An efficient method in breaking of dormancy from Bunium persicum (Boiss) Fedtsch seeds: a valuable herb of Middle East and Central Asia
2014-03-22YousefEmamipoorMahmoodMaziah
Yousef Emamipoor, Mahmood Maziah
1Department of Natural Resources, Agricultural and Natural Resources Research Center, 7617913935, Kerman, Iran
2Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, 43300, Selangor, Malaysia
An efficient method in breaking of dormancy from Bunium persicum (Boiss) Fedtsch seeds: a valuable herb of Middle East and Central Asia
Yousef Emamipoor1,2*, Mahmood Maziah2
1Department of Natural Resources, Agricultural and Natural Resources Research Center, 7617913935, Kerman, Iran
2Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, 43300, Selangor, Malaysia
PEER REVIEW
Peer reviewer
Prof. Shunhai Qu, Hainan Medical University, Haikou, China.
E-mail: qu.sh@163.com
Comments
This is a valuable research work in which authors investigated the effect of temperature and moisture, different plant growth regulators as well as combinations of GA3and TDZ under chilling temperature on breaking of B. persicum seed dormancy. The methods adopted are appropriate and the findings are interesting.
Details on Page 648
Objective:To develop a protocol for breaking of seed dormancy and increasing the seed germination rate of Bunium persicum.
Bunium persicum, Seed germination, Thidiazuron, Benzyl aminopurine, Gibberellic acid 3, Forchlorfenuron, Chilling temperature
1. Introduction
Bunium persicum (B. persicum)(Boiss) Fedtsch. is a herbaceous perennial plant distributed in mountainous steppes and shrub lands of Iran, Turkmenistan, Tajikistan, Afghanistan, Pakistan, Kashmir and India[1]. This plant is distributed at 1 700-2 900 m in the semi-arid region of Iran. However, March is the rainiest month in the distributed area and annual temperature is about 15 °C. Fruits are edible parts of the plant and are used in treatment for digestive and urinary systems.
Hypoglycemic, anticonvulsant, antiemetic and antiasthma activities are some important effects of the plant seeds[2].
Economic plantation ofB. persicumis confronted with two major problems including seed dormancy and long juvenile time. The plants show different methods for germination of dormant seeds[3]. Seed dormancy has been classified in many classes including physiological, morphological, morphophysiological, physical and combinational dormancy[4]. Dormancy ofB. persicumseeds is an interesting area of research. Some researchers believe that stratification is the only factor to influence the breaking of seed dormancy. In contrast, evidence shows that germination rate is increased in the presence of benzyl aminopurine (BAP) plus polyethylene glycol and gibberellic acid (GA3)[5]. There are some reports of successful application of forchlorfenuron (CPPU) for germination of under stress seeds such as rice seeds[6]. The next important member of these chemical groups, thidiazuron (TDZ), can be used for maturity and germination of seeds in plants such asPaphiopedilum hangianum[7]. In addition, TDZ have been used for the treatment of the immature seeds ofEpimedium alpinum[8]. Reports showed that GA3is a common plant growth regulator (PGR) that regulates the seed development and germination[9,10]. However, GA3alone or in combination with chilling treatment are common factors in breaking of seed dormancy[11]. Evidence shows that seed germination ofFerula gummosaincreased to 75% when seeds were treated with 2.9 mmol/L of GA3and under pre-chilling condition[12]. However, seed dormancy is the common problem of the umbelliferous family[13]. Also report revealed that nitric oxide could break dormancy from seeds only when combined with GA3[14]. InParthenium argentatum, breakage of seed dormancy happened in the present of 0.7-1.4 mmol/L of GA3, light and abscisic acid[15].
Two important cytokinins, BAP and N6-furfurylaminopurine (kinetin), alone or in combination with chilling temperature have been used to break the seed dormancy of umbelliferous plants[16] . BAP has also been found to be more effective in seed dormancy breaking than kinetin[17]. Moreover, chilling or hot temperature affects the breaking of seed dormancy in many plants. InFerula asafetida(Apiaceae) chilling temperature breaks seed dormancy while inHypericum aviculariifolium(Hypericaceae) hot temperature breaks seed dormancy[17]. InB. persicum, application of chilling temperature shortens the seed germination period[18]. Sometimes, chilling temperature is the only treatment for seed dormancy breaking[19]. However, seed dormancy, apart from physical, physiological or morphophysiological types, is one of the most significant problems in domestication ofB. persicum. The common factors in breaking of seed dormancy including gibberellins (GA3), cytokinins (BAP) and phenylureas alone or combined with stratification (chilling treatment) are used for increasing the efficiency of breaking the seed dormancy from the plant seeds.
2. Materials and methods
2.1. Seed collection
Seeds ofB. persicumwere collected from the mountains of Kerman, Iran, in June 2008. This area, situated 26 km from Sirch, is called the Bolboloie’s Mountains. In this area, the herbs ofB. persicumare distributed between 2 800 and 3 100 m from sea level. The area is an arid region and mean of precipitation is about 200 mm/year.
2.2. Seed sterilization method
Seeds were washed under running tap water for six hours. These seeds were shaken for 60 seconds with 70% ethanol followed by shaking in 100 mL of 2 g/L sodium hypochlorite solution with 2 drops of Tween 20 for 15 min. The sodium hypochlorite was removed with three times shaking in sterile distilled water at intervals of 10 min.
2.3. Experimental procedure
2.3.1. Effects of temperature and moisture on breaking of seed dormancy
Although stratification alone influences the breaking of dormancy inB. persicumseeds but the germination rate is low[18]. In this experiment the effects of chilling temperature alone (dry-chilling) and chilling temperature with moisture (moist-chilling) have been studied. For conducting the experiment, three month old seeds were divided into four parts as follows:
1. Two grams of non-sterile seeds were placed in a dark plastic bag and put at 25 °C (dry-room condition).
2. Two grams of non-sterile seeds were placed in a dark plastic bag and put at 2-5 °C (dry-chilling condition).
3. Two grams of sterile seeds were placed on three layers of moist Whatman paper in Petri dishes and put at 25 °C (dark moist-room condition).
4. Two grams of sterile seeds were transferred onto three layers of moist Whatman paper on to Petri dishes and put at 2-5 °C (moist-chilling condition).
At 20-day intervals, 300 non-sterile seeds from dry-room condition (a) and or dry-chilling condition (b) were sterilized. The imbibed seeds were transferred onto three separate Petri dishes with moist-sterile Whatman paper and put at 25 °C temperature in the PROTECH growth chamber, model GC-500. In addition, the same amount of seeds from moist-room condition and also moist-chilling condition was transferredto the growth chamber. The percentage of seed germination was calculated with the following formula:

2.3.2. Effects of plant growth regulators on breaking of seed dormancy
Evidence showed that exogenous chemicals had increased the breaking of seed dormancy in the umbelliferous family[17]. BAP and GA3are known as important chemicals in the umbelliferous family for this reason[20]. In addition, gibberellins, especially GA3play a critical role in seed germination and are an important factor in classification of seed dormancy.
In this study, the influence of two natural (BAP and GA3) and two synthesized plant growth regulators including TDZ and CPPU on six-month old seeds ofB. persicumhas been studied. Seeds were sterilized and transferred to 150 mL Erlenmeyer flasks. Each flask included 0, 3.1, 6.3, 12.5, 25, 50 and 100 µmol/ L of TDZ, CPPU, BAP or GA3. The flasks were then transferred to a shaker at room temperature. The seeds were shaken at 120 r/min. After 24 h, the seeds were transferred to bottles with provided moisture (Figure 1) and put under two different temperature conditions including room temperature (25 °C) and chilling temperature (2-5 °C) and the germinated seeds were counted.
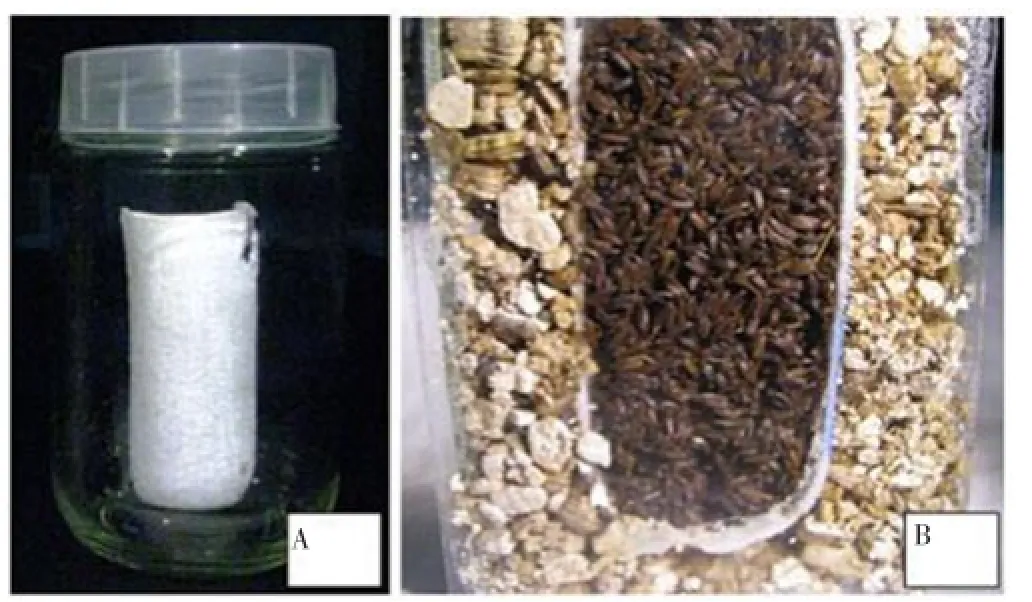
Figure 1. Seed germination bottle were filled with seeds of B. persicum.(A) A thimble has been used to separate seeds from vermiculite. (B) Longitudinal section of bottle showing the position of the seeds surrounded by vermiculite layer.
2.3.3. Effect of GA3and TDZ combinations on breaking of seed dormancy under chilling temperature
About 10 g seeds were sterilized and transferred into flasks with combinations of different concentrations of TDZ and GA3including: 0, 3.1, 6.3 and 12.5 µmol/L TDZ and 0, 25 and 50 µmol/L GA3. Seeds were treated for 24 h and transferred onto Whatman paper. The treated seeds with 0.0 µmol/L TDZ and 0.0 µmol/L GA3were used as control. Then, 100 seeds were transferred to 300 mL sterilized bottles with moist vermiculite. The bottles were put at 2-5 °C. The randomized complete design with three replicates was used. The seeds were observed after two weeks and the number of germinated seeds was compared after 30 d.
2.4. Seedling acclimatization
For hardening, seedlings were planted in 300 mL plastic pots filled with a mix of potting, burnt and high quality black soils in proportions of 1: 1: 1 (v: v: v). The lengths of two month-old seedlings were recorded.
2.5. Statistical analysis
The data were analysed by One-way ANOVA and the least significant difference test (LSD) was at 5% level. SAS version 9.1 and MSTATC computer program were used for significance of rate of seed germination. Results are expressed as mean±SD of independent experiments.
3. Results
3.1. Effects of temperature and moisture on breaking of seed dormancy
The dry and moist seeds did not germinate under room temperature condition, and therefore were dormant[4]. Analysis of variances about the influence of chilling temperature and moisture showed that these factors had a highly significant effect on breaking of seed dormancy (Table 1). Dry seedsat 2-5 °C (dry-chilling condition) germinated from Day 40 and maximum germination was 9.3% after 120 d chilling treatment (Figure 2).

Table 1 Analysis of variance of the effects of temperature and moisture on seed germination of B. persicum.

Figure 2. Seed germination of B. persicum under dry-chilling condition for 120 d, followed by transferring to moist-room condition for one month.(A) Seeds before imbibition. (B) Non-germinated seeds after 40 d treatment. (C) Germinated seeds after 60 d treatment. (D) Germinated seeds after 80 d treatment. (E) Germinated seeds after 100 d treatment. (F) Germinated seeds after 120 d treatment. Scale bar=5 mm.
The seeds under moisture and chilling temperature (moist-chilling condition) germinated from Day 20 and maximum germination was 54.7% after 60 d stratification (Table 2).

Table 2 Seed germination rate of B. persicum seeds exposed to temperature and moisture conditions for 20, 40, 60, 80, 100 and 120 d.
Results of this experiment showed that breaking of seed dormancy was related to temperature and moisture. In addition, results showed that more exposure time for the seeds under dry-chilling condition increases the germination rate but for seeds under moist-chilling condition, there was a decrease in seed germination rate (Figure 3).
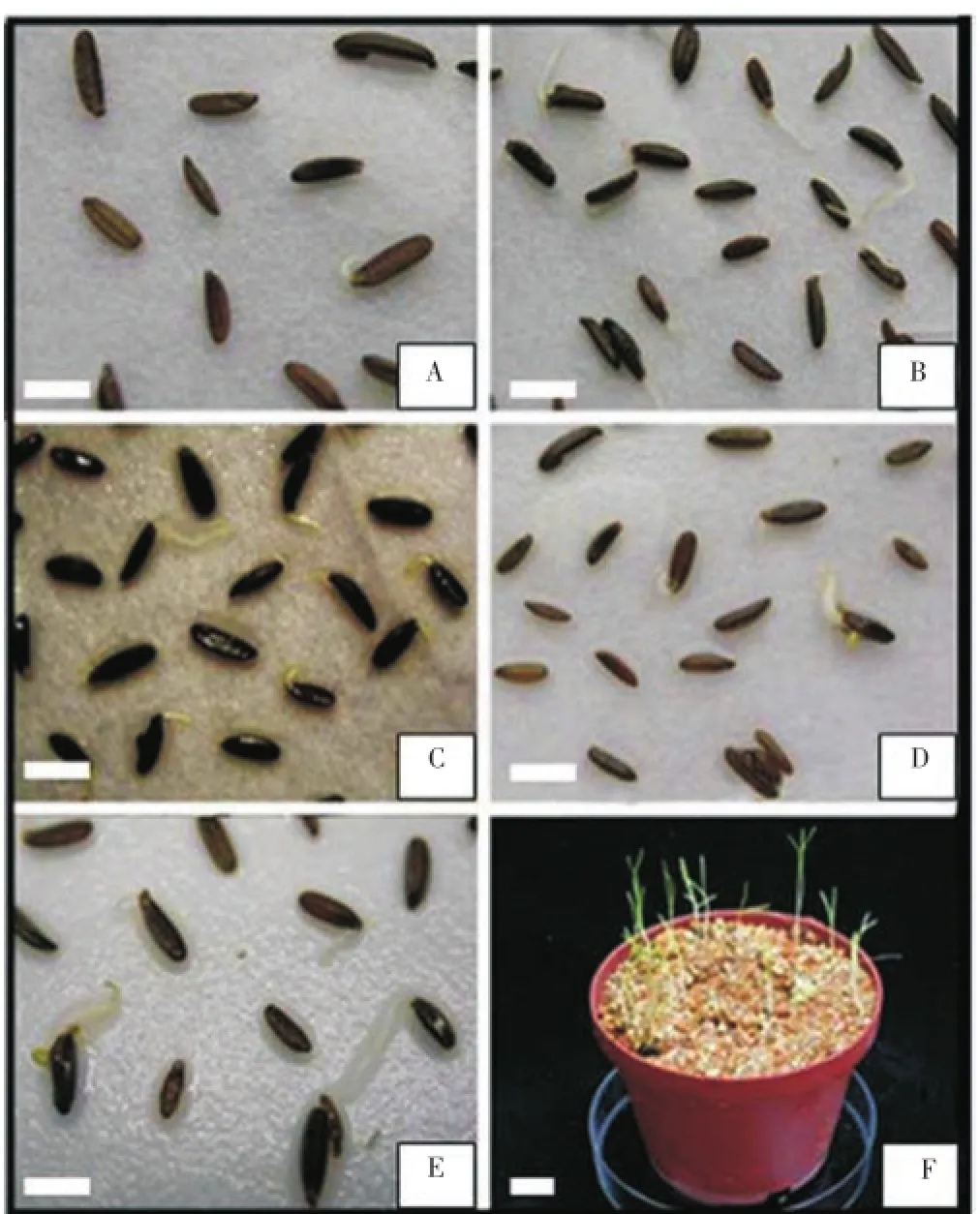
Figure 3. Seed germination of B. persicum under moist-chilling condition for 120 d, followed by transferring to moist-room condition for one month.(A) Germinated seeds after 20 d treatment. (B) Germinated seeds after 40 d treatment. (C) Germinated seeds after 60 d treatment. (D) Germinated seeds after 80 d treatment. (E) Germinated seeds after 100 d treatment. (F) Seedlings after two months in plastic pod. Scale bar=5 mm.
3.2. Influence of plant growth regulators on breaking of seed dormancy under moist-room condition
Analysis variances showed that PGRs had a high significant influence on breaking of dormancy ofB. persicumseeds (Table 3). The results showed that cytokinins, including BAP and synthetic ones TDZ and CPPU have different effects on seed germination. The results indicated that the seeds treated with BAP showed no effect on seed germination under room temperature while TDZ and CPPU influenced seed germination in different concentrations. The maximum seed germination under cytokinins treatments was 23.3% and 20.0% obtained from 12.5 µmol/L TDZ and 6.3 or 12.5 µmol/L CPPU respectively. Also, seeds treated with 100 µmol/L GA3showed 46.7% seed germination which was the most effective exogenous growth regulator in breaking of seed dormancy and germination (Figure 4).

Table 3 Analysis of variance for the breaking of seed dormancy by various concentrations of PGRs under moist-room condition.
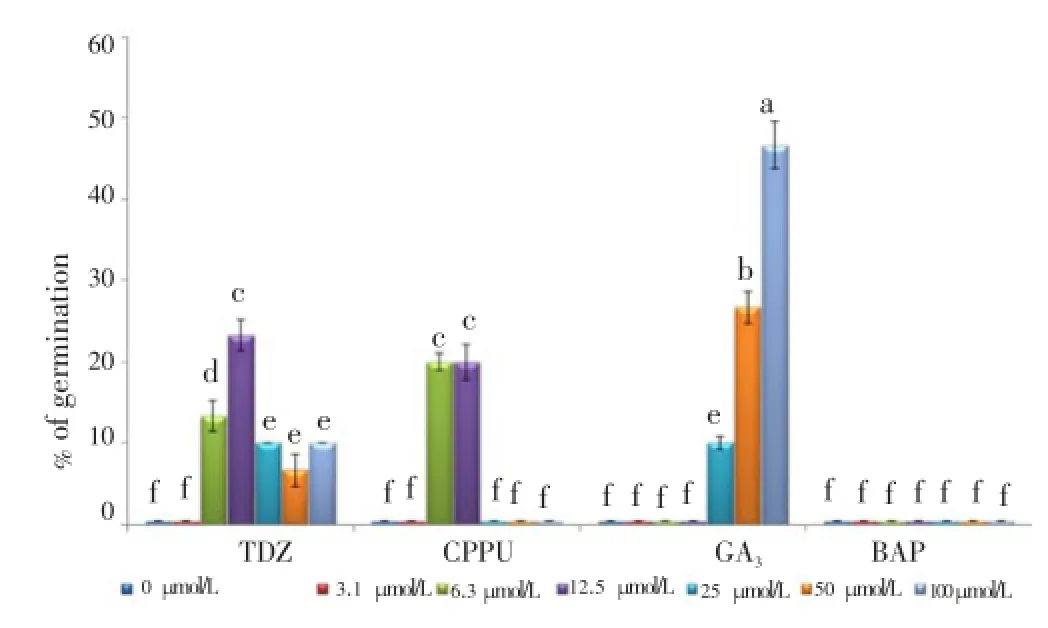
Figure 4. Effect of different levels of TDZ, CPPU, GA3, and BAP on seed germination of B. persicum under moist-room condition.Data were shown as mean±SD. Mean values±SD with the same letter (a-f) were not significantly different (P≤0.05).
3.3. Influence of plant growth regulators on breaking of seed dormancy under moist-chilling condition
The ANOVA table showed that PGRs had a highly significant effect on breaking of seed dormancy and germination (Table 4).
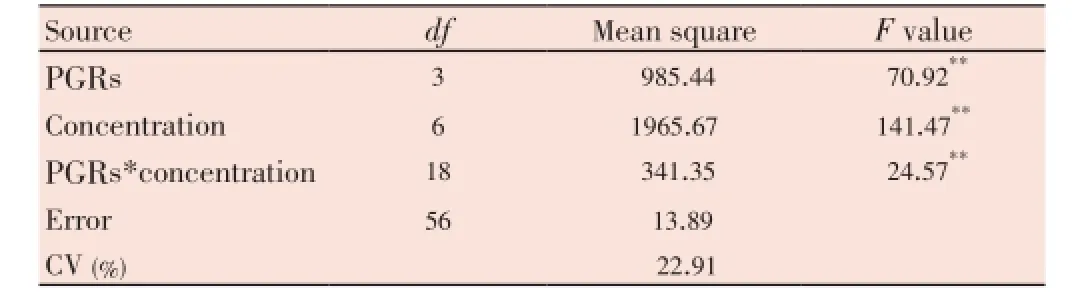
Table 4 Analysis of variance related to the effects of different concentrations of TDZ, CPPU, GA3and BAP on seed germination of B. persicum under chilling temperature (2-5 °C).
Results of seed germination under room and chilling temperature conditions showed that chilling temperature at 2-5 °C (stratification) increase the percentage of seed germination of three-month-old seeds. Accordingly, the combination of stratification and PGRs on six months old seeds was studied. The results showed that stratification alone is able to break seed dormancy, although it was less than that of three-month-old seeds. Germination of seeds was recorded after 30 d inoculation. The results showed that TDZ at 6.3 µmol/L highly influenced breaking of seed dormancy with higher seed germination of 53.3%. In addition, 12.5 µmol/L TDZ, 12 µmol/L BAP, 25, and 50 µmol/L GA3influenced seed germination with rate of 36.7%, 36.7%, 30%, and 30% respectively (Figure 5). Results of this experiment showed that stratification of dormant seeds (moist-chilling condition) was the main factor in breaking of seed dormancy (control). In addition, results revealed that under moist-chilling condition, cytokinins and gibberellins (GA3) had more influence on breaking of seed dormancy and germination. Furthermore, results showed that under that condition, BAP influenced seed germination with maximum rate of 23.3% at 6.3 µmol/L. It should be mentioned that under stratification condition, lower concentration of growth regulators gave a higher effect on breaking of seed dormancy and seed germination. Thus, it seemed that stratification was the main factor in seed germination and growth regulators were co-factors inB. persicumseed germination.

Figure 5. Effect of different levels of TDZ, CPPU, GA3, and BAP on seed germination of B. persicum under moist-chilling condition.Data were shown as mean±SD. Mean values±SD with the same letter (a-f) were not significantly different (P≤0.05).
3.4. Effect ofTDZand GA3on seed germination
In the previous experiments, the findings were: 1.B. persicumseeds were dormant and did not germinate under normal physical conditions (proper light and room temperature). 2. The moisture and chilling temperature were two important factors in breaking of seed dormancy. 3. GA3at 50 and 100 µmol/L showed maximum effect on breaking of seed dormancy at moist-room condition. 4. TDZ at 3.1 and 6.3 µmol/L showed maximum effect on breaking of seed dormancy under moist chilling condition.
In this experiment, the synergic effect of GA3and TDZ under moist chilling condition was investigated (Figure 6). Analysis of variance showed that GA3and TDZ have a highly significant influence on breaking of seed dormancy and seed germination (Table 5).

Table 5 Analysis of combinational variance of the effect of TDZ and GA3on seed germination of B. persicum under stratification condition (2-5 °C) .

Figure 6. Germinated seeds under treatment with chilling temperature and a combination of TDZ and GA3.(A) 0.0 µmol/L TDZ+0.0 µmol/L GA3, (B) 0.0 µmol/L TDZ+25 µmol/L GA3, (C) 0.0 µmol/L TDZ+50 µmol/L GA3, (D) 3.1 µmol/L TDZ+0.0 µmol/L GA3, (E) 3.1 µmol/L TDZ+25 µmol/L GA3, (F) 3.1 µmol/L TDZ+50 µmol/L GA3, (G) 6.3 µmol/L TDZ+0.0 µmol/L GA3, (H) 6.3 µmol/L TDZ+25 µmol/L GA3, (I) 6.3 µmol/L TDZ+50 µmol/L GA3, (J) 12.5 µmol/L TDZ+0.0 µmol/L GA3, (K) 12.5 µmol/L TDZ+25 µmol/L GA3, (L) 12.5 µmol/L TDZ+50 µmol/L GA3. Seed length=5 mm.
The results showed that the maximum seed germination, 93.7%, was from treatment of seeds with 6.3 µmol/L TDZ and 50 µmol/L GA3. The minimum synergism was from treatment with 3.1 µmol/L TDZ and 25 µmol/L GA3(Table 6).
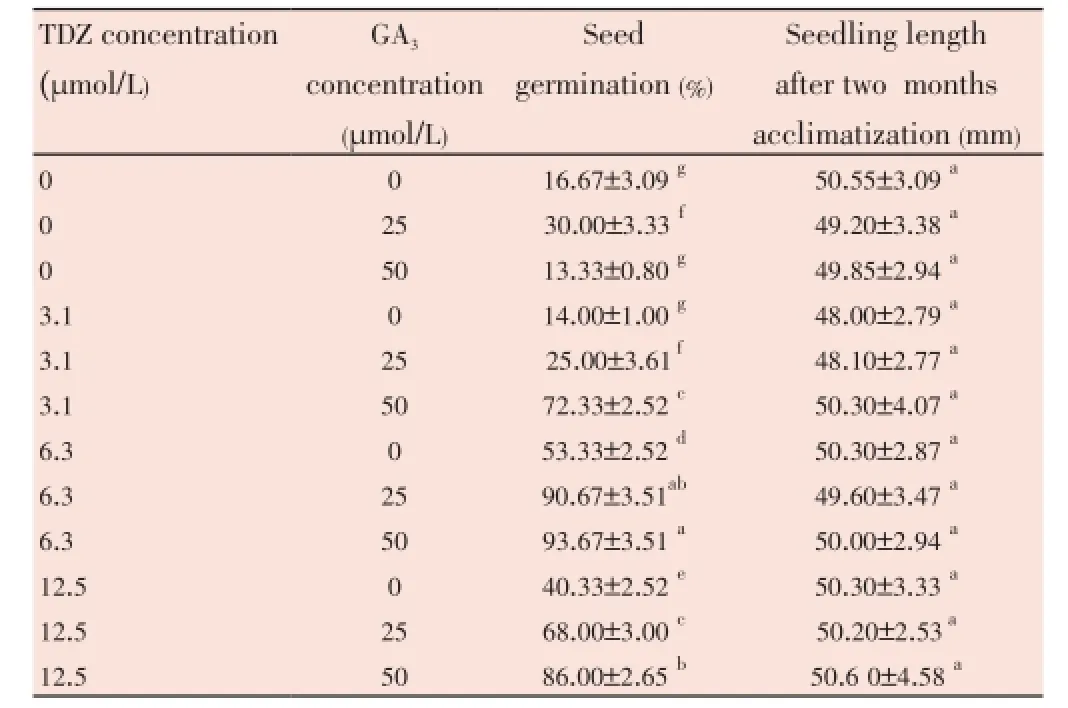
Table 6 The responses of seed germination and the seedling length of B. persicum to different level of TDZ and GA3.
3.5. Acclimatization of one-month-old seedlings
The seedlings were transferred to 300 mL plastic pots filled with potting, burnt and high quality black soils in a ratio of 1: 1: 1 (v: v: v) (Figure 7). All seedlings showed normal growth. Also, the variance analysis of data showed that the effect of TDZ and GA3on seedlings length was not significant (Table 7). It is therefore, suggested that TDZ and GA3have no effect on the normal growth of seedlings.

Table 7 Analysis of variance about the effects of different levels of TDZ and GA3on seedling length of B. persicum after one month acclimatization.

Figure 7. Seed acclimatization of B. persicum after treatment with chilling temperature and a combination of TDZ and GA3in the 300 mL pots.(A) 3.1 µmol/L TDZ+0.0 µmol/L GA3, (B) 3.1 µmol/L TDZ+25 µmol/L GA3, (C) 3.1 µmol/L TDZ+50 µmol/L GA3, (D) 6.3 µmol/L TDZ+0.0 µmol/L GA3, (E) 6.3 µmol/L TDZ+25 µmol/L GA3, (F) 6.3 µmol/L TDZ+50 µmol/L GA3, (G) 12.5 µmol/ L TDZ+0.0 µmol/L GA3, (H) 12.5 µmol/L TDZ+25 µmol/L GA3, (I) 12.5 µmol/L TDZ+50 µmol/L GA3.
4. Discussion
Seed germination behavior shows that stratification is the most effective factor and imbibed seeds germinate with the highest rate only occur in chilling temperature. It indicates that respiratory systems, including the citric acid cycle, glycolysis, and pentose phosphate pathway and or protein synthesis do not start in dry seeds at room temperature[21]. Normally byseed imbibition, respiration and protein synthesis will provide necessary energy (adenosine triphosphate) for germination[22]. Evidence shows that under moist-chilling condition many genes in dormant seeds are active and some of these genes are under control of plant hormones[23]. Thus, maybe low percent of germination in imbibed seeds under room temperature is related to inner cell membrane of mitochondria. Since only pentose phosphate pathway and glycolysis are active, cell membrane related photosynthesis, that is, citric acid cycle is not active. In fact, under chilling temperature, cell membrane structure permits respiratory enzymes to retain their active structure.
The effect of GA3on seed germination under room temperature condition showed that seed dormancy inB. persicumwas from intermediate physiological type to nondeep complex morphophysiological dormancy[4]. According to Hossainet al., by exogenous application of GA3, not only the ratio of endogenous germination promoters (such as GA3) to germination inhibitors increased, but also, the cell metabolism increaseed[16]. Therefore, it is suggested that high seed germination under moist-chilling condition pre-treated with combination of TDZ and GA3, is related to increasing in the ratio of GA3and or activity of endogenous phytohormones. In addition, according to Ferreiraet al., the effect of TDZ on breaking of seed dormancy could be related to increasing the endogenous auxins and cytokinins of the seeds[24].
These results show that seed germination ofB. persicumdepends on the age of seeds after harvesting. Seeds in the early harvesting time (three months old) were under physiological dormancy. However, behavior of seed germination changed after six months and seed dormancy changed to morphophysiological dormancy. This result was consistent with results showing effects of stratification and chemicals on seed germination ofB. persicum[18].
The aim of this project was to determine the factors associated with propagation ofB. persicumby seed intermediate. The seeds are dormant and our finding suggests that breaking of seed dormancy could be helpful for domestication of this wild plant. The following conclusions can be drawn from the present study. The first major finding is that 60 d stratification alone causes 54.7% seed germination. Seed germination increases to more than 93.67% when seeds are treated with a combination of GA3and TDZ under 2-5 °C stratification after one month. Future studies are needed to decrease the juvenile time for successful domestication ofB. persicum.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The authors would like to send gratitude to Universiti Putra Malaysia for supporting the project via Research University Grant Scheme (Vote No. 9322400) and Dr. Zand, the Deputy Minister and Head of Agricultural Research, Education and Extension Organization, and also Dr. Bahman Panahi, the Dean of Kerman Agricultural and Natural Resources Research Centre for providing the seeds.
Comments
Background
Economic plantation ofB. persicumis confronted with two major problems including seed dormancy and long juvenile time. Seed dormancy has been classified in many classes including physiological, morphological, morphophysiological, physical and combinational dormancy. Dormancy ofB. persicumseeds is an interesting area of research.
Research frontiers
The present study makes an attempt to investigate the effect of temperature and moisture, different plant growth regulators as well as combinations of GA3and TDZ under chilling temperature on breaking ofB. persicumseed dormancy.
Related reports
Some researchers believe that stratification is the only factor to influence the breaking of seed dormancy. In contrast, it is reported that germination rate is increased in the presence of BAP plus polyethylene glycol and GA3. There are some reports of successful application of CPPU for germination of under stress seeds such as rice seeds. Some reports showed that GA3is a common plant growth regulator that regulates the seed development and germination. Also another report revealed that nitric oxide could break dormancy from seeds only when combined with GA3.
Innovations and breakthroughs
In this research work, authors found that the moistureand chilling temperature were two important factors in breaking of seed dormancy. In addition, authors investigated the synergic effect of GA3and TDZ on breaking ofB. persicumseed dormancy under moist chilling condition.
Applications
The findings of this study suggest that breaking of seed dormancy could be helpful for domestication of this wild plantB. persicum.
Peer review
This is a valuable research work in which authors investigated the effect of temperature and moisture, different plant growth regulators as well as combinations of GA3and TDZ under chilling temperature on breaking ofB. persicumseed dormancy. The methods adopted are appropriate and the findings are interesting.
[1] Jahansooz F, Sefidkon F, Najafi A, Ebrahimzadeh H, Najafi MS. Comparison of essential oils of Bunium persicum (Boiss.) populations grown in Iran, Pakistan and India. J Essent Oil Bear Plants 2012; 15(5): 761-765.
[2] Mandegary A, Arab-Nozari M, Ramiar H, Sharififar F. Anticonvulsant activity of the essential oil and methanolic extract of Bunium persicum (Boiss). B. Fedtsch. J Ethnopharmacol 2012; 140: 447-451.
[3] Gul B, Ansari R, Flowers TJ, Khan MA. Germination strategies of halophyte seeds under salinity. Environ Exp Bot 2013; 92: 4-18.
[4] Zhou ZQ, Bao WK. Levels of physiological dormancy and methods for improving seed germination of four rose species. Sci Hortic 2011; 129: 818-824.
[5] Sharifi M, Pouresmael M. Breaking seed dormancy in Bunium persicum by stratification and chemical substances. Asian J Plant Sci 2006; 5(4): 695-699.
[6] Gashaw A, Theerawitaya C, Samphumphuang T, Cha-um S, Supaibulwatana K. CPPU elevates photosynthetic abilities, growth performances and yield traits in salt stressed rice (Oryza sativa L. spp. indica) via free proline and sugar accumulation. Pestic Biochem Physiol 2013; 108: 27-33.
[7] Zeng SJ, Wang J, Wu KL, Teixeira da Silva JA, Zhang JX, Duan J. In vitro propagation of Paphiopedilum hangianum Perner & Gruss. Sci Hortic 2013; 151: 147-156.
[8] Mihaljevic S, Vrsek I. In vitro shoot regeneration from immature seeds of Epimedium alpinum induced by thidiazuron and CPPU. Sci Hortic 2009; 120: 406-410.
[9] Cohn MA. Seed development, dormancy and germination. Annual Plant Reviews, Volume 27. Ann Bot 2008; 102: 877-878.
[10] Kouakou KL, Zoro Bi IA, Abessika YG, Kouakou TH, Baudoin JP. Rapid seedlings regeneration from seeds and vegetative propagation with sucker and rhizome of Eremospatha macrocarpa (Mann & Wendl.) Wendl and Laccosperma secundiflorum (P. Beauv.) Kuntze. Sci Hortic 2009; 120: 257-263.
[11] Guleryuz G, Kirmizi S, Arslan H, Sakar FS. Dormancy and germination in Stachys germanica L. subsp. bithynica (Boiss.) Bhattacharjee seeds: effects of short-time moist chilling and plant growth regulators. Flora Morphol Distribution Funct Ecol Plants 2011; 206: 943-948.
[12] Rahnama-Ghahfarokhi A, Tavakkol-Afshari R. Methods for dormancy breaking and germination of galbanum seeds (Ferula gummosa). Asian J Plant Sci 2007; 6(4): 611-616.
[13] Navie SC, Adkins SW, Ashmore S. Seeds: biology, developmnt and ecology. Wallingford, UK: CABI; 2007.
[14] Sírova J, Sedlarova M, Piterkova J, Luhova L, Petrivalsky M. The role of nitric oxide in the germination of plant seeds and pollen. Plant Sci 2011; 181: 560-572.
[15] Dissanayake P, George DL, Gupta ML. Effect of light, gibberellic acid and abscisic acid on germination of guayule (Parthenium argentatum Gray) seed. Ind Crops Prod 2010; 32: 111-117.
[16] Hossain MM, Sharma M, Teixeira da silva JA, Pathak P. Seed germination and tissue culture of Cymbidium giganteum Wall. ex Lindl. Sci Hortic 2010; 123: 479-487.
[17] Otroshy M, Zamani A, Khodambashi M, Ebrahimi M, Struik PC. Effect of exogenous hormones and chilling on dormancy breaking of seeds of asafoetida (Ferula assafoetida L.). Res J Seed Sci 2009; 2(1): 9-15.
[18] Bonyanpour AR, Khosh-Khui M. Factors influencing seed germination and seedling growth in black zira [Bunium persicum (Boiss.) B. Fedtsch.]. J Herbs Spices Med Plants 2001; 8(1): 79-85.
[19] Vahdati K, Aslamarz AA, Rahemi M, Hassani D, Leslie C. Mechanism of seed dormancy and its relationship to bud dormancy in Persian walnut. Environ Exp Bot 2012; 75: 74-82.
[20] Nadjafi F, Bannayan M, Tabrizi L, Rastgoo M. Seed germination and dormancy breaking techniques for Ferula gummosa and Teucrium polium. J Arid Environ 2006; 64: 542-547.
[21] Nonogaki H, Bassel GW, Bewley JD. Germination-still a mystery. Plant Sci 2010; 179: 574-581.
[22] Bewley JD, Black M, Halmer P. The encyclopaedia of seeds: science, technology and uses. Wallingford, UK: CABI; 2006.
[23] El-Dengawy ER. Promotion of seed germination and subsequent seedling growth of loquat (Eriobotrya japonica, Lindl) by moistchilling and GA3applications. Sci Hortic 2005; 105: 331-342.
[24] de Melo Ferreira W, Barbante Kerbauy G, Elizabeth Kraus J, Pescador R, Mamoru Suzuki R. Thidiazuron influences the endogenous levels of cytokinins and IAA during the flowering of isolated shoots of Dendrobium. J Plant Physiol 2006; 163: 1126-1134.
10.12980/APJTB.4.2014APJTB-2014-0042
*Corresponding author: Dr. Yousef Emamipoor, Department of Natural Resources, Agricultural and Natural Resources Research Center, 7617913935, Kerman, Iran.
Tel: +983412112391-3, +989132968790
Fax: +983412110395
E-mail address: u.emami@areo.ir
Foundation Project: Supported by Universiti Putra Malaysia, the project via Research University Grant Scheme (RUGS) (Vote No. 9322400).
Article history:
Received 12 May 2014
Received in revised form 20 May, 2nd evised form 28 May, 3ed evised form 9 Jun 2014
Accepted 26 Jul 2014
Available online 28 Aug 2014
Methods:The seeds were treated with 3.1, 6.3, 12.5, 25, 50 and 100 µmol/L of benzyl aminopurine, gibberellic acid (GA3), thidiazuron (TDZ) and forchlorfenuron. Then, seeds were transferred to two different temperature conditions including room temperature (25 °C) and chilling temperature (2-5 °C).
Results:The treatment of moist seeds with chilling temperature (2-5 °C) broke seed dormancy and showed maximum germination, which was 54.7% after 60 d treatment. Also, the treatment of dry seeds with chilling temperature broke seed dormancy with 9.3% germination rate after 120 d. Treatment of seeds with different level of plant growth regulators showed that under moistroom condition, there was evidence of higher and lower seed germination rate: GA3(100 µmol/L) with 46.7% and TDZ (50 µmol/L) with 6.67% respectively. In addition, the results showed that under moist-chilling condition, TDZ (6.3 µmol/L) with 53.3% seed germination rate had higher influence on breaking seed dormancy. Treatment of seeds with combination of TDZ and GA3under moistchilling condition revealed higher rate of breaking of seed dormancy when 6.3 µmol/L TDZ was combined with 100 µmol/L GA3, showing 93.7% germination rate.
Conclusions:The effect of plant growth regulators coupled with chilling temperature on breaking of seed dormancy could provide a large number of seedlings while the long juvenile time which is the next restricting factor of plantation still remained. Thus, the subsequent growth of seedlings to provide a large number of corms is necessary for successful plantation.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- An overview of travel-associated central nervous system infectious diseases: risk assessment, general considerations and future directions
- Genetic diversity of the msp-1, msp-2, and glurp genes of Plasmodium falciparum isolates along the Thai-Myanmar borders
- Impact of antibacterial drugs on human serum paraoxonase-1 (hPON1) activity: an in vitro study
- High levels of Zinc-α-2-Glycoprotein among Omani AIDS patients on combined antiretroviral therapy
- Toxicity effects of water extracts of Holothuria atra Jaeger in mice
- Prevention of renal dysfunction by nutraceuticals prepared from oil rich plant foods
