Cloning and Sequence Analysis of Y-box Binding Protein Gene in Min Pig
2014-03-07ZhangDongjieLiuDiWangLiangHeXinmiaoandWangWentao
Zhang Dong-jie, Liu Di, Wang Liang, He Xin-miao, and Wang Wen-tao
Institute of Animal husbandry, Heilongjiang Academy of Agricultural Sciences, Harbin 150086, China
Cloning and Sequence Analysis of Y-box Binding Protein Gene in Min Pig
Zhang Dong-jie, Liu Di*, Wang Liang, He Xin-miao, and Wang Wen-tao
Institute of Animal husbandry, Heilongjiang Academy of Agricultural Sciences, Harbin 150086, China
In order to study the gene sequence of Min pig Y-box binding protein (YB-1) gene, the complete coding sequence of Min pig YB-1 gene was cloned by RT-PCR, the sequence features were analyzed by some software and online website. The results showed that the complete CDS of Min pig Y-box was found to be 975 bp long, encoding 324 amino acids. It contained a conserved cold shock domain and several phosphorylation sites, but had no transmembrane domains, and was consistent with a protein found in the cytoplasm. Min pig YB-1 nucleotides shared high similarity (61.37% - 97.66%) with other mammals.
Min pig, Y-box binding protein, sequence feature
Introduction
YB-1, a transcription factor first identified by its ability to bind to the inverted CCAAT box (Y-box), has been implicated in gene transcription, cell proliferation, and cisplatin resistance (Uramoto et al., 2002). YB-1 is composed of three domains, an aminoterminal alanine-proline-rich region, a centrally located cold shock domain (CSD), and a carboxyl-terminal region characterized by four alternating clusters of basic and acidic amino acids (Bader et al., 2008). CSD sequence of human YB-1 gene exhibits >40% identity and >60% similarity to the major Escherichia coli coldshock protein CspA (Matsumoto et al., 1998). A blocked mRNA 5' end is required for YB-1-mediated stabilization (Valentina et al., 2001). YB-1 is an oncogenic transcription and translation factor and is overexpressed in several types of cancer. It is an important transcription factor regulating proliferation, survival, migration, invasion and chemosensitivity of cancer cells. It is activated and translocated to the nucleus after Ser102-phosphorylation in DNA binding domain (Sinnberg et al., 2012).
Factors that create stress during the winter months are cold, wind, snow, rain and mud. The primary effect on animals is due to the temperature. Swine are rather cold-susceptible and therefore are usually kept in heated housing when raised in colder regions. Low temperature has multiple effects on internal structure and function in animals. Primary among these is an increased resting metabolic rate, and hence an increased energy requirement for maintenance, which results in reduced digestive efficiency (Young, 1981). Heilongjiang Province is the northernmost part of China, average temperatures remain below freezing for up to 120 days each winter, and mean daily temperatures of January and February range from–10℃ to –20℃, with temperatures of –30℃ and below not uncommon. Min pig is a major breed of the North-China type and Min pigs are noted for their tolerance to cold. In severely cold weather (–20℃), Min pigscan stay in the open much longer without shivering or squealing. The objective of the present study was to clone and characterize the complete coding sequence of Min pig Y-box binding protein and compare the obtained cDNA sequence with those of other species.
Materials and Methods
Animals
75-day-Min pigs were killed for the cloning and spatial expression pattern analysis of YB-1 gene. Large intestine, muscle, lung, fat, heart, spleen and liver were harvested, snap-frozen in liquid nitrogen and stored at–70℃. Total RNA was extracted from various tissues using a Trizol reagent (Invitrogen, Grand Island, USA), according to the manufacturer's instructions.
cDNA cloning of Min pig YB-1
Based on EST sequence available in GenBank (AK239202), primers were designed to amplify the entire coding region of the pig YB-1 gene. Total cDNA from muscle was used as template to amplify YB-1 gene by reverse transcription (RT)-polymerase chain reaction (PCR). Reverse transcription of total RNA was performed by Superscript II (Gibco) according to the manufacturer's protocol, and one primer pair was designed to amplify the full coding region of YB-1 (primers A, forward 5'-ATGAGCAGCGAGGCCG AG-3' and reverse 5'- TTACTCAGCCCCGCCCT G -3'). 50 μL PCR mixtures contained 2.0 μL of 50 ng • μL-1cDNA, 5 μL of 10×buffer, 4.0 μL of 2.5 mmol • L-1dNTPs, 0.4 μL rTaq polymerase (TaKaRa Bio Inc.), and the remaining volume of ddH2O. PCR was performed in Bio-Rad thermal cyclers in a program of 94℃ predenaturing for 5 min, followed by 30 cycles of 30 s at 94℃, 30 s at 54℃, 1 min at 72℃, followed by a final extension of 10 min at 72℃. PCR products were checked by DNA electrophoresis in 1.0% agarose gel and purified by D2500-01 reaction kit (Omega Bio-tek). Target DNAs were then cloned into PMD18-T Easy plasmid vector (TaKaRa Bio Inc.) and sent for sequencing to Invitrogen Co., Ltd.
Sequence analysis
Overlapping fragments amplified by RT-PCR were assembled by DNAMAN package Version 5.2.2. The conserved domain was analyzed using the Prosite Database (http://cn.expasy.org/prosite/). Homologous nucleotide sequence was searched by using BLAST (Blastp, TBlastX, http://www.ncbi.nlm.nih.gov/ BLAST) and Pfam (http://www.sanger.ac.uk/Software/ Pfam/).
Results
Sequence analysis ofYB-1 gene
Based on sequence homology, the designed primers amplified a single 1.0 kb fragment which showed homology to other mammalian YB-1 when sequenced. The sequence analysis showed 975 bp long open reading frame of YB-1 gene encoding 324 amino acids. The deduced 324 amino acid sequences showed that the predicted protein had an estimated 35.9 ku molecular weight. The conserved domains using the NCBI (Conserved Domains program) and comparing with human YB-1 showed a highly conserved cold shock domain (located between amino acids 52-133) from Min pig YB-1 mRNA sequence. TMpred and TMHMM software predicted YB1 protein did not contain any transmembrane regions. It was mainly present in the cytoplasm. The domain signature using PROSITE showed a cold-shock domain signature among amino acids 72-91 of YB-1 mRNA coding sequence. Likewise, NetPhos 2.0 Server software predicted the localization of the phosphorylation sites: seven serine phosphorylation sites (located in the amino acid residues 21, 36, 102, 167, 174, 176 and 314), four threonine phosphorylation sites (located in the amino acid residues 25, 30, 62 and 80), and three tyrosine phosphorylation sites (located in the amino acid residues162, 197 and 208) (Fig. 1).
BLAST analysis showed that Min pig YB-1 nucleotides shared high similarity (61.37%-97.66%) with other mammals (Table 1).

Fig. 1 Nucleotide and predicted amino acid sequence of YB-1
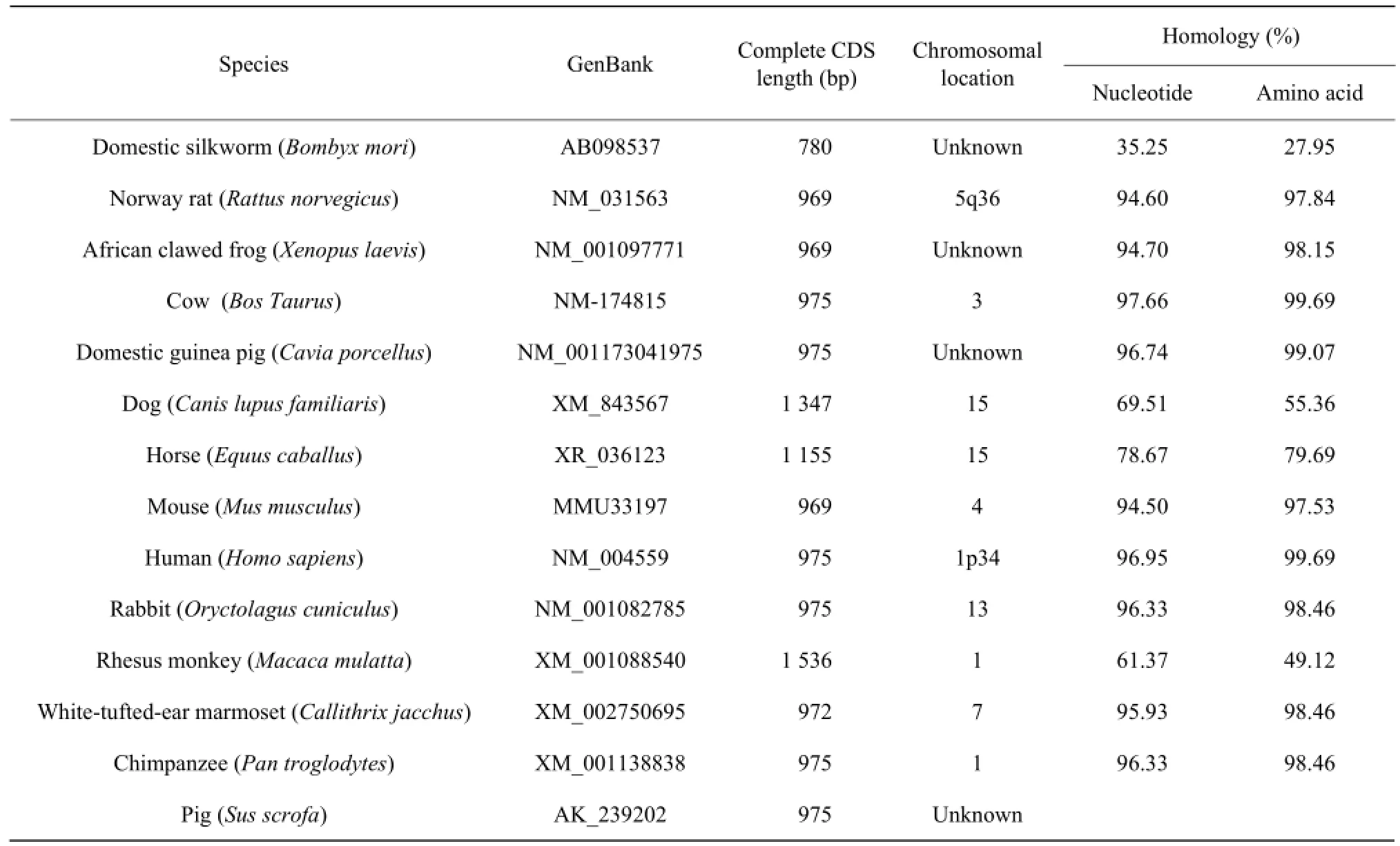
Table 1 Nucleotide and protein sequence homology of Min pig YB-1 with other species
Discussion
In plants and bacteria, YB-1 mainly acts as a molecular chaperone (Karlson et al., 2002) at low temperature or a protein which responds to low temperature environment (Salvetti et al., 2002). In invertebrates and vertebrates, it mainly acts as a transcription factor and promotes cell proliferation (Skehel and Bartsch, 1994; Zhang et al., 2006).
Sequence analyses of YB-1 gene of Min pig revealed a CSD conserved region between amino acids 52-133. We also found multiple phosphorylation sites, suggesting that post-translational processing is complex, which may be related to the variety of biological functions of YB-1 (Jin et al., 2008). Ser102, which located in DNA-binding motif of YB-1, is a known important phosphorylate site. YB-1 plays a key role in both PI3K/Akt and MAPK pathways by this site (To et al., 2010). In addition, on phosphorylation by AKT or RSK at Ser102, YB-1 translocates into nucleus where it regulates transcription by binding to Y-box (sequence motif CTGATTGG) in the promoter regions of growth-promoting genes, such as Her-2 and EGFR (Gao et al, 2009). Y-box motif is located in 3'-UTR of YB-1 gene, not in ORF. Sequence alignment revealed that pig YB-1 proteins are closer to cow and human YB-1 than to those of the other species. Some studies have suggested that gene duplication and alternative splicing events might be an important mechanism of the evolution of vertebrate YB-1 gene family (Ulusu and Tezcan, 2001).
Bader A G, Felts K A, Jiang N, et al. 2008. Y box-binding protein 1 induces resistance to oncogenic transformation by the phosphatidylinositol 3-kinase pathway. Proc Natl Acad Sci U S A, 100(21): 12384-12389.
Gao Y, Fotovati A, Lee C, et al. 2009. Inhibition of Y-box binding protein-1 slows the growth of glioblastoma multiforme and sensitizes to temozolomide independent O6-methylguanine-DNA methyltransferase. Mol Cancer Ther, 8(12): 3276-3284.
Jin P, Zhang W W, Huang H F, et al. 2008. Cloning and analysis of Lyb gene from Lampetra japonica. Hereditas (Beijing), 30(12): 1597-1602.
Karlson D, Nakaminami K, Toyomasu T. 2002. A cold-regulated nucleic acid-binding protien of winter wheat shares a domain with bacterial cold shock proteins. J Biol Chem, 277(38): 35248-35256.
Matsumoto K, Wolffe A P. 1998. Gene regulation by Y-box proteins: coupling control of transcription and translation. Trends Cell Biol, 8(8): 318-323.
Salvetti A, Lena A, Rossi L, et al. 2002. Characterization of DeY1, a novel Y-box gene specifically expressed in differentiating male germ cells of planarians. Gene Expr Patterns, 2(3-4): 195-200.
Sinnberg T, Sauer B, Holm P, et al. 2012. MAPK and PI3K/AKT mediated YB-1 activation promotes melanoma cell proliferation which is counteracted by an autoregulatory loop. Exp Dermatol, 21(4): 265-270.
Skehel P A, Bartsch D. 1994. Characterization of a Y-Box factor from Aplysia californica. Gene, 145(2): 231-235.
To K, Fotovati A, Reipas K M, et al. 2010. YB-1 induces expression of CD44 and CD49f leading to enhanced self-renewal, mammosphere growth, and drug resistance. Cancer Res, 70(7): 2840-2851.
Ulusu N N, Tezcan E F. 2001. Cold shock proteins. Tr J Med Sci, 31: 283-290.
Uramoto H, Izumi H, Ise T, et al. 2002. p73 interacts with c-Myc to regulate Y-box-binding protein-1 expression. J Biol Chem, 277(35): 31694-31702.
Valentina E, Peter R, Hiroaki I, et al. 2001. The major mRNA-associated protein YB-1 is a potent 5' cap-dependent mRNA stabilizer. EMBO J, 20(19): 5491-5502.
Young B A. 1981. Cold stress as it affects animal production. J Anim Sci, 52: 154-163.
Zhang W W, Huang H F, Li Q W, et al. 2006. Functions of Y-box binding protein and its role in tumorigenicity. Hereditas (Beijing), 28(9): 1153-1160.
Q78
A
1006-8104(2014)-01-0052-04
Received 21 January 2013
Supported by China Agricultural Research System (CARS-36)
Zhang Dong-jie (1980-), female, associate professor, Ph. D, engaged in the research of animal genetics and breeding. E-mail: djzhang8109@163.com
* Corresponding author. Liu Di, professor, supervisor of Ph.D student, engaged in the research of animal genetics and breeding. E-mail: liudi1963@163.com
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Study on Relationship Between Cucumber Germplasm and Propamocarb Residue Using Subjective Rating Technique
- Cloning and Expression Analysis of Mlo Gene from Pericallis hybrida B. Nord.
- Simulation of in situ Root Decomposition of Two Barley Cultivars
- Vertical Migrating and Cluster Analysis of Soil Mesofauna at Dongying Halophytes Garden in Yellow River Delta
- Effect of MSTN Propeptide and shRNA Co-expression Vector on Proliferation of Skeletal Muscle Satellite Cells
- Application of Protein Feed Processed by Microbial Fermentation to Dairy Cow
