437.].
GC-MS is routinely used for the analysis of volatile oil and fixed oil compositions and the compounds identified have minimal role to play in exhibiting antimicrobial activity.
Innovations and breakthroughs
he study only identified volatile constituents that can help in Phytochemical profiling.
Applications
The data obtained can help in standardization of non polar root extract.
Peer review
I reckon this is a pilot study aimed to identify the fatty/volatile constituents in the petroleum ether and dichloromethane extracts. This plant has been extensively explored phytochemically well as for different pharmacological activitires due to its wide spread use in traditional medicines throughout the world.
[1] Navie S, Csurhes S. In: Horseradish tree: Moringa oleifera. Queensland: Department of Employment, Economic Development and Innovation; 2010.
[2] Kuben KN, Roger MC. Review on herbal remedies used by the 1 860 South African Indian settlers. Afr J Biotechnol 2011; 10: 8533-8538.
[3] Anthonia OO. Evaluation of antimicrobial properties and nutritional potentials of Moringa oleifera Lam. leaf in South Western Nigeria. Mal J Microbiol 2012; 8: 59-67.
[4] Dhakar RC, Maurya SD, Pooniya BK, Bairwa N, Gupta M. Moringa: the herbal gold to combat malnutrition. Chron of Young Scientists 2011; 2: 119-125.
[5] Mbikay M. Therapeutic potential of Moringa oleifera leaves in chronic hyperglycemia and dyslipidemia: a review. Front Pharmacol 2012; 3: 24.
[6] Garima M, Pradeep S, Ramesh V, Sunil K, Saurabh S, Jha KK, et al. Traditional uses, phytochemistry and pharmacological properties of Moringa oleifera plant: an overview. Der Pharmacia Lett 2011; 3: 141-164.
[7] Subrata KB, Anusua C, Joysree D, Ajoy R, Hosen S. Pharmacological potentials of Moringa oleifera Lam.: a review. Int J Pharma Sci Res 2012; 3: 305-310.
[8] Sashidhara KV, Rosaiah JN, Tyagi E, Shukla R, Raghubir R, Rajendran SM. Rare dipeptide and urea derivatives from roots of Moringa oleifera as potential anti-inflammatory and antinociceptive agents. Eur J Med Chem 2009; 44(1): 432-436.
[9] Gupta R, Mathur M, Bajaj VK, Katariya P, Yadav S, Kamal R, et al. Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes. J Diabetes 2012; 4: 164-171.
[10] Mass Spectrometry Data Centre. Eight peak index of mass spectra: the eight most abundant ions in 31,101 mass spectra, indexed by molecular weight, elemental composition and most abundant ions (4 volume set). 2nd ed. Aldermaston: Mass Spectrometry Data Centre; 1974.
[11] Penczek S, Duda A. Anionic copolymerization of elemental sulfur. Pure Appl Chem 1981; 53: 1679-1687.
[12] Li N, Li X, Yang SL, Zhang P. [Isolation and identification of heteroatom-containing and polyols constituents from Comptosorus sibiricus Rupr]. Chin J Med Chem 2004; 14: 368-369. Chinese.
[13] Gao YT, Yang XW, Ai TM. [Studies on the chemical constituents in the ethanolic extract from the herbs of Dicliptera chinensis]. Zhongguo Zhong Yao Za Zhi 2006; 31: 985-987. Chinese.
[14] Lognay G, Seck D, Marlier M, Haubruge E, Gaspar C, Severin M. Identification of elemental sulfur (S8) in Boscia senegalensis leaves. Bull Rech Agron Gembloux 1993; 28: 501-505.
[15] Zhao J, Muhammad II, Dunbar DC, Mustafa J, Khan A. New alkamides from macca (Lepidium meyeni). J Agric Food Chem 2005; 53: 690-693.
10.12980/APJTB.4.201414B141
*Corresponding author: Muhammad Ali Versiani, Department of Chemistry, Federal Urdu University of Arts, Sciences and Technology, Gulshan-e-Iqbal, Science Campus, Karachi-75300, Pakistan.
Tel: 92 21 99244141-146 (Ext. 2150)
Fax: 92 21 99244272
E-mail: mali.versiani@fuuast.edu.pk
Article history:
Received 26 Jun 2014
Received in revised form 27 Jun, 2nd revised form 7 Jul, 3rd revised form 19 Jul 2014
Accepted 11 Aug 2014
Available online 28 Aug 2014
Methods:A total of 5.11 kg fresh and undried crushed root of M. oleifera were cut into small pieces and extracted with petroleum ether and dichloromethane (20 L each) at room temperature for 2 d. The concentrated extracts were subjected to their GC-MS analysis.
Results:The GC-MS analysis of the petroleum ether and dichloromethane extracts of M. oleifera roots, which showed promising biological activities, has resulted in the identification 102 compounds. These constituents belong to 15 classes of compounds including hydrocarbons, fatty acids, esters, alcohols, isothiocyanate, thiocyanate, pyrazine, aromatics, alkamides, cyanides, steroids, halocompounds, urea and N-hydroxyimine derivatives, unsaturated alkenamides, alkyne and indole. GC/GC-MS studies on petroleum ether extract of the roots revealed that it contained 39 compounds, belonging to nine classes. Cyclooctasulfur S8 has been isolated as a pure compound from the extract. The major compounds identified from petroleum ether extract were trans-13-docosene (37.9%), nonacosane (32.6%), cycloartenol (28.6%) nonadecanoic acid (13.9%) and cyclooctasulfur S8 (13.9%). Dichloromethane extract of the roots was composed of 63 compounds of which nasimizinol (58.8%) along with oleic acid (46.5%), N-benzyl-N-(7-cyanato heptanamide (38.3%), N-benzyl-N-(1-chlorononyl) amide (30.3%), bis [3-benzyl prop-2-ene]-1-one (19.5%) and N, N-dibenzyl-2-ene pent 1, 5-diamide (11.6%) were the main constituents.
Conclusions:This study helps to predict the formula and structure of active molecules which can be used as drugs. This result also enhances the traditional usage of M. oleifera which possesses a number of bioactive compounds.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- Adult Klebsiella pneumoniae meningitis in Qatar: clinical pattern of ten cases
- Iron-chelating and anti-lipid peroxidation properties of 1-(N-acetyl-6-aminohexyl)-3-hydroxy-2-methylpyridin-4-one (CM1) in longterm iron loading β-thalassemic mice
- Digestive fungal flora in asymptomatic subjects in Bobo-Dioulasso, Burkina Faso
- Glucose-6-phosphate dehydrogenase (G6PD) deficiency is associated with asymptomatic malaria in a rural community in Burkina Faso
- An efficient method in breaking of dormancy from Bunium persicum (Boiss) Fedtsch seeds: a valuable herb of Middle East and Central Asia
- Physico-chemical analysis and antimicrobial potential of Apis dorsata, Apis mellifera and Ziziphus jujube honey samples from Pakistan
