Simvastatin inhibits apoptosis of endothelial cells induced by sepsis through upregulating the expression of Bcl-2 and downregulating Bax
2014-03-20HuiFuQiaoshengWangQiongLuoSiTanHuaSuShilinTangZhengliangZhaoLipingHuang
Hui Fu, Qiao-sheng Wang, Qiong Luo, Si Tan, Hua Su, Shi-lin Tang, Zheng-liang Zhao, Li-ping Huang
1Department of Critical Care Medicine, First Af fi liated Hospital of University of South China, Hengyang 421001, China
2Department of Infection, Third Hospital of Hengyang City, Hengyang 421001, China
Corresponding Author:Qiao-sheng Wang, Email: docwqs@163.com
Simvastatin inhibits apoptosis of endothelial cells induced by sepsis through upregulating the expression of Bcl-2 and downregulating Bax
Hui Fu1, Qiao-sheng Wang1, Qiong Luo1, Si Tan2, Hua Su1, Shi-lin Tang1, Zheng-liang Zhao1, Li-ping Huang1
1Department of Critical Care Medicine, First Af fi liated Hospital of University of South China, Hengyang 421001, China
2Department of Infection, Third Hospital of Hengyang City, Hengyang 421001, China
Corresponding Author:Qiao-sheng Wang, Email: docwqs@163.com
BACKGROUND:Many studies have showed that apoptosis of endothelial cells plays a curial role in the progress of sepsis. But the role of simvastatin in apoptosis of endothelial cells induced by sepsis is not clear. The present study aimed to investigate the role of simvastatin in apoptosis of endothelial cells induced by sepsis and its mechanism.
METHODS:Human umbilical vein endothelial cells (HUVECs) were randomly divided into three groups: control group, sepsis serum intervention group (sepsis group) and simvastatin+sepsis serum intervention group (simvastatin group). After 24-hour incubation with corresponding culture medium, the relative growth rate of HUVECS in different groups was detected by MTT assay; the apoptosis of HUVECs was detected by Hoechst33258 assay and fl ow cytometry; and the expression of the Bcl-2 and Bax genes of HUVECs was detected by PCR.
RESULTS:Compared with the sepsis group, HUVECs in the simvastatin group had a higher relative growth rate. Apoptotic HUVECs decreased significantly in the simvastatin group in comparison with the sepsis group. Expression of the Bcl-2 gene in HUVECs decreased obviously, but the expression of the Bax gene increased obviously after 24-hour incubation with sepsis serum; however, the expression of the Bcl-2 and Bax genes was just the opposite in the simvastatin group.
CONCLUSIONS:Our study suggests that simvastatin can inhibit apoptosis of endothelial cells induced by sepsis through upregulating the expression of Bcl-2 and downregulating Bax. It may be one of the mechanisms for simvastatin to treat sepsis.
Simvastatin; Sepsis; Endothelial cells; Apoptosis; Bcl-2 gene; Bax gene
INTRODUCTION
Sepsis is an excessive body inflammation reaction caused by infection, and its speci fi c pathogenesis is very complex.[1]One of the important hallmarks of sepsis is microvascular dysfunction, in which the activation and dysfunction of endothelial cells seem to play a pivotal role. In infection and subsequent sepsis, components of the bacterial cell wall, such as lipopolysaccharide (LPS), activate pattern recognition receptors on the endothelial surface.[2,3]Once an inflammatory response has been instigated, a large number of host-derived mediators, including cytokines, chemokines, and products of the complement system, are also able to activate endothelial cells. The endothelium responds to these mediators with structural changes such as cytoplasmic swelling and detachment and importantly, also with functional changes such as the expression of adhesion molecules, resulting in increased platelet adhesion and leukocyte traf fi cking. An important feature of endothelial dysfunction in sepsis is increased vascular permeability, resulting inredistribution of body fluid and edema. Fluid leakage from the intravascular space contributes to hypovolemia and hypotension, which are the important signs of sepsis syndrome.[4]
Normally, only a small portion of endothelial cells is apoptotic (<0.1%). LPS has been shown to induce endothelial apoptosis in vitro and also in vivo, although not in all studies.[5]Interaction with other cells may in fl uence endothelial apoptosis; for example, LPS-activated monocytes have been shown to contribute to endothelial apoptosis in vitro.[6]Endothelial apoptosis has been shown to contribute to a procoagulant state in vitro.[7]It plays a central role in the pathogenesis of some diseases, and the prevention of endothelial apoptosis can cause significant improvements in the patient's outcome.[8]
Recent studies[9,10]have shown that simvastatin has immunomodulatory and anti-in fl ammatory effects, which were not dependent on the lipid-lowering effect. An animal study[11]showed that simvastatin is an effective agent that reduces cytokines levels and leukocyte count in sepsis, and is well-known for the lipid-lowering effects. Simvastatin has important anti-inflammatory effects on abdominal sepsis in rats. Another study[12]demonstrated that high-dose simvastatin (80 mg) may be useful for patients with acute systemic in fl ammation and associated vascular hyporeactivity during endotoxemia. Simvastatin reduces recruitment and activation of neutrophils, hereby protecting from LPS-induced acute lung injury (ALI).
So far, though a great deal of studies have shown that simvastatin has an obvious anti-inflammatory effect on sepsis, but its specific mechanism for the treatment of sepsis is not clear. Apoptosis of endothelial cells plays an important role in the pathogenesis of sepsis. Few studies have been reported whether simvastatin can reduce apoptosis of endothelial cells induced by sepsis. The present study aimed to investigate the role of simvastatin in apoptosis of endothelial cells induced by sepsis as well as its mechanism.
METHODS
Animals and experimental groups
Fifty adult male Sprague-Dawley (SD) rats weighing 250–300 g (body weight) (Hunan SJA Laboratory Animal Co.Ltd, PR China) were used as the sepsis model of cecal ligation and puncture (CLP) as described previously.[13]The serum was collected at 6 hours following CLP. The serum from the rat sepsis model was prepared for another part of the experiment. Animals handling and experiments were approved by the Institutional Animal Care and Use Committee, University of South China, China.
Human umbilical vein endothelial cells (HUVECs), ECV-304 cells, were randomly divided into three groups: control group, rat sepsis serum intervention group (sepsis group) and simvastatin+sepsis serum intervention group (simvastatin group). The HUVECs in the control group were cultured in DMEN, in the sepsis group in DMEN at the concentration of 20% for sepsis serum, and in the simvstatin group in DMEN at the concentration of 20% for sepsis serum and 1 µmol/L simvastatin. The HUVECs were used in the experiments 24 hours after the onset of culture.
MTT assay
MTT assay was used to measure cell proliferation as described previously.[14]The specific MTT assay was carried out with the kit (Nanjing KeyGEN Biotech CO., LTD, China) according to the manufacturer's protocol. Briefly, cells were seeded in 96-well tissue culture plates at a concentration of 4X103cells/well. When cells reached approximately 70% con fl uence, the medium was changed from DMEM-15 to DMEM at the concentration of 20% of rat sepsis serum, the concentration of 20% of rat sepsis serum including 1 µmol/L smvastatin, respectively, and maintained for 24 hours. The serum-starved cells were maintained for 24 hours in each culture condition. At the end of culture, the MTT (50 μg/mL) was added and further maintained for 2 hours at 37 °C. The MTT-containing medium was discarded, and 100 μL of undiluted dimethyl sulfoxide was added to the cells. After 30-minute incubation at room temperature, absorbance (A value) of the converted dye was measured at a wavelength of 450 nm with background subtraction at 650 nm using a spectrophotometric plate reader (Benchmark Plus Microplate Spectrophotometer; Bio-Rad Laboratories, Hercules, CA). Measurements were repeated three times to get a mean value. Relative growth rate = (intervention group A value / control group A value) X 100%.
Hoechst staining
Apoptosis was determined by incubating the cells with 10 μg/mL Hoechst 33 342 for 30 minutes at 37 °C using the Hoechst staining kit (Nanjing KeyGEN Biotech, CO.,LTD, China) according to the manufacturer's protocol and scoring the percentage of cells having intensely condensed chromatin and/or fragmented nuclei by fl uorescence microscopy. Apoptotic cells showed with strong blue fl uorescence, normal cells did with only weak fluorescence, while dead cells were not stained in the fl uorescence microscopy.
Flow cytometry
Cells were incubated with fl uorescein isothiocyanate (FITC)-conjugated Annexin-V according to the recommendations of the manufacturer (Nanjing KeyGEN Biotech. CO., LTD, China). Nuclei were counterstained with propidium iodide (PI) and screened by flow cytometry (approximately 10 000 events). A cell scattergram which was composed of four quadrants would be given by flow cytometry. And analysis of apoptosis rate (including early apoptotic cells and late apoptotic cells) would refer to Wendelina method.[15]
RT-PCR analysis
Logarithmic phase cells were collected, and a single cell suspension of 1X105/mL was prepared with culture medium. Of the suspension, 300 μL per well was seeded in a 6-well plate. After 24 hours' adherent growth, the culture medium was drained, and washed with PBS three times. Then, the cells were incubated with conditioned medium for 24 hours. The cells of each group were incubated in three different wells and the experiment repeated 3 times.
Total RNA was extracted from the cells using RNAesy mini kit (TAKARA Biotechnology (Dalian) Co. Ltd, China) according to the manufacturer's protocol. The quantity of total RNA was measured with a UV spectrophotometer (Biochrom Ltd, England). For the reverse transcription, 2 μg of total RNA was combined with 1 μm of Oligo (dT) 15 primer (Invitrogen, CA, USA). The mixture was then heated at 70 °C for 5 minutes and then placed on ice. Single-strand cDNA was synthesized from the RNA by adding the following reagents (final concentrations): 1Xfirst strand buffer, 1 U/μL RNAsin, 25 mL of each dNTP and 200 U M-MLV reverse transcriptase (Promega, WI, USA). The reaction mixture (20 μL) was incubated at 42 °C for 50 minutes and the mixture was heated to 95 °C for 5 minutes in order to terminate the reaction. The samples were stored at –20 °C for PCR analysis.
PCR was carried out on a Life Eco Thermocycler (Bio-Rad Laboratories, Inc, USA) using kit (TAKARA Biotechnology (Dalian) Co. Ltd, China) according to the manufacturer's instructions. Briefly, a total volume of 20 μL mix was prepared with 2 μL cDNA, 10 μL PCR Master mix, 0.5 μL forward primer, 0.5 μL reverse primer, and ddH2O 7 μL. The oligonucleotide primer sequences used for Bcl-2, Bax and b-actin are as follows: Bcl-2 (242 bp), forward primer: 5CTCGTCGC TACCGTCGTGACTTCG3; reverse primer: 5CAGATG CCGGTTCAGGTACTCAGTC3; Bax (542 bp), forward primer: 5ATGGAGGGGTCCGGGGAG3, reverse primer: 5TGGAAGAAGATGGGCTGA3; β-actin (852 bp), forward primer: 5CACGATGGAGG GGCCGGACTCATC3, and reverse primer 5'TAAAGACCTCTATGCCAACACAGT3.Thermal cycling was carried out. The first segment of the amplification cycle consisted of a pre-denaturation programme at 95 °C for 3 minutes, denaturation programme at 95 °C for 30 seconds. The second segment consisted of denaturation, primer annealing, elongation and a quanti fi cation programme repeated for 34 cycles, and ultimately extends to 72 °C for 5 minutes. The products preserved at 4 °C. Amplifications were confirmed by standard submarine gel electrophoresis, using 2% w/v low-melting agarose/TBE gels (NuSieve, FMC BioProducts), stained with ethidium bromide. The relative mRNA levels were determined with β-actin as the internal control.
Statistical analysis
All values were presented as mean±SD. Statistical analyses were conducted with commercially available software SPSS 13.0. Difference of the relative growth rate of HUVECs in each group was determined by the results of independent-samples t test, The difference between the absorbance values and the apoptotic rate of HUVECs was determined by one-way ANOVA. P<0.05 was considered statistically signi fi cant.
RESULTS
Relative growth rate of HUVECs in each group detected by MTT assay
The relative growth rate of HUVECs was reduced significantly in the sepsis group compared with the control group. After the intervention with simvastatin, the relative growth rate of HUVECs was increased significantly in the simvastatin group compared with the sepsis group. The rates in the sepsis group and simvastatin group were (64.06±1.70)% and (37.59±2.13)% respectively (t=–16.846, P=0.000) (Figure 1). These results showed that simvastatin can alleviate injury of endothelial cells induced by sepsis.
Apoptosis detected by staining with Hoechst 33342
Normal living cells with Hoechst staining showed blue fluorescence uniformly in the control group, while apoptotic cells showed hyperchromatic and dense fl uorescent particles within the massive apoptotic nucleior cytoplasm in the fl uorescence microscopy in the sepsis group and the simvastatin group. Apoptotic HUVECs reduced signi fi cantly in the simvastatin group compared with the sepsis group (P<0.05) (Figure 2).
Apoptosis detected by fl ow cytometry

Figure 1. The relative growth rate of HUVECs in each group was detected by MTT assay. The relative growth rate of HUVECs in both sepsis and simvastatin groups decreased signi fi cantly compared with the control group#P<0.05,*P<0.05). However, the relative growth rate of HUVECs increased in the simvastain group compared with the sepsis group (#P<0.05).
Flow cytometry showed the significant difference of apoptosis rates of HUVECs between the two groups (F=15.317, P=0.004). The apoptosis rate of HUVECs in the sepsis group increased signi fi cantly compared with the control group (P=0.001). Meanwhile, the apoptosis rate of the simvastatin group was signi fi cantly reduced compared with that of the sepsis group (P=0.027) (Figure 3).
Expression of Bcl-2 and Bax mRNA
PCR analysis showed significant difference of Bcl- 2 and Bax mRNA in HUVECs of each group. The expression of Bcl- 2 mRNA decreased in the sepsis group compared with the control group, whereas the Bcl-2 expression increased in the simvastatin group compared with the sepsis group (Figure 4 A, C). The expression of Bax in the sepsis group and the simvastatin group was conversely changed. The expression of Bax mRNA increased significantly in the sepsis group compared with the control group. The epression of Bax mRNA decreased in the simvastatin group compared with the sepsis group (Figure 4 B, D).
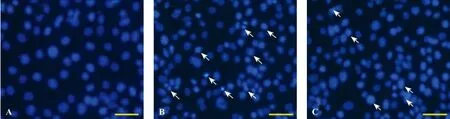
Figure 2. Apoptosis was detected by staining with Hoechst 33342. The apoptosis of HUVECs showed that intensely condensed chromatin and/or fragmented nuclei in the cells were detected by fl uorescence microscopy. The apoptosis increased signi fi cantly in the sepsis group (B) compared with the control group (A); however, the apoptosis in the simvastatin group (C) decreased significantly compared with the sepsis group. Scale bars: A–C, 20 µm.

Figure 3. Apoptosis was detected by fl ow cytometry.The apoptosis of HUVECs increased signi fi cantly in the sepsis and simvastatin groups (B) compared with the control group (A); however, the apoptosis in the simvastatin group decreased signi fi cantly compared with the sepsis group (C). The apoptotic rate of HUVECs in both sepsis and simvastatin groups signi fi cantly increased compared with the control group (#P<0.05,*P<0.05). However, the apoptotic rate decreased in the simvastain group (#P<0.05) (D) compared with the sepsis group.
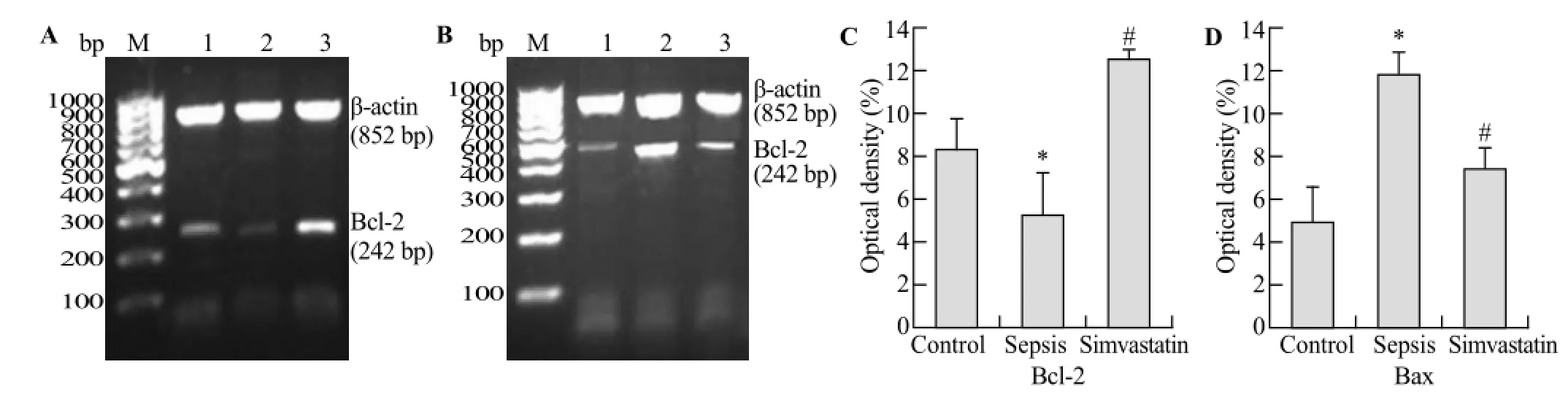
Figure 4. Bcl-2 mRNA and Bax mRNA were detected respectively by RT-PCR in the two groups (A-D). A showed that the changes of density of Bcl-2 mRNA in the control group (band 1), the sepsis group (band 2) and the simvastatin group (band 3), respectively. The bar grah (C) showed that the optical density of Bcl-2 mRNA decreased in the sepsis group compared with the control group (*P<0.05) and the optical density of Bcl-2 Mrna increased in the simvastatin group compared with the sepsis group (#P<0.05). However, B showed that the changes of density of Bax mRNA in the control group (band 1), the sepsis group (band 2) and the simvastatin group (band 3), respectively. The bar grah (D) showed that the optical density of Bcl-2 mRNA increased in the sepsis group compared with the control group (*P<0.05) and that the opical density of Bcl-2 mRNA decreased in the simvastatin group compared with the sepsis group (#P<0.05).
DISCUSSION
Injury of endothelial cells induced by sepsis play a crucial role in the progress of sepsis and its severity is significantly related to prognosis. Studies[16–21]have shown that endothelial activation and dysfunction seem to play a pivotal role in sepsis. It has been reported that endothelial apoptosis could be the pivotal event in the pathogenesis of systemic inflammatory response syndrome, sepsis, acute respiratory distress syndrome, and multiple-organ dysfunction syndrome.[19]Endothelial apoptosis plays a central role in the pathogenesis of some diseases, and the prevention of endothelial apoptosis can signi fi cantly improve the patient's outcome.[8]
At the same time, microparticles are circulating cell fragments derived from activated or apopototic cells. In sepsis, circulating microparticles derived from platelets, monocytes, and endothelial cells have been identi fi ed.[17,22,23]Although many aspects of microparticle function are still unclear, they are increasingly recognized as important regulators of cell-to-cell interactions, and play an important role in inflammation, coagulation, and vascular function.[17]A recent study[24]found they may play important roles not only in homeostasis but also in the pathogenesis of a number of diseases such as vascular diseases, cancer, infectious diseases and diabetes mellitus.
HMG-CoA reductase inhibitors (statins) such as simvastatin have pleiotropic effects independent of lipid lowering.[25–27]Statin therapy has clinically beneficial effects on cardiovascular, cerebrovascular and acute and chronic kidney diseases via diverse effects.[26,28,29]The protective effects of statins on both human and animal sepsis have been recently reported. A retrospective study in humans reported that statin therapy reduced both overall and attributable mortality in patients with bacteremia.[30]A retrospective study[31]found that statin therapy is bene fi cial for patients with sepsis. A controlled study[32]revealed that prior statin therapy was associated with a reduction of severe sepsis and intensive care unit admission. Simvastatin improved survival of animals in a murine CLP model.[33]Merx et al[34]reported that the average survival rate of septic mice using statin was 4 times higher than that of controls. Simvastatin reduces recruitment and activation of neutrophils, thereby protecting from LPS-induced ALI. Simvastatin improved sepsis-induced AKI by direct effects on the renal vasculature, reversal of tubular hypoxia, and a systemic anti-inflammatory effect.[35]Statins might improve microvascular dysfunction in sepsis. In rats, simvastatin attenuated the development of sinusoidal endothelial dysfunction induced by LPS administration through restoring a physiologic phosphorylated endothelial nitric oxide synthase (PeNOS)/endothelial nitric oxide synthase (eNOS) ratio, suggesting that statins might have potential for liver protection during endotoxemia.[36]Pretreatment with simvastatin protected against alphatoxin-induced sepsis associated with reduced p53, TNF-alpha, apoptosis, and necrosis.[37]
Apoptosis is termed programmed cell death because it is a series or program of coordinated processes.[38]Apoptosis is induced through two major signaling cascades, which are termed the extrinsic and intrinsic pathways. In the extrinsic pathway, external proteins bind to cell-surface receptors that subsequently induce apoptosis. Eventually caspase-3 becomes activated; caspase-3 has been termed the master executioner. The intrinsic pathway is the mitochondrial mediated pathway of apoptosis. A balance exists between (a) antiapoptotic proteins such as BCL-2, BCL-XL, and several others;and (b) proapoptotic proteins such as Bim, Bax, and PUMA. The proapoptotic proteins induce mitochondria to release cytochrome c, which activates caspase-9 through a series of events. As in the extrinsic pathway, caspase-3 becomes activated as a terminal event.[1]
The Bc1-2 family plays an important role in transduction apoptotic signal within the cell. Bcl-2 and Bax genes in the Bcl-2 family have a close relationship with the apoptosis, the former is anti-apoptotic genes, the latter is pro-apoptotic genes. The balance of them affect the apoptosis.[39]A study has showed that Bcl-2 inhibits cell apoptosis through various ways: Bax-mediated apoptosis, inhibition of release of calcium ion in endoplasmic reticulum, antioxidant, etc.[40]
Our study showed that apoptotic HUVECs increased signi fi cantly, Bcl-2 mRNA upregulated, and Bax mRNA of HUVECs downregulated after incubation with septic serum of rats. The results suggested that sepsis may induce apoptosis after change of the balance of Bcl-2 mRNA and Bax mRNA.
However, after incubation with simvastatin, apoptotic HUVECs decreased significantly and the expression of Bcl-2 mRNA and Bax mRNA was changed inversely. This change shows that simvastatin inhibits sepsisinduced endothelial cell apoptosis by upregulating the Bcl-2 gene and downregulating the Bax gene.
At present, the effect of statins on apoptosis is still controversial. A study[41]showed that simvastatin decreased the TNF-alpha-induced apoptosis in endothelial progenitor cells by promoting the expression of silent information regulator type-1. Another study[42]showed that simvastatin triggers cell death in LPS-activated RAW 264.7 mouse macrophage cells through both caspase-dependent and independent apoptotic pathways. The nuclear orphan receptor NR4A1 expression and mitochondrial translocation of Bax are related to simvastatin-induced apoptosis in LPS-activated RAW 264.7 macrophages. These studies suggested that statins play different roles in apoptosis or anti-apoptosis through different pathways because of different pathophysiological causes. However, the speci fi c mechanisms still need further study. If the role of statins in reducing mortality and improving the prognosis of sepsis patients is proved, it will be a signi fi cant basis for the clinical use of statins for sepsis.
In conclusion, our study suggests that simvastatin can inhibit apoptosis of endothelial cells induced by sepsis through upregulating the expression of Bcl-2 and downregulating Bax. It may be one of the mechanisms for simvastatin to treat sepsis.
Funding:This study was supported by grants from the Medical Research Foundation of Hunan Province (B2013-040) and the Science and Technology Development Foundation of Hengyang City (2010kj38).
Ethical approval:The study was approved by Institutional Animal Care and Use Committee, University of South China, China.
Conflicts of interest:The authors have no competing interests relevant to the present study.
Contributors:Fu H and Wang QS contributed equally to this work. All authors read and approved the final version of the manuscript.
REFERENCES
1 Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol 2011; 6: 19–48.
2 Henneke P, Golenbock DT. Innate immune recognition of lipopolysaccharide by endothelial cells. Crit Care Med 2002; 30: S207–S213.
3 Zhang FX, Kirschning CJ, Mancinelli R, Xu XP, Jin Y, Faure E, et al. Bacterial lipopolysaccharide activates nuclear factorkappaB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem 1999; 274: 7611–7614.
4 Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 2003; 101: 3765–3777.
5 Hotchkiss RS, Tinsley KW, Swanson PE, Karl IE. Endothelial cell apoptosis in sepsis. Crit Care Med 2002; 30: S225–S228.
6 Lindner H, Holler E, Ertl B, Multhoff G, Schreglmann M, Klauke I, et al. Peripheral blood mononuclear cells induce programmed cell death in human endothelial cells and may prevent repair: role of cytokines. Blood 1997; 89: 1931–1938.
7 Bombeli T, Karsan A, Tait JF, Harlan JM. Apoptotic vascular endothelial cells become procoagulant. Blood 1997; 89: 2429–2442.
8 Stefanec T. Endothelial apoptosis: could it have a role in the pathogenesis and treatment of disease? Chest 2000; 117: 841–854.
9 Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med 2001; 7: 687–692.
10 Pruefer D, Makowski J, Dahm M, Guth S, Oelert H, Darius H, et al. Aprotinin inhibits leukocyte-endothelial cell interactions after hemorrhage and reperfusion. Ann Thorac Surg 2003; 75: 210-215, 215–216.
11 Souza Neto JL, Araújo Filho I, Rego AC, Dominici VA, Azevedo IM, Egito ES, et al. Effects of simvastatin in abdominal sepsis in rats. Acta Cir Bras 2006; 21 Suppl 4: 8–12.
12 Boyd AR, Hinojosa CA, Rodriguez PJ, Orihuela CJ. Impact of oral simvastatin therapy on acute lung injury in mice during pneumococcal pneumonia. BMC Microbiol 2012; 12: 73.
13 Kono Y, Inomata M, Hagiwara S, Shiraishi N, Noguchi T, Kitano S. A newly synthetic vitamin E derivative, E-Ant-S-GS, attenuates lung injury caused by cecal ligation and punctureinduced sepsis in rats. Surgery 2012; 151: 420–426.
14 Lee JG, Kay EP. Common and distinct pathways for cellular activities in FGF-2 signaling induced by IL-1beta in corneal endothelial cells. Invest Ophthalmol Vis Sci 2009; 50: 2067–2076.
15 Raju J, Bird RP. Energy restriction reduces the number of advanced aberrant crypt foci and attenuates the expression of colonic transforming growth factor beta and cyclooxygenase isoforms in Zucker obese (fa/fa) rats. Cancer Res 2003; 63: 6595–6601.
16 Darwish I, Liles WC. Emerging therapeutic strategies to prevent infection-related microvascular endothelial activation and dysfunction. Virulence 2013; 4: 572–582.
17 Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis, thrombosis and organ dysfunction. Thromb Res 2012; 129: 290–295.
18 Calderari B, Liaudet L. Pathophysiological mechanisms of organ dysfunction in sepsis. Rev Med Suisse 2010; 6: 2406–2409.
19 Schouten M, Wiersinga WJ, Levi M, van der Poll T. In fl ammation, endothelium, and coagulation in sepsis. J Leukoc Biol 2008; 83: 536–545.
20 Matsuda N, Yamamoto S, Hatakeyama N, Hattori Y. Vascular endothelial dysfunction in septic shock. Nihon Yakurigaku Zasshi 2008; 131: 96–100.
21 Boos CJ, Goon PK, Lip GY. The endothelium, inflammation, and coagulation in sepsis. Clin Pharmacol Ther 2006; 79: 20–22.
22 Meziani F, Delabranche X, Asfar P, Toti F. Bench-to-bedside review: circulating microparticles-a new player in sepsis? Crit Care 2010; 14: 236.
23 Walenta KL, Link A, Friedrich EB, Böhm M. Circulating microparticles in septic shock. Am J Respir Crit Care Med 2009; 180: 100, 100–101.
24 Wu ZH, Ji CL, Li H, Qiu GX, Gao CJ, Weng XS. Membrane microparticles and diseases. Eur Rev Med Pharmacol Sci 2013; 17: 2420–2427.
25 Mason JC. The statins-therapeutic diversity in renal disease? Curr Opin Nephrol Hypertens 2005; 14: 17–24.
26 Epstein M, Campese VM. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors on renal function. Am J Kidney Dis 2005; 45: 2–14.
27 Khanal S, Attallah N, Smith DE, Kline-Rogers E, Share D, O'Donnell MJ, et al. Statin therapy reduces contrast-induced nephropathy: an analysis of contemporary percutaneous interventions. Am J Med 2005; 118: 843–849.
28 Pierre-Paul D, Gahtan V. Noncholesterol-lowering effects of statins. Vasc Endovascular Surg 2003; 37: 301–313.
29 Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005; 352: 20–28.
30 Liappis AP, Kan VL, Rochester CG, Simon GL. The effect of statins on mortality in patients with bacteremia. Clin Infect Dis 2001; 33: 1352–1357.
31 Kruger P, Fitzsimmons K, Cook D, Jones M, Nimmo G. Statin therapy is associated with fewer deaths in patients with bacteraemia. Intensive Care Med 2006; 32: 75–79.
32 Almog Y, Shefer A, Novack V, Maimon N, Barski L, Eizinger M, et al. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation 2004; 110: 880–885.
33 Merx MW, Liehn EA, Graf J, van de Sandt A, Schaltenbrand M, Schrader J, et al. Statin treatment after onset of sepsis in a murine model improves survival. Circulation 2005; 112: 117–124.
34 Grommes J, Vijayan S, Drechsler M, Hartwig H, Mörgelin M, Dembinski R, et al. Simvastatin reduces endotoxin-induced acute lung injury by decreasing neutrophil recruitment and radical formation. PLoS One 2012; 7: e38917.
35 Yasuda H, Yuen PS, Hu X, Zhou H, Star RA. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int 2006; 69: 1535–1542.
36 La Mura V, Pasarín M, Meireles CZ, Miquel R, Rodríguez-Vilarrupla A, Hide D, et al. Effects of simvastatin administration on rodents with lipopolysaccharide-induced liver microvascular dysfunction. Hepatology 2013; 57: 1172–1181.
37 Buerke U, Carter JM, Schlitt A, Russ M, Schmidt H, Sibelius U, et al. Apoptosis contributes to septic cardiomyopathy and is improved by simvastatin therapy. Shock 2008; 29: 497–503.
38 Pinheiro da Silva F, Nizet V. Cell death during sepsis: integration of disintegration in the in fl ammatory response to overwhelming infection. Apoptosis 2009; 14: 509–521.
39 Chittenden T, Harrington EA, O'Connor R, Flemington C, Lutz RJ, Evan GI, et al. Induction of apoptosis by the Bcl-2 homologue Bak. Nature 1995; 374: 733–736.
40 Schulz JB, Weller M, Moskowitz MA. Caspases as treatment targets in stroke and neurodegenerative diseases. Ann Neurol 1999; 45: 421–429.
41 Du G, Song Y, Zhang T, Ma L, Bian N, Chen X, et al. Simvastatin attenuates TNFalpha induced apoptosis in endothelial progenitor cells via the upregulation of SIRT1. Int J Mol Med 2014; 34: 177–182.
42 Kim YC, Song SB, Lee SK, Park SM, Kim YS. The Nuclear Orphan Receptor NR4A1 is Involved in the Apoptotic Pathway Induced by LPS and Simvastatin in RAW 264.7 Macrophages. Immune Netw 2014; 14: 116–122.
Received April 10, 2014
Accepted after revision October 6, 2014
World J Emerg Med 2014;5(4):291–297
10.5847/wjem.j.issn.1920–8642.2014.04.009
杂志排行
World journal of emergency medicine的其它文章
- Current pre-hospital traumatic brain injury management in China
- Emergency bedside ultrasound for the diagnosis of pediatric intussusception: a retrospective review
- How to secure the connection between thoracostomy tube and drainage system?
- Clinical probability and risk analysis of patients with suspected pulmonary embolism
- Thyroid hormone alterations in trauma patients requiring massive transfusion: An observational study
- The incidence of oxygen desaturation during rapid sequence induction and intubation
