Clinical features, survival and prognostic factors of primary testicular diffuse large B-cell lymphoma
2014-03-21BoJiaYuankaiShiMeiDongFengyiFengShengYangHuaLinLiqiangZhouShengyuZhouShanshanChenJianliangYangPengLiuYanQinChanggongZhangLinGuiLinWangXueWangXiaohuiHe
Bo Jia, Yuankai Shi, Mei Dong, Fengyi Feng, Sheng Yang, Hua Lin, Liqiang Zhou, Shengyu Zhou, Shanshan Chen, Jianliang Yang, Peng Liu, Yan Qin, Changgong Zhang, Lin Gui, Lin Wang, Xue Wang, Xiaohui He
1Department of Medical Oncology,2Department of Medical Record Library,3Department of VIP ward, Cancer Institute & Hospital, Chinese Academy of Medical Sciences (CAMS) & Peking Union Medical College (PUMC), Beijing 100021, China
Correspondence to: Prof. Xiaohui He. Department of Medical Oncology, Cancer Institute & Hospital, Chinese Academy of Medical Sciences (CAMS) & Peking Union Medical College (PUMC), Beijing 100021, China. Email: xiaohuih2008@163.com.
Clinical features, survival and prognostic factors of primary testicular diffuse large B-cell lymphoma
Bo Jia1, Yuankai Shi1, Mei Dong1, Fengyi Feng1, Sheng Yang1, Hua Lin2, Liqiang Zhou1, Shengyu Zhou1, Shanshan Chen1, Jianliang Yang1, Peng Liu1, Yan Qin1, Changgong Zhang1, Lin Gui1, Lin Wang1, Xue Wang3, Xiaohui He1
1Department of Medical Oncology,2Department of Medical Record Library,3Department of VIP ward, Cancer Institute & Hospital, Chinese Academy of Medical Sciences (CAMS) & Peking Union Medical College (PUMC), Beijing 100021, China
Correspondence to: Prof. Xiaohui He. Department of Medical Oncology, Cancer Institute & Hospital, Chinese Academy of Medical Sciences (CAMS) & Peking Union Medical College (PUMC), Beijing 100021, China. Email: xiaohuih2008@163.com.
Objective:To assess the clinical features, survival and prognostic factors of primary testicular diffuse large B-cell lymphoma (DLBCL).
Methods:A retrospective study of 37 patients with primary testicular DLBCL was carried out from November 2003 to May 2012. Their clinical features, survival and prognostic factors were analyzed.
Results:During a median follow-up period of 39.8 months (5.4-93.0 months), the median progression-free survival (PFS) was 26.2 months (95% CI: 0-65 months) and the 3-year overall survival (OS) rate was 78.4%. Within the whole cohort, the factors signifcantly associated with a superior PFS were limited stage (stage I/II), lactate dehydrogenase (LDH) ≤245 U/L, international prognostic index (IPI) ≤1, primary tumor diameter<7.5 cm, and patients who had complete response (CR) and received doxorubicin-contained chemotherapy (P<0.05). There was a trend toward superior outcome for patients who received combined therapy (surgery/ chemotherapy/radiotherapy) (P=0.055). Patients who had CR, primary tumor diameter <7.5 cm and IPI score≤1 were signifcantly associated with longer PFS at multivariate analysis.
Conclusions:Primary testicular DLBCL had poorer survival. CR, primary tumor diameter and IPI were independent prognostic factors. The combined therapy of orchectomy, doxorubicin-contained chemotherapy and contralateral testicular radiotherapy (RT) seemed to improve survival.
Diffuse large B-cell lymphoma (DLBCL); testicular; survival; prognostic factor; chemotherapy; radiotherapy (RT)
View this article at:http://dx.doi.org/ 10.3978/j.issn.1000-9604.2014.08.12
Introduction
Primary testicular lymphoma (PTL) is an uncommon disease, and accounts for approximately 1-2% of non-Hodgkin’s lymphomas and less than 5% of all testicular malignancies, among which primary testicular diffuse large B-cell lymphoma (DLBCL) is the most common type, which incidence was estimated to be 0.26/100,000 per year (1). According to the previous studies, the median overall survival (OS) of PTL is 4-5 years and a continuous risk of relapse exists after diagnosis (2,3). Distant relapse at extranodal sites, especially the central nervous system (CNS) and the contralateral testis, remains the greatest challenge in PTL (4,5). The international extranodal lymphoma study group (IELSG) 10 trial was the first prospective study in PTL, which revealed that a trimodality treatment with RCHOP chemotherapy, contralateral testicular radiotherapy (RT) and CNS prophylaxis was associated with a good outcome in patients with PTL (6). However, the treatment for PTL is still variable and the prognostic factors remain unclear in Chinese patients.
Herein, we performed a retrospective study to assessclinical characteristics, treatment modalities, survival and prognostic factors of 37 cases of primary testicular DLBCL. As the disease is rare and the related data in China is scarce, this paper can provide a relative deep insight into primary testicular DLBCL for Chinese patients.
Materials and methods
Patients
Thirty-seven cases of primary testicular DLBCL, who were admitted in Cancer Institute & Hospital, Chinese Academy of Medical Sciences (CAMS) & Peking Union Medical College (PUMC) from November 2003 to May 2012, were involved in the study. Initial staging was performed for each patient according to the Ann Arbor classifcation. All tumor tissue specimens were reviewed. Hans’ algorithm was used to determine germinal center (GC) or non-germinal center (non-GC) classifcation (7). Initial responses at the end of primary treatment were recorded. The study was approved by the Ethic Committee of Cancer Institute & Hospital, CAMS & PUMC.
Research endpoint
According to the International Workshop Criteria, initial responses include complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). When the responses could not be determined, the cases were classified as not evaluable (NE). OS was calculated from the date of diagnosis to the date of either last followup or deaths from any cause. Patients who were alive at last follow-up visit were referred to as censored data. Progression-free survival (PFS) was calculated from the date of the diagnosis to the date of disease progression, frst relapse, deaths from any cause or last follow-up. The patients without relapse, disease progression or deaths at last follow-up visit were recognized as censored data.
Statistical analysis
Survival rates and PFS were estimated by the Kaplan-Meier method, and the differences between the curves were analyzed using the Log-rank test. The Cox proportional hazards model was used for multivariate analysis of PFS. P<0.05 (two-sided test) was considered statistically signifcant. The statistical analyses were conducted using the SPSS 19.0 software package (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Thirty-seven cases were included in our study. Their characteristics are shown in Table 1. The median age at diagnosis was 61 years (range, 29-85 years). Thirty-one patients presented with early stage disease (stage I/II), and 6 patients with advanced stage (stage III/IV). International prognostic index (IPI) score was calculated in all patients, with 0-1 in 28 patients and ≥2 in 9 patients. Among the 33 cases examined, 29 (88%) belonged to the non-germinal center B cell (GCB) like type. Of the 25 cases evaluated, Bcl-2 expression was positive in 22 cases (88%).
Thirty-six patients underwent surgery as frst therapeutic and diagnostic intervention (testicular mass resection in 6 cases; orchidectomy in 9 cases; high inguinal orchidectomy in 21 cases). Thirty-one patients received chemotherapy, including doxorubicin-contained regimen in 31 patients, etoposide-contained regimen in 7 and rituximab-contained regimen in 17. The median number of cycles was six (range, 1-8). Twenty-two patients received RT, among which 8 cases underwent RT at the contralateral testis alone and 14 cases at the contralateral testis plus abdominal lymph nodes. Twenty patients received CNS prophylaxis. Specifically, 16 patients received intrathecal methotrexate or cytarabine, and 4 patients received intravenous methotrexate (Table 1).
Response
Of 32 evaluable patients, 25 patients achieved CR (78%), 6 PR (19%) and 1 SD (3%). No patients experienced PD during treatment (Table 2).
Follow-up and survival
The median follow-up period of the 37 patients was 39.8 months (5.4-93.0 months). The median PFS was 26.2 months [95% confidence interval (95% CI): 0-65 months]. The 3-year OS rate was 78.4% (Figure 1).
Nineteen of 37 patients relapsed or progressed at the time of analysis. Sites of failure are available in Table 3. CNS progression and death occurred in fve patients even though two of them received intrathecal prophylaxis.
Prognostic factors
Using PFS (as defned above) as the primary endpoint, the potential prognostic factors were examined by Kaplan-Meier method and Log-rank test analysis (Table 4). Within the whole cohort, the factors significantly associated with a superior PFS were limited stage (stage I/II), lactate dehydrogenase (LDH) ≤245 U/L, IPI score ≤1, primary tumor diameter <7.5 cm, and patients who had CR and received doxorubicin-contained chemotherapy (P<0.05). There was a trend toward superior outcome for patients who received combined therapy (surgery/chemotherapy/ radiotherapy) (P=0.055) (Figure 2). Patients who had CR, primary tumor diameter <7.5 cm and IPI score ≤1 were significantly associated with longer PFS at multivariate analysis (Table 5).

Table 1 Pathological and clinical features of 37 primary DLBCL patients
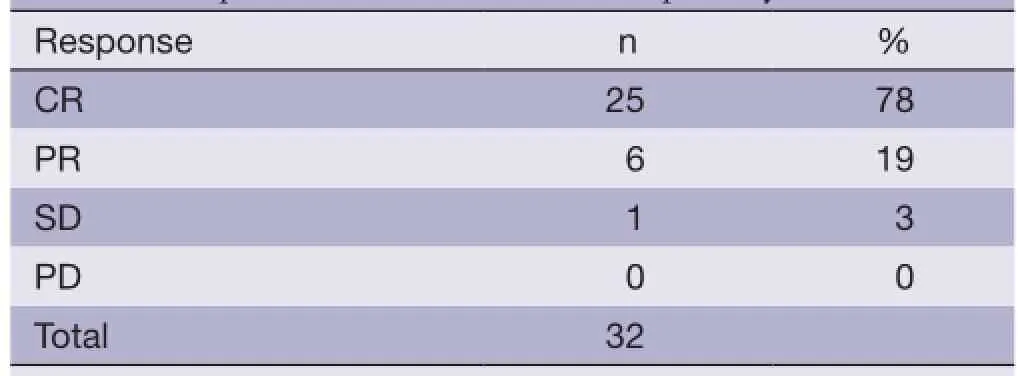
Table 2 Response evaluated at the end of primary treatment
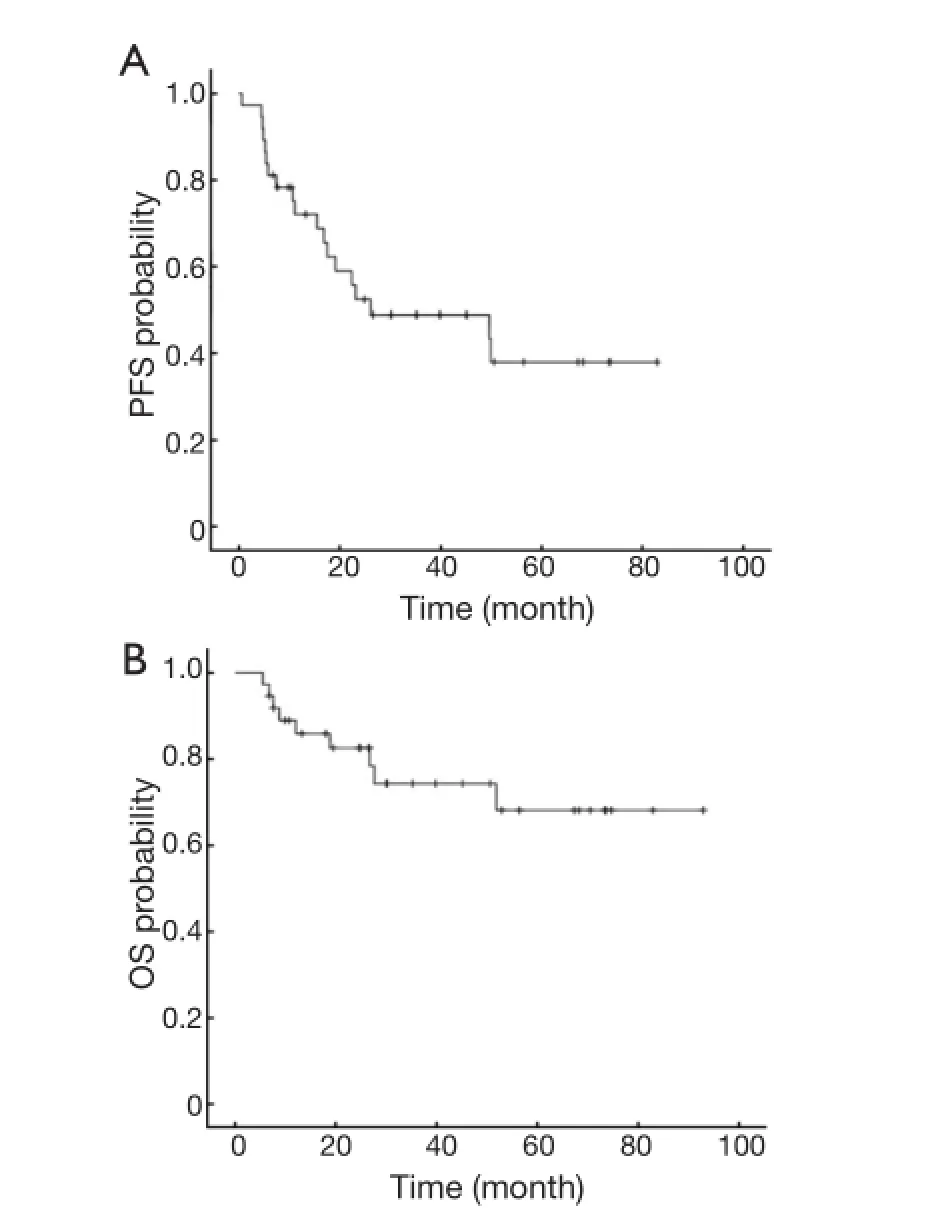
Figure 1 PFS (A) and OS (B) in all patients. PFS, progression-free survival; OS, overall survival.
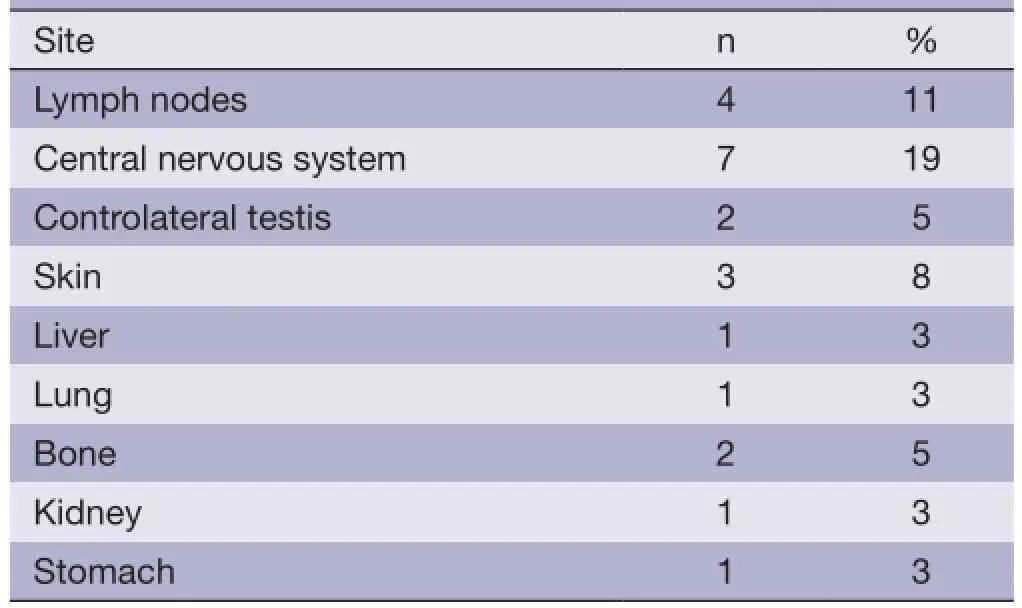
Table 3 Recurrence sites
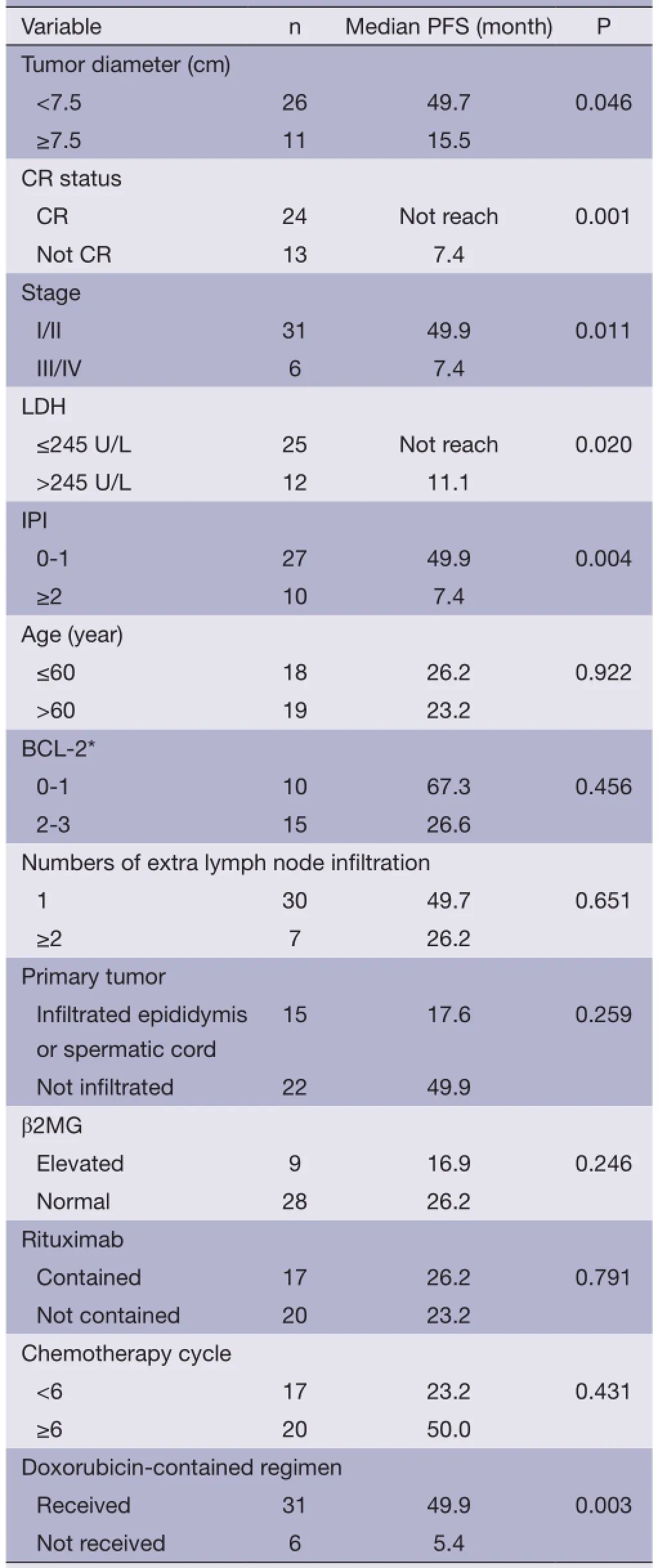
Table 4 Univariate analysis of impact of various clinical variables on PFS

Table 4 (continued)
Restricting the analysis to the patients with stage I/II disease (n=31), primary tumor size <7.5 cm (P=0.030), the patients who had CR (P=0.006) and received doxorubicinecontained chemotherapy (P<0.01) were associated with significantly superior PFS at univariate analysis. There was an association between better outcomes and combined therapy (chemotherapy/radiotherapy/surgery) at univariate analysis (Figure 2).
Discussion
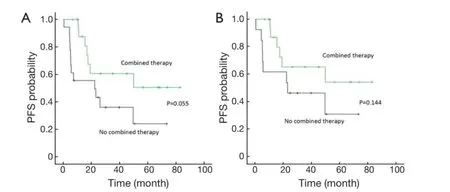
Figure 2 PFS in all patients (A) and stage I/II patients (B) treated by combined therapy or not. PFS, progression-free survival.
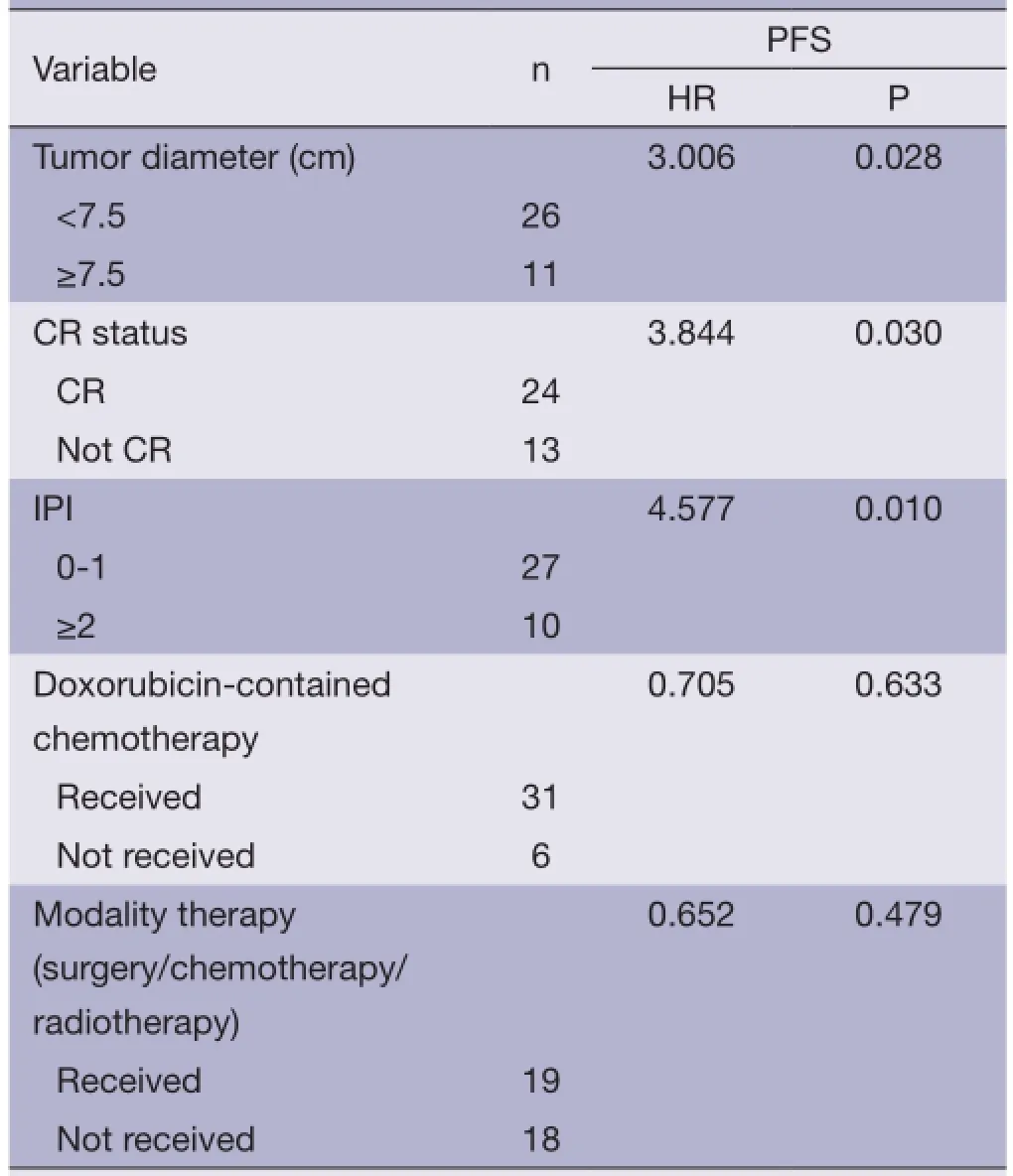
Table 5 Multivariate analysis of impact of various clinical variables on PFS
PTL is a rare disease with poor prognosis, of which primary testicular DLBCL is the most common type (1). In our institute, the incidence of primary testicular DLBCL accounts for 3.9% of all DLBCL patients. In the previous IELSG retrospective study (2), the 5- and 10-year PFS rates were 48% and 33%, respectively. Similarly, a recent population-based retrospective study (3) revealed that the 5- and 10-year disease-free survival (DFS) were 62% and 50%, respectively. In our study, the median PFS was 26.2 months and the 3-year OS was 78.4%, but a longer follow-up is required for better assessment. A previous study in our institute assessed the clinical characteristics and survival of 23 PTL patients from 1987 to 2000, indicating that the median PFS was 19 months with the 3-year PFS and OS of 42.3% and 59.8%, which were all lower than those in this study (8). The improved comprehensive therapy modality within these years in our institute might account for the difference. With a median followup of 39.8 months in this study, relapse was widespread with involvement of multiple extranodal sites. The most frequently affected sites were CNS, skin, bone, contralateral testis, lung, liver, kidney and stomach, consistent with the report before (5). This observation provided major implications for the optimal initial treatment. There is very strong data supporting the benefit of RT in preventing disease recurrence in the contralateral testis. According to the previous retrospective IELSG study (2), prophylactic RT at the contralateral testis appeared to prevent testicular relapses, and the recurrence rate was reduced to 8% compared with 35% among those not irradiated. Also a prospective phase II study showed that no contralateral testis relapses occurred in 47 PTL patients who received contralateral testis RT (6). Consistently, our study also revealed that contralateral testis RT was associated with longer PFS even though the difference was not statistically signifcant.
Based on a review of 176 cases of irradiated patients from 14 series, Shahab and Doll (9) concluded that although RT provided excellent in-field control, ultimately 70% of patients still relapsed systemically, Therefore, systemic chemotherapy is recommended. ISELG study showed that patients who received anthracyclines-based chemotherapy had longer survival. In our study, there was also an association between an improved PFS and doxorubicincontained chemotherapy, with signifcant difference. Strong evidence showing the benefit of CHOP implied that apotential beneft may also be present in PTL. Addition of rituximab to chemotherapy has been established in DLBCL, which implied that a potential beneft may also be present in PTL (10-12). Among a retrospective series of 24 patients with PTL, a similar trimodality strategy (doxorubicin-based chemotherapy, testicular RT, and intrathecal methotrexate) showed the 5-year PFS and OS were 78% and 66%, respectively (6). A comparison of these results with those of the IELSG-10 study may suggest that the addition of rituximab could be beneficial (6). However, rituximabcontained chemotherapy was not associated with superior PFS in our study. A longer follow-up period is needed to clarify the exact role of rituximab in PTL treatment. Although a randomized double-blinded controlled clinical trial was the most persuasive approach, it was impossible for PTL because of the rarity of this disease.
The CNS relapse is more common in PTL in contrast to other aggressive lymphomas. In the previous IELSG series, the 5- and 10-year risks of CNS relapse were 20% and 35%, respectively (2). The best strategy to prevent CNS relapse is still a matter to debate. The value of prophylactic intrathecal chemotherapy is controversial because CNS relapse occurs more frequently in brain parenchyma than in meninges and also prefers the patients who have received intrathecal prophylaxis (13). In our study, CNS progression and death occurred in fve patients even though two of them received intrathecal prophylaxis. But in IELSG 10 study, the cumulative incidence of CNS relapse at 5 years was only 6% in patients who received CNS prophylaxis (6), and the lower incidence of CNS relapse might be ascribed to the introduction of CNS prophylaxis.
IELSG 10 was the first prospective study in PTL and showed that a trimodality treatment with RCHOP chemotherapy, contralateral testicular RT and CNS prophylaxis was associated with better outcomes and the 5-year PFS and OS rates were 74% and 85%, respectively (6). In our study, there was also a trend of better outcomes for the patients who received combined therapy (P=0.055), but a longer follow-up is needed to compare our results with previous IELSG 10 study.
Prior studies have found a number of prognostic factors including age, performance status, stage, IPI score, elevated serum LDH, bulky disease, infltration of adjacent tissues, the lack of systemic chemotherapy, etc. (2,14-16). In our study, only patients who have CR, IPI score ≤1 and primary tumor diameter <7.5 cm were significantly associated with longer PFS at multivariate analysis. Song et al. (17) reported that patients of extranodal maximum tumor diameter ≥7.5 cm had lower PFS and OS. Hence we used the maximum tumor diameter of 7.5 cm as the critical value for survival, and demonstrated that primary tumor diameter ≥7.5 cm was an independent prognostic factor.
Limitation is also prominent in the current study. Firstly, this is a retrospective trial which covers all the shortcomings of retrospective study. Secondly, this is not a large case series due to rarity of the disease. But this paper also provided a relative deep insight into clinical characteristics, treatment modalities, survival and prognostic factors of primary testicular DLBCL in the Chinese population.
Acknowledgements
Disclosure: The authors declare no confict of interest.
1. Vural F, Cagirgan S, Saydam G, et al. Primary testicular lymphoma. J Natl Med Assoc 2007;99:1277-82.
2. Zucca E, Conconi A, Mughal TI, et al. Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J Clin Oncol 2003;21:20-7.
3. Gundrum JD, Mathiason MA, Moore DB, et al. Primary testicular diffuse large B-cell lymphoma: a populationbased study on the incidence, natural history, and survival comparison with primary nodal counterpart before and after the introduction of rituximab. J Clin Oncol 2009;27:5227-32.
4. Seymour JF, Solomon B, Wolf MM, et al. Primary largecell non-Hodgkin's lymphoma of the testis: a retrospective analysis of patterns of failure and prognostic factors. Clin Lymphoma 2001;2:109-15.
5. Fonseca R, Habermann TM, Colgan JP, et al. Testicular lymphoma is associated with a high incidence of extranodal recurrence. Cancer 2000;88:154-61.
6. Vitolo U, Chiappella A, Ferreri AJ, et al. First-line treatment for primary testicular diffuse large B-cell lymphoma with rituximab-CHOP, CNS prophylaxis, and contralateral testis irradiation: fnal results of an international phase II trial. J Clin Oncol 2011;29:2766-72.
7. Hans CP, Weisenburger DD, Greiner TC, et al. Confrmation of the molecular classifcation of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275-82
8. Yang JL, Shi YK, He XH, et al. Clinical and pathologicalfeatures of 23 patients with primary lymphoma of the testis. Zhonghua Zhong Liu Za Zhi 2003;25:498-500.
9. Shahab N, Doll DC. Testicular lymphoma. Semin Oncol 1999;26:259-69.
10. Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 2008;9:105-16.
11. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol 2005;23:5027-33.
12. Pfreundschuh M, Kuhnt E, Trümper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 2011;12:1013-22.
13. Pfreundschuh M, Trümper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with goodprognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 2006;7:379-91.
14. Pectasides D, Economopoulos T, Kouvatseas G, et al. Anthracycline-based chemotherapy of primary non-Hodgkin’s lymphoma of the testis: the hellenic cooperative oncology group experience. Oncology 2000;58:286-92.
15. Tondini C, Ferreri AJ, Siracusano L, et al. Diffuse largecell lymphoma of the testis. J Clin Oncol 1999;17:2854-8.
16. Wang Y, Li ZM, Huang JJ, et al. Three prognostic factors infuence clinical outcomes of primary testicular lymphoma. Tumour Biol 2013;34:55-63.
17. Song MK, Chung JS, Sung-Yong O, et al. Clinical impact of bulky mass in the patient with primary extranodal diffuse large B cell lymphoma treated with R-CHOP therapy. Ann Hematol 2010;89:985-91.
Cite this article as:Jia B, Shi Y, Dong M, Feng F, Yang S, Lin H, Zhou L, Zhou S, Chen S, Yang J, Liu P, Qin Y, Zhang C, Gui L, Wang L, Wang X, He X. Clinical features, survival and prognostic factors of primary testicular diffuse large B-cell lymphoma. Chin J Cancer Res 2014;26(4):459-465. doi: 10.3978/ j.issn.1000-9604.2014.08.12
10.3978/j.issn.1000-9604.2014.08.12
Submitted Apr 24, 2014. Accepted for publication Jun 24, 2014.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Aberrant DNA methyltransferase 1 expression in clear cell renal cell carcinoma development and progression
- In vitro effect of iASPP on cell growth of oral tongue squamous cell carcinoma
- Long-term survival outcomes of video-assisted thoracic surgery for patients with non-small cell lung cancer
- Embolization of symptomatic renal angiomyolipoma with a mixture of lipiodol and PVA, a mid-term result
- Decline of serum CA724 as a probable predictive factor for tumor response during chemotherapy of advanced gastric carcinoma
- TPX2 knockdown suppressed hepatocellular carcinoma cell invasion via inactivating AKT signaling and inhibiting MMP2 and MMP9 expression
