HMGB1基因多态性与HBV相关性肝癌的关联性研究*
2014-03-11王丹邓春青赵龙凤
王丹邓春青赵龙凤
HMGB1基因多态性与HBV相关性肝癌的关联性研究*
王丹①邓春青①赵龙凤①
目的:探讨高迁移率族蛋白B1(HMGB1)第4内含子1176G/C与HBV感染后肝癌是否存在关联。方法:采用聚合酶链反应-限制性片段长度多态性分析(PCR-RFLP)方法检测110例HBV感染后肝癌患者及316例HBV感染后非肝癌患者HMGB1 1176G/C多态性,采用 χ2检验及非条件logistic回归统计方法进行分析。结果:乙肝肝癌组3种基因型及G、C等位基因分布与慢性乙型肝炎组比较差异有统计学意义(χ2=6.152,P=0.046;χ2=5.605,P=0.018)。在显性模式下乙肝肝癌组与慢性乙型肝炎组比较差异有统计学意义(P=0.023),在隐性模式下与乙肝病毒携带组、轻型肝病组比较差异有统计学意义(P=0.048,P=0.028),在共显性模式下与轻型肝病组比较差异有统计学意义(P=0.03)。结论:HMGB1基因多态性与HBV感染后肝癌易感性相关。
高迁移率族蛋白B1; 单核苷酸; 乙型肝炎病毒; 多态性; 肝癌细胞
原发性肝癌(HCC,简称肝癌)是我国最常见的恶性肿瘤之一,尤以东南沿海地区多见,自20世纪90年代以来已上升为恶性肿瘤的第2位,全国每年13万人死于肝癌,其中约有1/3的患者有乙肝病史[1-2]。HBV感染遗传易感性的相关基因已被不断发现[3-5]。在HCC发病的相关因素中,遗传易感性同样起着重要作用,与之有关的基因多态性指标有:GST、VDR、IL28B、HLA、STAT4、CXCL14等[6-11]。深入研究HBV及HCC遗传易感性的分子机制对降低HCC的发病率及死亡率水平均具有重要意义。HMGB1是一类非组蛋白染色体结合蛋白,具有诱导炎性反应,调控基因转录,调节免疫等多种功能。大量实验证明,HMGB1参与了肝癌的发生发展过程[12-13]。HMGB1基因多态性与多种疾病的遗传易感性相关,但与HBV感染背景下HCC的发生是否有关联,目前报道甚少[14-19]。
1 资料与方法
1.1 一般资料 426例HBV感染者均为本院门诊及住院患者,男334例,女92例,年龄(42.15±11.87)岁,均为汉族,且无亲缘关系。所有病例诊断符合2005年中华医学会肝病与感染病学分会修订的病毒性肝炎诊断标准,同时排除HIV、TP及其他肝炎病毒感染。样本收集均获患者知情同意。按疫病类型将患者分为四组,其中乙肝病毒携带组98例,男62例,女36例;慢性乙型肝炎组116例,男92例,女24例;乙肝硬化组102例,男86例,女16例;乙肝肝癌组110例,男86例,女24例。
1.2 基因组DNA的提取 采用硅胶柱纯化方式,从700 μL抗凝血液中提取淋巴细胞基因组DNA。UV计测定DNA浓度及纯度,并将样本稀释至10 ng/μL,分装保存。
1.3 PCR-RFLP 从SNPs数据库下载SNP的标准序列,Primer Premier 6.0软件设计引物:上游:5’-3’CCTTTGCCCAGTGTATC,下游:5’-3’TGTATGCCAAGCCATTTG(上海英潍捷基公司合成),内切酶BclI位点T/GATCA(NEB公司提供)。PCR反应条件:95 ℃预变性3 min,94 ℃变性30 s,55 ℃退火45 s,72 ℃延伸30 s,循环次数35次,72 ℃延迟5 min结束PCR反应。产物37 ℃过夜酶切,65 ℃ 30 min,终止反应。2%琼脂糖凝胶电泳分离酶切产物,送上海英潍捷基公司测序验证。
1.4 统计学处理 SPSS 17.0软件进行数据处理,计算等位基因频率和基因型频率,Hardy-Weinberg平衡检验及各组间比较均采用 χ2检验,以P>0.01表示符合H-W平衡,以P<0.05表示各组间比较差异有统计学意义,非条件Logistic回归校正年龄及性别等因素,进行关联分析,计算比值比(Odds Ratios, OR)及其95%可信区间(Confidence Intervals,CI)表示相对危险度。
2 结果
2.1 基因型的判定及测序验证 PCR产物及酶切产物见图1~2。
2.2 H-W平衡判定及各基因型、等位基因的频率分布 诊断明确的乙型肝炎患者总计426例,样本经 χ2检验,P=0.85,统计学检验符合Hardy-Weinberg平衡,具有代表性,见表1。GG、CC和GC 3种基因型频率及G、C等位基因频率在样本中的分布比较差异无统计学意义(P=0.113,P=0.104)。乙肝肝癌组GG基因型频率低于乙肝携带组、乙肝肝硬化组、慢性乙型肝炎组,与前两组比较差异无统计学意义(P=0.086,P=0.285),与慢性乙型肝炎组比较差异均有统计学意义(χ2=6.152,P=0.046);GC、CC基因型频率高于其余三组,仅与慢性乙型肝炎组比较差异有统计学意义(P=0.046)。慢性乙型肝炎组G等位基因频率高于乙肝肝癌组,比较差异有统计学意义(χ2=5.605,P=0.018);C等位基因频率低于其余三组,仅与乙肝肝癌组比较差异有统计学意义(P=0.018)。
根据非条件Logistic回归校正年龄、性别混杂因素,以乙肝肝癌组为病例组分别与其他组比较进行分层分析,HMGB1基因位点1176G/C基因多态性在乙肝肝癌组与乙肝携带者组比较差异有统计学意义(OR=0.301,95%CI:0.092-0.989,P=0.048,Recessive model);乙肝肝癌组与慢性乙型肝炎组(OR=3.792,95%CI:1.206-11.919,P=0.023,Dominant model)比较差异有统计学意义;肝癌组与轻型肝病组(乙肝病毒携带组+慢性乙型肝炎组)(OR=0.227,95%CI:0.061-0.852,P=0.028,Dominant model;OR=0.225,95%CI:0.058-0.866,P=0.03,Codominant model)比较差异有统计学意义,见表2。

图1 PCR产物

图2 酶切产物
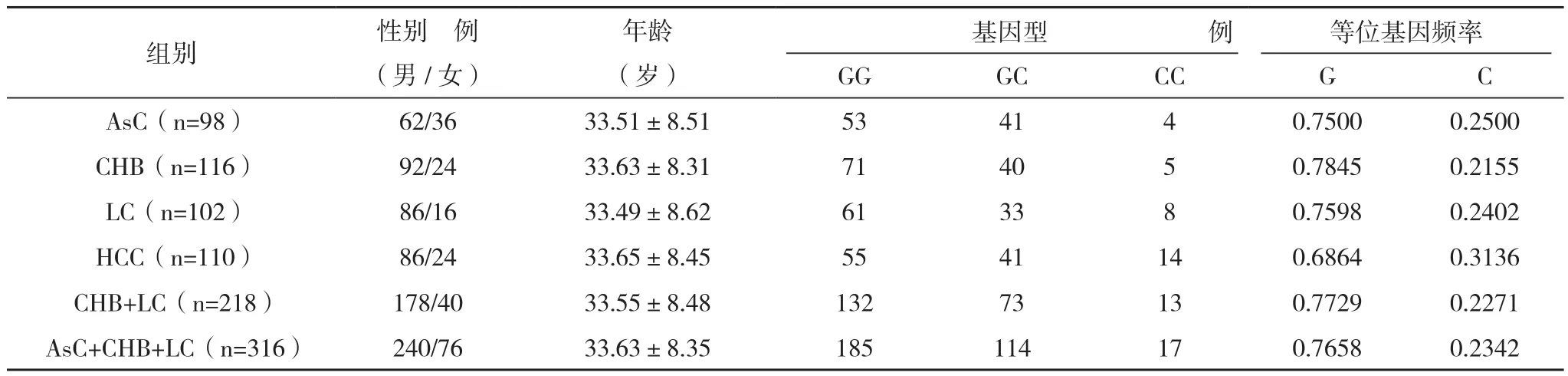
表1 HBV感染人群的HMGB1 1176G/C基因多态性
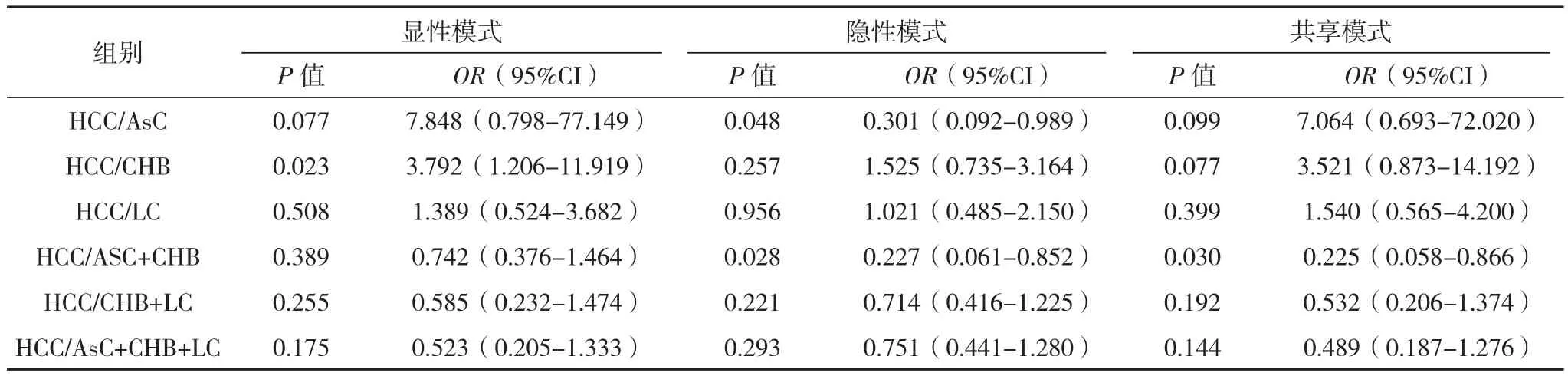
表2 HMGB1基因位点1176G/C与HBV感染关联分析
3 讨论
原发性肝癌的发生机制复杂,病因尚未确定,目前主要认为与肝炎病毒、致癌物质、饮水污染、寄生虫病等众多环境因素及遗传因素有关。肝癌有明显的家族聚集现象,其本质是因为遗传因素和肝癌之间存在明显的相关性。通过遗传学和表观遗传学改变引起原癌基因活化和抑癌基因灭活是导致肝癌发生的重要生物学过程。我国学者发现人体内存有导致肝癌的易感基因,此举拉开了研究肝癌相关易感基因的帷幕[20]。
HMGB1基因位于13q12染色体上,编码产物HMGB1已被证实参与了HBV感染后肝癌发生的过程,Yan等[12]指出HMGB1通过激活TLR4和RAGE信号通路,促进肝癌的浸润和转移。Jiang等[13]发现HMGB1 mRNA及蛋白在肝癌组织中表达最高,指出HMGB1的过度表达是肝癌发病的一个重要因素。继Kornblit等[14]人首次报道HMGB1基因总共存在6个SNP和4种基因突变后,其基因多态性与SIRS、同种异体T细胞移植免疫反应、产后脓毒症、MODS等多种疾病的相关性已被陆续报道[15-18]。Deng等[19]在研究HMGB1 1176G/C多态性与HBV感染临床表型的关联性分析中指出GG基因型人群对慢性乙型肝炎、肝硬化、急性乙型肝炎的易感性高于CC、GC基因型人群,G等位基因的HBV感染风险明显高于C等位基因,但与HBV感染后肝癌的关联性未予报道。
本研究结果提示,HMGB1 intron4 1176G/C多态性与HBV感染后HCC的发生有关联。携带GG基因型的人群对HBV感染后HCC的患病风险度增加,该慢性乙型肝炎人群更易发展成乙肝后肝癌,而携带CC基因型的人群感染HBV后发生HCC的风险较低。G等位基因不仅与HBV感染严重肝病密切相关,而且与HBV感染后肝癌的发生相关。HBV感染后HCC的发生,影响因素繁多,遗传背景尤为复杂。在分析其与HMGB1基因多态性的关联性时,种族和地域的差异、样本量不足、来源局限、等位基因连锁不平衡现象、基因突变特征、其他微效基因的相关影响、环境及宿主因素等诸多因素均可干扰研究结果,要考证其准确性,仍需要多中心、大样本的临床观察及研究。
[1] Tanaka M, Katayama F, Kato H, et al. Hepatitis B and C virus infection and hepatocellular carcinoma in China: a review of epidemiology and control measures[J].J Epidemiol, 2011, 21(2): 401-416.
[2] The Ministry of Health of the People's Republic of China. 2011 Chinese Health Statistics Yearbook (Section 9-3-1)[M].http://61.49.18.65/ htmlfiles/zwgkzt/ptjnj/year2011/index2011.html(accessed November 15,2012).
[3]晏泽辉,邓国宏,王宇明.乙型肝炎的宿主遗传易感性的研究进展及前景[J].世界华人消化杂志,2005,13(8):1002-1007.
[4] Deng C Q,Deng G H,Wang Y M. Relationship between polymorphisms of 3-hydroxy-s-methyglutaryl coenzyme A reductase gene and hepatitis B virus infection[J].World Journal of Gastroenterology,2005,17(5):2086-2089.
[5] Deng C Q, Deng G H, Wang Y M. eNOS gene 894G/T polymorphisms among patients infected with HBV[J].Virologica Sinica, 2005, 20(6):476-479.
[6] Liu K, Zhang L, Lin X, et al. Association of GST genetic polymorphisms with the susceptibility to hepatocellular carcinoma (HCC) in Chinese population evaluated by an updated systematic meta-analysis[J].PLoS One, 2013, 8(2):e57043.
[7] Yao X, Zeng H, Zhang G, et al. The associated ion between the VDR gene polymorphisms and susceptibility to hepatocellular carcinoma and the clinicopathological features in subjects infected with HBV[J]. Biomed Res Int, 2013, 20(9):953-974.
[8] Wang Y, Zhang H H, Chen Y H, et al. Correlation between interleukin-28B genetic polymorphisms and primary hepatocellular carcinoma[J]. Chinese Journal of Preventive Medicine, 2012, 46(6):527-532.
[9] Pan N, Chen K, Qiu J, et al. Human leukocyte antigen class I alleles and haplotypes associated with primary hepatocellular carcinoma in persistent HBV-infected patients[J].Hum Immunol, 2013, 74(6):758-763.
[10] Clark A, Gerlach F, Tong H V, et al. A trivial role of STAT4 variant in chronic hepatitis B induced hepatocellular carcinoma[J]. Nfect Genet Evol, 2013, 18(6): 257-261.
[11] Gu X, Wang H, Wang A, et al. An intronic polymorphism rs2237062 in the CXCL14 gene influences HBV-related HCC progression in Chinese population[J]. Mol Biol Rep, 2012, 39(2): 797-803.
[12] Yan W, Chang Y, Liang X, et al. High-mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases[J].Hepatology, 2012, 55(6): 1863-1875.
[13] Jiang W, Wang Z, Li X, et al.High-mobility group box 1 is associated with clinicopathologic features in patients with hepatocellular carcinoma[J].Pathol Oncol Res, 2012, 18(2): 293-298.
[14] Kornblit B, Munthe-Fog L, Petersen S L, et al. The genetic variation of the human HMGB1 gene[J]. Tissual Antigens, 2007, 70(2):151-156.
[15] Brian K, Munthe-Fog L, Madsen H O, et al. Association of HMGB1 polymorphisms with outcome in patients with systemic inflammatory response syndrome[J].Critical care, 2008, 12(3): R83.
[16] Kornbit B, Masmas T, Petersen S L, et al. Association of HMGB1 polymorphisms with outcome after allogeneic hematopoietic[J].Cell Transplantation, 2010, 16(2): 239-252.
[17] Davis S M, Clark E A, Nelson L T, et al. The association of innate immune response gene polymorphisms and puerperal group A streptococcal sepsis[J].American Journal of Obstetrics and Gynecology, 2010, 202(3): 308.
[18] Ling Z, Zhang A Q, Wei G, et al. Clinical relevance of single nucleotide polymorphisms of the high mobility group box 1 protein gene in patients with major trauma in Southwest China[J].Surgery Volume, 2012, 151(3):427-436.
[19] Deng C Q, Wang Y M.HMGB1 gene polymorphisms in patients with chronic hepatitis B virus infection[J]. World J Gastroenterol, 2013,19(31): 5144-5149.
[20]周钢桥,贺福初,张红星.中国人群肝癌的易感基因研究[J].中国科学杂志, 2011, 41(5): 785-789.
The Correlation of Genetic Polymorphisms of HMGB1 to Hepatitis B Virus-related Hepatocellular Carcinoma/
WANG Dan,DENG Chun-qing,ZHAO Long-feng.//Medical Innovation of China,2014,11(12):013-016
Objective:To study the possible association of the genetic polymorphism of high mobility group protein B1 (HMGB1) 1176 G/C intron 4 with the susceptibility to hepatocellular carcinoma (HCC)after hepatitis B virus (HBV)infection.Method:110 patients with HBV-related HCC and 316 patients of HBV infection without HCC after HMGB1 intron4 1176G/C polymorphism were detected by polymerase chain reaction-restriction fragment length pdymo-rphism (PCR-RFLP)method, chi-square test and unconditioned logistic regression model were applied to analysis results.Result:There were significant statistically difference in the three genotypes among the HBV-related HCC group and the chronic HBV infection group(χ2=6.152,P=0.046;χ2=5.605,P=0.018). There were significant difference (P=0.023) between HCC and CHB under the dominant model either, hepatitis group of AsC,AsC+CHB different from HCC group(P =0.048, 0.028)under the recessive model respectively. There were significant difference between HCC and AsC+CHB under codominant model(P=0.03).Conclusion:The results suggested that the genotype of HMGB1 intron4 1176 G/C is associated with the susceptibility to HBV-induced hepatocellular carcinoma.
High mobility group protein B1; Mononucleotide; Hepatitis B virus; Polymorphisms;Hepatocellular carcinoma
10.3969/j.issn.1674-4985.2014.12.005
2014-02-12) (本文编辑:黄新珍)
山西医科大学博士科研启动基金(YB0605)
①山西医科大学第一附属医院 山西 太原 030001
邓春青
First-author’s address: The First Affiliated Hospital of Shanxi Medical University,Taiyuan 030001,China
