Effects of mild hypothermia on the ROS and expression of caspase-3 mRNA and LC3 of hippocampus nerve cells in rats after cardiopulmonary resuscitation
2014-03-20JianLuYiShenHuiyinQianLijunLiuBaochunZhouYanXiaoJinningMaoGuoyinAnMingzhongRuiTaoWangChanglaiZhu
Jian Lu, Yi Shen, Hui-yin Qian, Li-jun Liu, Bao-chun Zhou, Yan Xiao, Jin-ning Mao, Guo-yin An, Mingzhong Rui, Tao Wang, Chang-lai Zhu
1Department of Emergency Medicine, Suzhou Municipal Hospital, Suzhou 215002, China
2Department of Emergency and Critical Care Medicine, Second Af fi liated Hospital of Soochow University, Suzhou 215007, China
3Electron Microscope Laboratory, Medical College of Nantong University, Nantong 226200, China
Corresponding Author:Li-jun Liu, Email: lijunliusz@hotmail.com
Effects of mild hypothermia on the ROS and expression of caspase-3 mRNA and LC3 of hippocampus nerve cells in rats after cardiopulmonary resuscitation
Jian Lu1,2, Yi Shen1, Hui-yin Qian2, Li-jun Liu2, Bao-chun Zhou2, Yan Xiao2, Jin-ning Mao2, Guo-yin An2, Mingzhong Rui2, Tao Wang2, Chang-lai Zhu3
1Department of Emergency Medicine, Suzhou Municipal Hospital, Suzhou 215002, China
2Department of Emergency and Critical Care Medicine, Second Af fi liated Hospital of Soochow University, Suzhou 215007, China
3Electron Microscope Laboratory, Medical College of Nantong University, Nantong 226200, China
Corresponding Author:Li-jun Liu, Email: lijunliusz@hotmail.com
BACKGROUND:Cardiac arrest (CA) is a common and serious event in emergency medicine. Despite recent improvements in resuscitation techniques, the survival rate of patients with CA is unchanged. The present study was undertaken to observe the effect of mild hypothermia (MH) on the reactive oxygen species (ROS) and the effect of neurological function and related mechanisms.
METHODS:Sixty-five healthy male Sprague Dawley (SD) adult rats were randomly (random number) divided into 2 groups: blank control group (n=5) and CPR group (n=60). CA was induced by asphyxia. The surviving rats were randomly (random number) divided into two groups: normothermia CPR group (NT) and hypothermia CPR group (HT). Normothermia of 37 °C was maintained in the NT group after return of spontaneous circulation (ROSC), hypothermal intervention of 32 °C was carried out in the HT group for 4 hours immediately after ROSC. Both the NT and HT groups were then randomly divided into 2 subgroups 12 hours and 24 hours after ROSC (NT-12, NT-24, HT-12, HT-24 subgroups). During observation, the neurological de fi cit scores (NDSs) was recorded, then the bilateral hippocampi were obtained from rats' head, and monoplast suspension of fresh hippocampus tissue was made immediately to determine the level of intracellular ROS by flow cytometry. Transmission electron microscope was used to observe the ultramicro changes of cellular nucleus and mitochondria. Reverse transcription-polymerase chain reaction (RT-PCR) was used to determine the expression of caspase-3 mRNA, and western-blotting (WB) was used to determine the level of LC3 in frozen hippocampus tissue. Measured data were analyzed with paired sample t test and One-Way ANOVA.
RESULTS:Of 60 rats with CA, 44 (73%) were successfully resuscitated and 33 (55%) survived until the end of the experiment. The NDSs of rats in the NT and HT groups were more signi fi cantly reduced than those in the BC group (F=8.107, P<0.05), whereas the NDSs of rats in the HT-12 and HT-24 subgroups were significantly increased in comparison with those NDSs of rats in the NT-12 and NT-24 subgroups, respectively (t=9.692, P<0.001; t=14.374, P<0.001). The ROS in hippocampus nerve cells in the NT and HT groups signi fi cantly increased compared to the BC group (F=16.824, P<0.05), whereas the ROS in the HT-12 and HT-24 subgroups significantly reduced compared with that ROS in the NT-12 and NT-24 subgroups, respectively (t=9.836, P<0.001; t=7.499, P<0.001). The expression of caspase-3 mRNA in hippocampus nerve cells in the NT and HT groups were signi fi cantly increased compared to the BC group (F=24.527, P<0.05), whereas the expression of caspase-3 mRNA in rats of the HT-12 and HT-24 subgroups was signi fi cantly reduced compared to the NT-12 and NT-24 subgroups, respectively (t=6.935, P<0.001; t=4.317, P<0.001). The expression of LC3B-II/I in hippocampus nerve cells of rats in the NT and HT groups signi fi cantlyincreased compared to the BC group (F=6.584, P<0.05), whereas the expression of LC3B-II/I in rats of the HT-12 and HT-24 subgroups significantly reduced compared to the NT-12 and NT-24 subgroups, respectively (t=10.836, P<0.001; t=2.653, P=0.02). Ultrastructure damage of nucleus and mitochondria in the NT group was more evident than in the BC group, and eumorphism of nucleus and mitochondria were maintained in rats of the HT group compared with the NT group.
CONCLUSION:Mild hypothermia lessened the injury of nerve cells and improved the neurological function of rats that survived from cardiac arrest by reducing the ROS production of nerve cells and inhibiting the expression of caspase-3 mRNA and LC3, leading to cellular apoptosis and massive autophagy in rats that survived from cardiac arrest after CPR.
Mild hypothermia; Cardiopulmonary resuscitation; Reactive oxygen species; Caspase-3; LC3; Autophagy
INTRODUCTION
Cardiac arrest (CA) is a common and serious event in emergency medicine. Despite recent improvements in resuscitation techniques, the survival rate of patients with CA is unchanged. Following return of spontaneous circulation (ROSC), manifestations of poor neurological outcomes are closely linked to high post-resuscitation mortality and poor quality of life.[1]Studies[1–3]have revealed that apoptosis is the main pathophysiological process of the dysfunction of nerve cells in survived patients with CA. Hence, how to prevent and control the occurrence and development of apoptosis of cranial nerve cells after CA is one of the important measures to reduce nerve dysfunction.[2]The occurrence of nerve cells apoptosis after ischemia and hypoxia is closely related to the degree of cell autophagy,[3]but the relationship between autophagy and apoptosis and its regulating mechanism remain unclear. In the present study, we utilized a rat model of asphyxial CA to observe the effect of mild hypothermia (MH) on the quantity of reactive oxygen species (ROS) in rat hippocampal neurons after cardiopulmonary resuscitation (CPR) and the effect of neurological function and related mechanisms.
METHODS
Animal grouping
In this study, 65 male Sprague-Dawley rats (Shanghai Slac Laboratory Animal Co. Ltd, Shanghai, China), aged 16–18 weeks and weighing 450±45 g, were randomly divided into two groups: blank control group (BC, n=5) and CPR group (n=60). The rats of the BC group were only anesthetized with an intraperitoneal injection of chloral hydrate, and after endotracheal intubation, two trocars were placed into the left femoral artery and right femoral vein. After evaluation for neurologic de fi cit scores (NDS), the rats were euthanized and the hippocampus tissue was obtained. The rats of the CPR group were anesthetized with chloral hydrate and then the rat model of asphyxial CA was replicated (detailed in model manufacture). Immediately after ROSC, the rats were randomly divided into two groups again: normothermia CPR group (NT) and hypothermia CPR group (HT). The NT rats after ROSC were maintained at a body temperature of 37.0 °C, the HT rats after ROSC were actively cooled to a target body temperature of 32 °C, maintained at this temperature for 4 hours, then re-warmed slowly to normal temperature at a velocity of 0.5 °C/h. The rats in the CPR group were further divided into two subgroups based on the time of euthanasia, either 12 or 24 hours after ROSC (NT-12, HT-12, NT-24, HT-24).
Model manufacture and neurologic de fi cit scores (NDSs)
The model was established according to the method of Idris.[4]The rats of the CPR group were anesthetized with an intraperitoneal injection of 3.6% chloral hydrate (10 mL/kg) and then placed on a temperature-controlled carpet (RWD Life Science Co., Ltd, Shenzhen, China). After endotracheal intubation, two 24 G trocars were placed into the left femoral artery and right femoral vein of the rat, respectively. Sandard lead II electrocardiogram surface electrodes were used to monitor heart rhythm and respiration, a temperature sensor was placed rectally to monitor core temperature, and a heparin sodium fi lled catheter was connected to the left femoral artery tomonitor arterial blood pressure (ZhengHua Biological Instrument Equipment Co., LTD, Huaibei, Anhui, China). After operation, the rats were ventilated with a volume-controlled ventilator (A-CPR-IIC, Institute of Cardiopulmonary Cerebral Resuscitation, Guangdong, China), with a tidal volume of 6 mL/kg, a fraction of inspiration oxygen (FiO2) of 100% 1 minute, then 21% 4 minutes, and a ventilation rate of 100 breaths/ min. Asphyxia was induced by shutting the ventilator and clipping the endotracheal catheter. Onset of CA was denoted as the time where systolic arterial blood pressure was less than or equal to 25 mmHg. After the straight line of ECG appeared, animal CPR was initiated using endotracheal ventilation with 100% oxygen at 50 breaths/min. Mechanical chest compressions at 250 times/min at a depth of compression one-third of the rat's anteroposterior diameter of the chest were performed with an animal cardiopulmonary resuscitator (A-CPR-IIC, Institute of Cardiopulmonary Cerebral Resuscitation, Guangdong, China). After 10 seconds of CPR, epinephrine (0.01 mg/kg) was injected via the right femoral vein. ROSC was de fi ned by the presence of an autonomic cardiac rhythm and a mean arterial blood pressure greater than 60 mmHg, which was maintained more than 10 minutes.[4]The rectal temperature of rats of the CPR group was maintained at 37±0.5 °C before and during CPR. The rats of the HT group were actively cooled to a target body temperature of 32 °C, maintained at this temperature for 4 hours, then slowly re-warmed to 37.0 °C at a velocity of 0.5 °C/h. After evaluation for NDS according to the methods of Geocadin et al[5]at 12 or 24 hours after ROSC, the rats were anesthetized and euthanized to obtain the hippocampus tissue.
Sampling methods
Arterial blood gas analysis was performed before asphyxia, after 0.5 and 4 hours of ROSC from the femoral artery (added with the same amount of normal saline). The rats after ROSC accepted 5% GNS lavage (10 mL/kg, q6h). The NDS of rats was evaluated at corresponding observation time, and then the rats were euthanized by the intracardiac injection of 10% Kcl 2 mL under anesthesia. After sternal incision, separation of the pericardium and exposure of the heart, a needle was inserted into the aorta through the left ventricle. The right auricle was cut, and 250 mL 4 °C normal saline was perfused through the heart rapidly till the ef fl uent of the right room was limpid, the brain was removed quickly, and the bilateral hippocampus was separated. About 2/3 of the left hippocampus was grinded to produce monoplast suspension on the ice, and the remaining 1/3 was used for electron microscope observation. The right side of the hippocampus was stored at –80 °C, which was used for the determination of caspase-3 mRNA and LC3B expression.
Quantity of ROS determined by flow cytometry
2/3 of the left hippocampus was homogenated 10 times in a glass of cold PBS on ice, fi ltered by a 300 mesh nylon filter. The filtrate was centrifuged at 600Xg and 4 °C for 5 minutes, the supernatant was discarded, and the sediment was re-suspended with cold PBS repeatedly. The above operation was repeated three times, and finally the sediment was suspended with cold PBS. The monoplast suspension was loaded by DCFH-DA probes according to the concentration of 1:1000 with the active oxygen detection kit (Item No: S0033, Beyotime Institute of Biotechnology, Jiangsu, China). The monoplast was washed three times by PBS after 20 minutes of incubation at 37 °C, and finally the DCF fluorescence intensity of 100 000 single cell was analyzed by flow cytometry instrument (Model: FACSCalibur, BD company, USA).
Determination of caspase-3 mRNA expression
RT-PCR method was used to extract RNA with Trizol (batch number: 120 805, GIBCO Company, USA), reverse-transcribed mRNA to cDNA, amplified cDNA with PCR ampli fi cation (model: 9700, Biological Systems China). The synthesis of primers was provided by Shanghai Invitrogen Agent, caspase-3 primer: upstream 5'-GCA CTG GAA TGT CAG CTC GCA-3', and downstream 5'-GCC ACC TTC CGG TTA ACA CGA-3', 559 bp. β-actin primers were as follows: upstream 5'-CAT CTC TTG CTC GAA GTC CA-3', downstream 5'-ATC ATG TTT GAG ACC TTC AAC A-3', 300 bp. Reaction conditions were as follows: 94 °C modification 5 minutes; 94 °C modification 30 seconds, 30 seconds 50 °C annealing, extension 72 °C for 30 seconds, a total of 30 cycles. The last extension of 72 °C was 7 minutes. Amplification products were electrophorased by an electrophoresis apparatus, and the separated stripes were recorded by radiography after electrophoresis, and Vilber gel imaging analysis system (Type: Type T2A, France Lourmat company) was used to scan absorbance. The stripe to β-actin ratio was deemed as the relative expression of caspase-3 mRNA.
Western blot analysis of LC3B
Western blot was used to extract protein with RIPA (middle) (batch number: P0013C, Beyotime Institute ofBiotechnology, Jiangsu, China). Protein concentration was determined using the bicinchoninic acid (BCA) assay kit (Beyotime Institute of Biotechnology, Jiangsu, China). Proteins were resolved on a 6% or 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membrane. They were incubated at 4 °C overnight with primary antibodies, including rabbit polyclonal anti-LC3B (1:1 000; Abcam, Cambridge, MA, USA), and mouse polyclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:800; Sigma-Aldrich, St. Louis, MO, USA). Membranes were incubated at room temperature for 1 hour with secondary antibodies, including goat anti-rabbit IgG (1:3 000; Beyotime Institute of Biotechnology) and rabbit anti-mouse IgG (1:4 000; Sigma-Aldrich). Optical density of the immunoreactive bands was calculated by Quantity One 1-D analysis software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). LC3B-II and LC3B-I levels were normalized to GAPDH, and the level of intracellular autophagy was de fi ned as the ratio of LC3B-II/I.
Electron microscopy (EM)
Approximately 1 mm thick sections of the hippocampus were sliced on ice and fi xed overnight at 4 °C with 2.5% (v/v) glutaraldehyde, postfixed with 1% (v/v) osmic acid for 1 hour, and dehydrated and embedded with acetone and embedding medium. Ultra-thin sections (approximately 40–50 nm) were cut, stained with 2.0% (w/v) lead citrate, and blindly evaluated with regard to treatment condition with a HITACHI H-600 transmission electron microscope (Hitachi Scientific Instruments, Mountain View, CA, USA). In order to detect minor changes in cellular structure, at least four different electron microscopic micrographs representing independent areas in each section were selected for analysis.
Statistical analysis
Statistical analysis was performed using the software package IBM SPSS Statistics 19 and Microsoft Office Excel 2007. All data were expressed as mean±SD, and the mean differences between the two groups were compared with Student's t test. One-way ANOVA was used to assess overall differences among the groups for each variable. P<0.05 was considered statistically signi fi cant.
RESULTS
Asphyxiation, resuscitation time and survival rate of CPR groups
Of the 60 rats that underwent asphyxiation and resuscitation, 33 (55%) rats survived until the completion of the study. Mechanical ventilation and chest compression were main resuscitation measures in this study. Since there was no long-playing arrhythmia in the course of resuscitation, electric defibrillation was not used. The time of asphyxiation and resuscitation was not statistically different among the experimental groups (P>0.05; Table 1).
Basic vital signs and blood gas analysis of the CPR groups
Basic vital signs (except for Tc) at 0.5 and 4 hours after ROSC were not statistically different among the groups (P>0.05). Arterial blood lactate 4 hours after ROSC decreased more signi fi cantly in the HT group than in the NT group (t=2.146, P=0.036) (Tables 2, 3).
Neurological outcomes
The median NDS of rats was significantly lowerin the CPR group than in the BC group after ROSC (F=8.107, P<0.05). The median NDS of rats was signi fi cantly higher in the HT group than in the NT group at 12 hours (t=2.880, P=0.024) and 24 hours (t=5.104, P=0.001) after ROSC (Figure 1).

Table 2. Basic vital signs of the CPR group (mean±SD)

Table 1. Asphyxia time and data associated resuscitation in the CPR group (mean±SD)
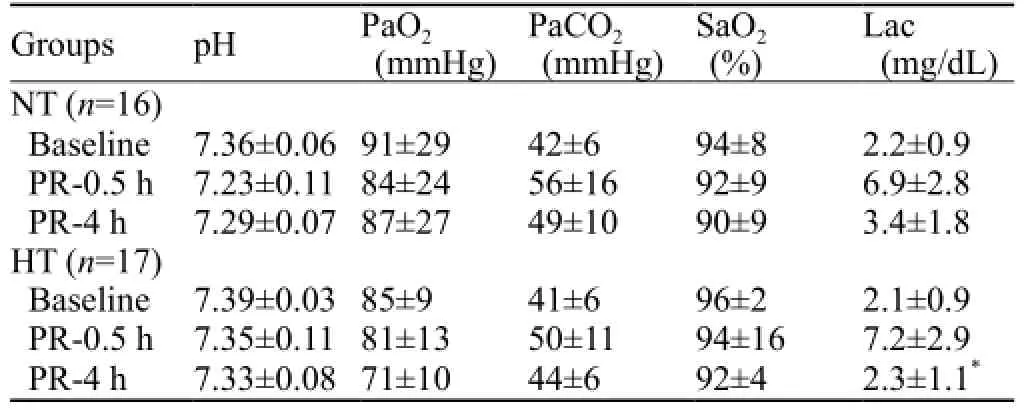
Table 3. Blood gas analysis of the CPR group before asphyxia, 0.5 and 4 hours after ROSC

Figure 1. NDS in each group. Results are presented as the mean±SD.*P<0.05 vs. BC group;#P<0.05 vs. NT group. HT: mild hypothermia group; NT: normothermia group; BC: blank control group; NDS: neurologic de fi cit scores.
ROS fl ow cytometry in hippocampus monoplast suspension
The mean fluorescent value of DCF was increased more signi fi cantly in the CPR group than in the BC group after ROSC (F=16.824, P<0.05). The median fl uorescent value of DCF was significantly lower in the HT group than in the NT group at 12 hours (t=9.836, P<0.01) and 24 hours (t=7.499, P<0.01) after ROSC (Figures 2, 3).
Caspase-3 mRNA expression in hippocampus nerve cells
The mean OD value relative to β-actin of caspase-3 mRNA was increased more significantly in the CPR group than in the BC group after ROSC (F=24.527, P<0.05). The median OD value relative to β-actin of caspase-3 mRNA was significantly lower in the HT group than in the NT group at 12 hours (t=6.935, P<0.01) and 24 hours (t=4.317, P<0.01) after ROSC (Figures 4, 5).

Figure 3. ROS flow cytometry in monoplast suspension of the hippocampus in each group. Results are presented as the mean±SD.*P<0.05 vs. the BC group;#P<0.05 vs. the NT group. HT: mild hypothermia group; NT: normothermia group; BC: blank control group.

Figure 2. ROS fl ow cytometry in monoplast suspension of the hippocampus in each group.

Figure 4. Caspase-3 mRNA expression in nerve cells of the hippocampus in each group.

Figure 5. Caspase-3 mRNA expression in nerve cells of the hippocampus in each group. The data of β-actin were normalized. The fold changes of caspase-3mRNA were calculated in the BC group, and the results are presented as the mean fold of the blank control±SD.*P<0.05 vs. the BC group;#P<0.05 vs. the NT group. HT: mild hypothermia group; NT: normothermia group; BC: blank control group.
LC3B-II/I expression in nerve cells of the hippocampus
The mean OD value relative to GAPDH of LC3BII/I increased more significantly in the CPR group than in the BC group after ROSC (F=6.584, P<0.05). The median OD value relative to GAPDH of LC3B-II/I was signi fi cantly lower in the HT group than in the NT group at 12 hours (t=10.836, P<0.01) and 24 hours (t=2.653, P=0.02) after ROSC (Figures 6 and 7).
Electron micrographs of nucleus and mitochondria
Analysis of intracellular structures by an electron microscope at 24 hours after ROSC showed that the extent of ultrastructural damage of nuclei in the hippocampus reduced in the HT group compared with the NT group. In the BC group, the nucleus appeared normal with intact nuclear membrane and smooth chromatin (Figure 8A). In contrast, the nuclei in the NT group were markedly damaged with chromatin margination and condensed nucleoplasm (Figure 8B). The nucleus appeared slightly damaged in the HT group (Figure 8C). In the BC group, the mitochondrion was maintained with intact membrane cristae and a smooth matrix (Figure 8D). In contrast, the mitochondrion was markedly damaged with disrupted cristae and a damaged matrix in the NT group (Figure 8E). The mitochondrion was slightly damaged in the HT group (Figure 8F). Autophagolysosomes were also observed in the NT group (Figure 8G).

Figure 6. LC3B-II/I expression in nerve cells of the hippocampus in each group.

Figure 7. LC3B-II/I expression in nerve cells of the hippocampus in each group. The data were normalized GAPDH. The fold changes of LC3B-II/I were calculated in the BC group, and the results were presented as the mean fold of the blank control ( mean±SD).*P<0.05 vs. the BC group;#P<0.05 vs. the NT group. HT: mild hypothermia group; NT: normothermia group; BC: control group.
DISCUSSION
Mild hypothermia (MH) has become one of the effective cerebral resuscitation measures for cardiac arrest (CA) patients; however, it is only conducive to ventricular fibrillation patients out of hospital. MH can be only applied to the patients with mild and moderate brain injury, but its mechanisms remain to be clari fi ed.

Figure 8. Representative electron micrographs of nuclei (original magnificationX8 000) and mitochondria (original magnificationX40 000). Tissues were isolated from the rat hippocampus at 24 hours after ROSC. A: normal nuclei with intact nuclear membrane and a smooth chromatin in the blank control group. B: markedly damaged nuclei with chromatin margination and condensed nucleoplasm in the normothermia group. C: slightly damaged nuclei with basically normal nuclear membrane and a slightly damaged chromatin in the hypothermia group. D: normal mitochondria with intact membrane cristae and a smooth matrix in the blank control group. E: markedly damaged mitochondrion with disrupted cristae and a damaged matrix in the normothermia group. F: slightly damaged mitochondrion with basically normal cristae and a slightly damaged matrix in the hypothermia group. G: damaged mitochondria engulfed by autophagolysosome in the normothermia group (black arrow: double membrane structure of autophagosome).
The organs, tissues and cells of the patients were ischemic and anoxic after CA which decreased the efficiency of respiratory chain electron transmission and reduced the number of electron acceptors, but the production of radical oxygen species (ROSs) in ischemic area did not increase.[6]After return of spontaneous circulation (ROSC) after cardiopulmonary resuscitation (CPR) treatment, reperfusion contained a large amount of oxygen in the blood, but the enzyme activity of mitochondrial respiratory chain failed to recover quickly, thus the ROSs were produced by the single electron restoration.[7]In addition, the overload of Ca2+also damaged mitochondrial function, inhibited the cytochrome oxidase system, and eventually the production capacity of ROSs was higher than clearing capacity,[6]leading to different degree of damage to cell membranes and organelles, including necrosis and apoptosis.[8]This study showed that intracellular ROSs increased remarkably in rat hippocampus nerve cells after ischemia/reperfusion (I/ R). This fi nding was consistent with the result of cascade after ischemia and hypoxia. MH can decrease more signi fi cantly the production of ROSs in the HT group than in the NT group (Figures 2 and 3); the mechanism may be related to that MH can reduce the production of ROSs and protect the enzyme system removing ROSs.
Caspase-3 was the last performer of apoptotic pathways, and deemed as the extent of cell apoptosis.[9]Research results have shown that MH can reduce the apoptosis of nerve cells and protect the neurologic function.[10]In the present study, we found that MH can significantly reduce the quantity of intracellular ROSs after cerebral I/R, which was correlated with the expression of caspase-3 mRNA. The possible mechanism is that the decrease of ROSs inhibited the cycle of excitatory amino acid between ROSs mediated by calcium overload, and reduced the expression of caspase-3 mRNA and the level of apoptosis (Figures 4 and 5).
In addition, autophagy is usually considered as a protective effect of cells, and it plays an important role in maintaining cell survival under ischemic and anoxic condition of stress[11]and removing aged organelles and misfolded proteins in cells.[12]In recent years, however, some studies[9–13]found that autophagy can also lead to cell death under certain conditions, which is called excessive autophagy. Besides observing the form of autophagosome via an electron microscope, there are also some other methods to judge the degree of autophagy in cells including determining LC3 by means of WB.[14]LC3B can be used as the molecular marker of intracellular autophagy which increased when autophagy generated.[15]In the process of autophagy, LC3-I was modified and transformed by the ubiquitin system, and produced LC3-II molecular weight of 16 000, which was positioned to autophagosome when autophagy occurred. Therefore, LC3-I and LC3-II on the autophagosome can be deemed as the molecular marker of autophagy, and the quantity of LC3-II was proportional to the level of autophagy.[16]This study also found that the expression of LC3B-II/I in the HT group decreased more signi fi cantly than in the NT group (Figures 6 and 7), indicating that MH reduced intracellular autophagy, one of the reasons for the improvement in rat neurologic function (Figure 1). The above results indicated that autophagy in rat brain nerve cells after resuscitation was excessive and harmful.
ACKNOWLEDGEMENTS
The authors would like to thank Professor Zi-tong Huang, Professor Xiang-shao Fang of the Af fi liated Sun Yat-sen Memory Hospital of Zhongshan University, Director Ai-dong Wang of the Experimental Center of the Second Af fi liated Hospital of Soochow University, the Experimental Center of Suzhou Health College for their technical assistance.
Funding:The study was supported by a grant from a science and technology project "Health of Science and Education" of Suzhou in 2013 (KJXW2013026).
Ethical approval:This study was carried out in accordance with the guidelines for animal care and use established by Soochow University Animal Care and Use Committee. The protocol of the study was approved by the Ethics Committee of Animal Experiments of Soochow University (Permiting Number: 120410).Con fl icts of interest:The authors declare they have no competing interests relevant to this study.
Contributors:Lu J proposed the study and wrote the first draft. Shen Y contributed to interpretation of the draft. All authors read and approved the fi nal manuscript.
REFERENCES
1 Wang XP, Lin QM, Zhao S, Lin SR, Chen F. Therapeutic bene fi ts of mild hypothermia in patients successfully resuscitated from cardiac arrest: A meta-analysis. World J Emerg Med 2013; 4: 260–265.
2 Xiong W, Hoesch RE, Geocadin RG. Post-cardiac arrest encephalopathy. Semin Neurol 2011; 31: 216–225.
3 Liu J, Huang L. The impact of mutual antagonism from AMPK and mTOR on the ischemic brain injury. Chin J Emerg Med 2012; 21: 1398–1400.
4 Idris AH, Becker LB, Ornato JP, Hedges JR, Bircher NG, Chandra NC, et al. Utstein-style guidelines for uniformreporting of laboratory CPR research. Circulation 1996; 94: 2324–2336.
5 Geocadin RG, Ghodadra R, Kimura T, Lei H, Sherman DL, Hanley DF, et al. A novel quantitative EEG injury measure of global cerebral ischemia. Clin Neurophysiol 2000; 111: 1779–87.
6 Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 2009; 417: 1–13.
7 Marchi S, Giorgi C, Suski JM, Agnoletto C, Bononi A, Bonora M, et al. Mitochondria-ros crosstalk in the control of cell death and aging. J Signal Transduct 2012; 329635.
8 Liu X, Wang M, Chen H, Guo Y, Ma F, Shi F, et al. Hypothermia protects the brain from transient global ischemia/reperfusion by attenuating endoplasmic reticulum response-induced apoptosis through CHOP. PLoS One 2013; 8: e53431
9 Wu X, Mao H, Liu J, Xu J, Cao J, Gu X, et al. Dynamic change of SGK expression and its role in neuron apoptosis after traumatic brain injury. Int J Clin Exp Pathol 2013; 6: 1282–1293.
10 Zgavc T, Ceulemans AG, Hachimi-Idrissi S, Kooijman R, Sarre S, Michotte Y. The neuroprotective effect of post ischemic brief mild hypothermic treatment correlates with apoptosis, but not with gliosis in endothelin-1 treated rats. BMC Neurosci 2012; 13: 105.
11 Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, et al. Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol 2006; 169: 566–583.
12 Jaeger PA, Wyss-Coray T. All-you-can-eat: autophagy in neurodegeneration and neuroprotection. Mol Neurodegener 2009; 4: 16.
13 Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, et al. Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol 2006; 169: 566–583.
14 Weidberg H, Shvets E, Shpilka T, Shimron F, Shinder V, Elazar Z. LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J 2010; 29: 1792–1802.
15 Bendix I, Schulze C, Haefen Cv, Gellhaus A, Endesfelder S, Heumann R, et al. Erythropoietin modulates autophagy signaling in the developing rat brain in an in vivo model of oxygentoxicity. Int J Mol Sci 2012; 13: 12939–12951.
16 Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 2008; 4: 151–175.
Received February 20, 2014
Accepted after revision September 10, 2014
World J Emerg Med 2014;5(4):298–305
10.5847/wjem.j.issn.1920–8642.2014.04.010
杂志排行
World journal of emergency medicine的其它文章
- Current pre-hospital traumatic brain injury management in China
- Emergency bedside ultrasound for the diagnosis of pediatric intussusception: a retrospective review
- How to secure the connection between thoracostomy tube and drainage system?
- Clinical probability and risk analysis of patients with suspected pulmonary embolism
- Thyroid hormone alterations in trauma patients requiring massive transfusion: An observational study
- The incidence of oxygen desaturation during rapid sequence induction and intubation
