Vertical Migrating and Cluster Analysis of Soil Mesofauna at Dongying Halophytes Garden in Yellow River Delta
2014-03-07HeFuxiaXieTongyinXieGuilinandFuRongshu
He Fu-xia, Xie Tong-yin, Xie Gui-lin, and Fu Rong-shu
1College of Life Sciences, Northeast Agricultural University, Harbin 150030, China
2College of Life Sciences, Shandong Normal University, Jinan 250014, China
Vertical Migrating and Cluster Analysis of Soil Mesofauna at Dongying Halophytes Garden in Yellow River Delta
He Fu-xia1, Xie Tong-yin1, Xie Gui-lin1, and Fu Rong-shu2
1College of Life Sciences, Northeast Agricultural University, Harbin 150030, China
2College of Life Sciences, Shandong Normal University, Jinan 250014, China
For the first time, we used Tullgren method made a study on vertical migrating and cluster analysis of the soil mesofauna in Dongying Halophytes Garden in the Yellow River Delta (YRD), Shandong Province. The results showed that the soil mesofauna tended to gather on soil surface in most samples at most times, but the vertical migrating greatly varied in different seasons or environment conditions. Acari was the dominant group. The index of diversity of the soil fauna was correlated with the index of evenness. The Acari's number of individuals infected other species and numbers. Dominant group-Acari made greater contribution to the result of cluster analysis, and there were significant differences between communities in different habitats by cluster analysis with both Bray-Curtis and Jaccard similarity coefficient.
halophytes, soil mesofauna, vertical migrating, cluster analysis
Introduction
Soil mesofauna are those which influence soil conditions and spend one or more life stages in the soil during their life history (Yin, 1992). It is well known that soil biota play very important roles in decomposition of soil organic matter and nutrient cycling (Wardle et al., 2004). Soil mesofauna are important components of soil ecosystem and play important roles in several aspects, such as participating in soil formation, keeping the stability of physical and chemical structure of soil, speeding the decomposition of organic matter, and improving the water holding capacity of soil (Ruiter et al., 1998; Rusek, 1998; Ponge et al., 2003; Wardle et al., 2004; Ge et al., 2005; Xu et al., 2007; Fu, 2007). In China, although some basic researches on soil biodiversity had been carried out during the last two decades (Yin, 1998, 2000; Liao and Chen, 1990; Qiu, 1999), it did not attract enough attention until recently. In order to understand the ecology of soil mesofauna in saline land, and uncover its vertical migration, the present paper studied the vertical distribution of soil mesofauna and made a clustering analysis in Dongying Halophytes Garden in the Yellow River Delta (YRD), Shandong Province (Fu and Liu, 2004; Chen, 1983).
Materials and Methods
Field investigation and specimen collection
Dongying Halophytes Garden of the Yellow River Delta (YRD) located in (118°04′-119°14′E, 35°15′-38°16′N). Nine halophyte species on the basis of artificial planting vegetation and 10 sampling points in total were selected. (I) Limonium bicolor, (II) Scorzonera mongolica Maxim, (III) Puccinellia tenuiflora, (IV) Lonicera japonica, (V) Tamarix chinensis Lour,(VI) Poplar sp., (VII) Suaeda salsa, (VIII) Iris lacteal, (IX) Atriplex centralasiatica, and (X) Iris lacteal continuous cropping 10 years. We divided the soil into three layers: 0-5, 5-10, and 10-15 cm, and took stochastic sampling each layer for 3 times. Then, we put soil samples in PE pocket for lab testing (Chen, 1983). We persisted in collecting specimens every month from October 2008 to October 2009, 13 times in total. Test procedure was followed by Yin (1992; 1998), Tian et al. (2001), and Xie et al. (2011).
Environmental factor
We used WET Sensor HH-2 tested three environmental factors: water content, temperature and conductivity.
Data analysis
We analyzed the data using Microsoft Excel and Bio-Diversity Software of Professional. The soil mesofauna' quantitative study was carried out utilizing the following ecological indexes:
Shannon-Weiner diversity index: H =–∑Pi • lnPi;
Pielou evenness index: E=H/lnS;
Simpson dominance index: C=∑(ni/N)2;
Jaccard similarity coefficient: PS= 2c/(a+b);
Where, S: number of animal's groups; N: number of individuals; ni: number of individuals in every group; Pi: the percent of which number of individuals in every group account for all total individuals of numbers; a: the number of individuals of a given species in the community A; b: the number of individuals of given species in the community B; c: the number of individuals of common species for the communities A and B.
Results
Vertical distribution
From October 2008 to October 2009, the soil mesofauna' vertical distribution trends obviously changed in (I) Limonium bicolor, (III) Puccinellia tenuiflora, (IV) Lonicera japonica, (V) Tamarix chinensis Lour, (VI) Poplar sp., (VIII) Iris lacteal, (X) Iris lacteal continuous cropping 10 years, but excepted for (II) Scorzonera mongolica Maxim, (VII) Suaeda salsa, and (IX) Atriplex centralasiatica (Table 1). The reason for this phenomenon was (II) Scorzonera mongolica Maxim in the region had not formed apparent edificators; people's activities in (VII) Suaeda salsa and (IX) Atriplex centralasiatica mightbe the cause.
The soil's vertical distribution was altered when the temperature changed at different seasons. In summer, the soil temperature in upper layer was higher than lower layer, it was one of good conditions for soil mesofauna to feed activities. The vertical distribution and surface aggregation phenomenon were obvious in October 2008, April 2009, May 2009, June 2009, July 2009, August 2009, September 2009, and October 2009 at all sampling points.
During the colder months, November 2008, December 2008, January 2009, February 2009, and March 2009, soil temperature reduced with the outside ambient temperature. The upper layer was reduced faster than lower layer, soil mesofauna had to passively adapt to the temperature changes in the soil, and the soil mesofauna lived in superficial soil phenomenon was not obvious and in turn inverse distribution phenomenon was more common.
Community diversity
The soil mesofauna' density was related to the soil electric conductivity. The soil mesofauna' density was lower when the soil contained much salt and the conductivity much higher. The salt-tolerant plants planting would decrease the soil salinity. Then the number of soil mesofauna and density in each group would be increased when the soil electric conductivity value located in lower level (Table 2).
The change of the community diversity index was correlated with number of communities and number of individuals in various groups. The more numbers of the groups in community and numbers of individuals in various groups had, the higher the diversity index was. The highest evenness index was (V) Tamarix chinensis Lour, E=0.4608, and the lowest evenness index was (X)Iris lacteal continuous cropping 10 years, E=0.1841. The dominance index was inversely proportional to the evenness index. In this text, the highest dominance index was (X) Iris lacteal continuous cropping 10 years, C=0.789 (Table 2). (IV) had the highest community diversity index for H=1.082, and the lowest community diversity index was (X) Iris lacteal continuous cropping 10 years, H =0.4575 (Table 2).
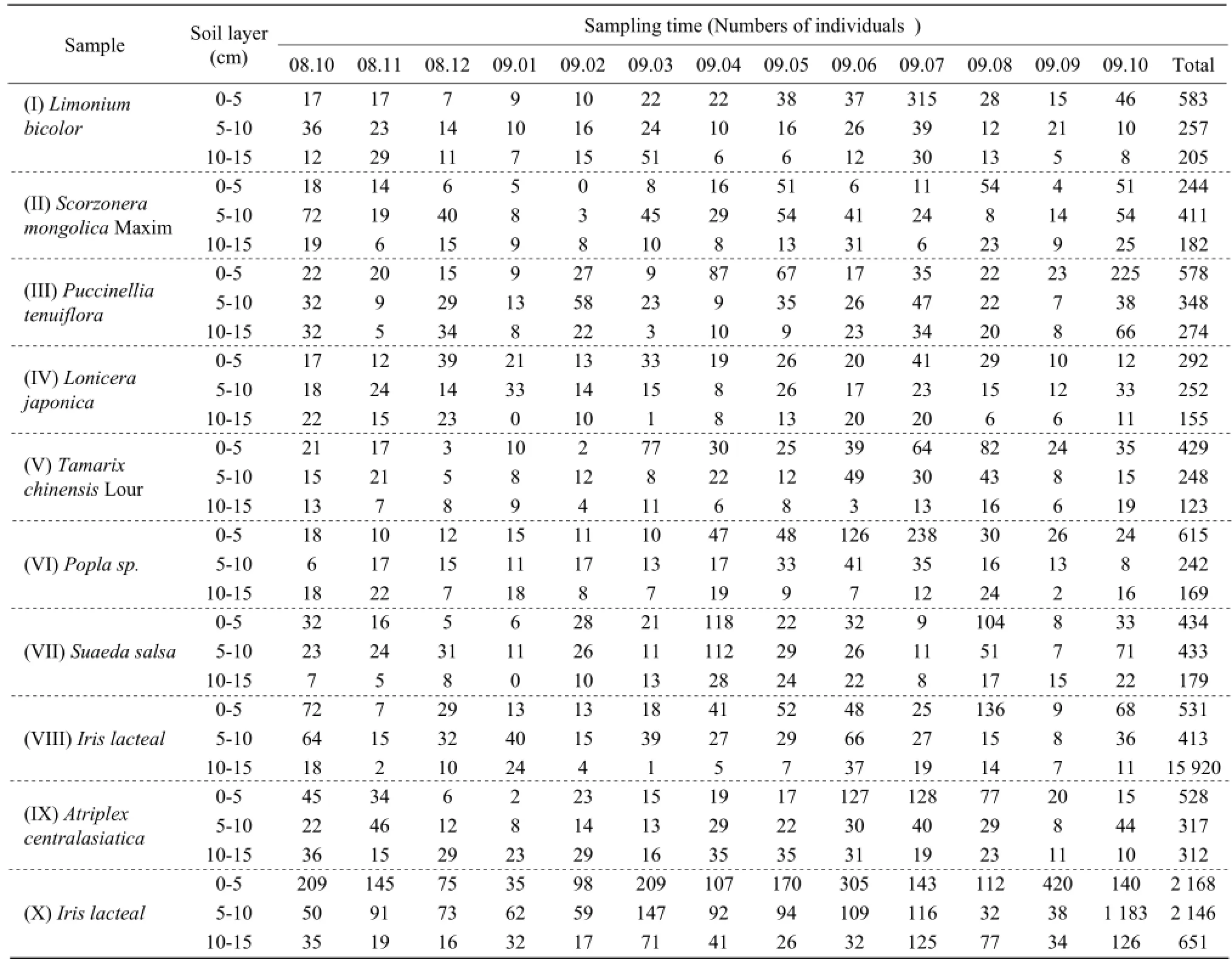
Table 1 Vertical distribution of soil mesofauna groups

Table 2 Indexes of soil mesofauna structure
Community similarity
According to the Jaccard coefficient similarity principle, community similarity was the lowest when q value between 0 and 0.25; community similarity was low when q value between 0.25 and 0.5; community similarity was moderate when q between 0.5 and 0.75; community similarity was large when q value between 0.75 and 1.0. Jaccard similarity coefficient (Table 3 and Fig. 1) with all the 45 groups q value were larger than 0.5 exhibited a moderate or large similar. Because in Jaccard coefficient formula, only counted both community common species number and ignored the number of animals' individual influence in each group, Jaccard similarity coefficient method could weaken the advantage group effecting in clustering results. Bray-Curtis similarity coefficient cluster (Table 4 and Fig. 2) showed the 10 sampling points could be divided into nine groups in 0.85 level. (I) Limonium bicolor and (VII) Suaeda salsa had the highest similarity, which was equal to 94.15%, (II) Scorzonera mongolica Maxim and (VII) Suaeda salsa was 85.33%. The lowest similar community was (V) Tamarix chinensis Lour and (X) Iris lacteal continuous cropping 10 years just only 23.28%. The number of Acari was large and affected Bray-Curtis similarity coefficient result. The density of dominant group made greater contribution to the result of cluster analysis.
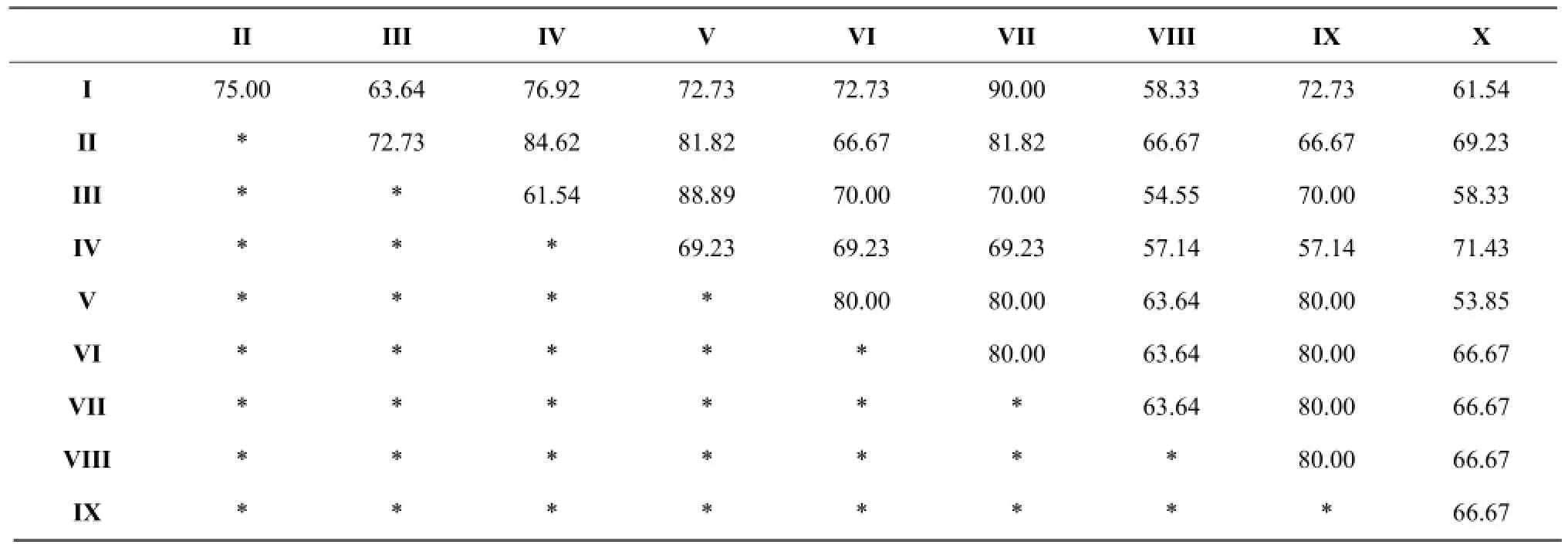
Table 3 Jaccard cluster analysis of soil mesofauna
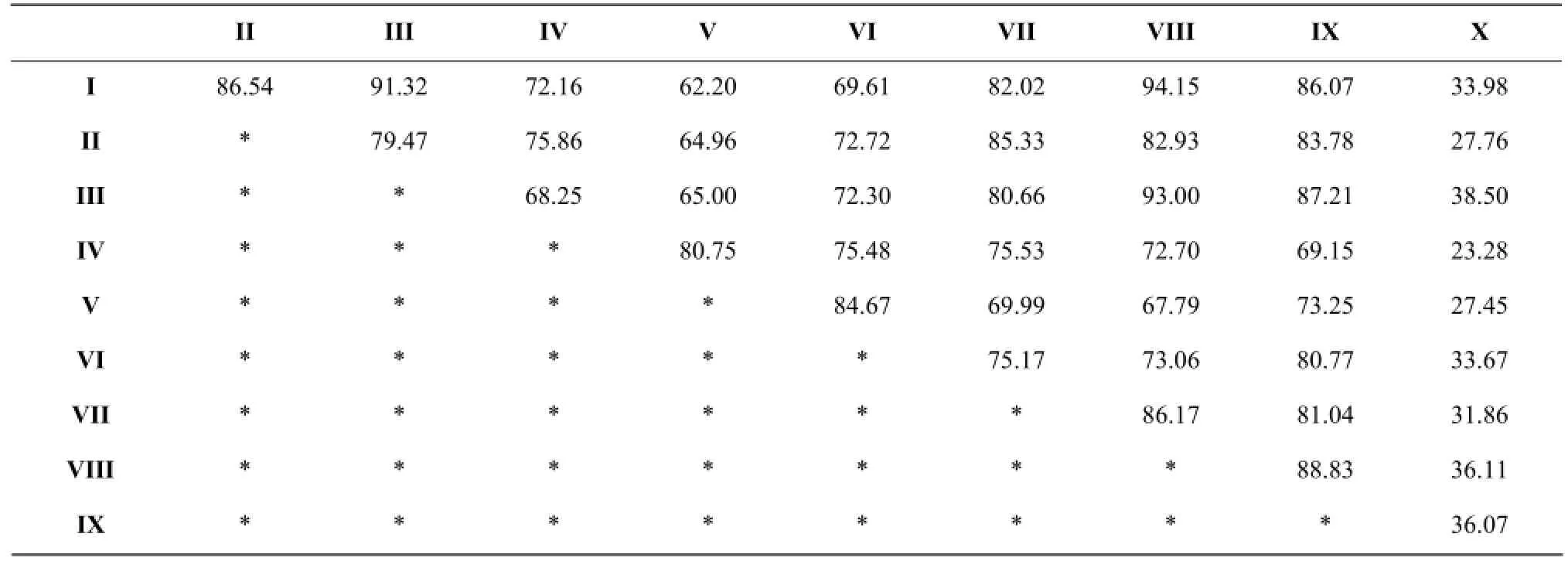
Table 4 Bray-Curtis cluster analysis of soil mesofauna
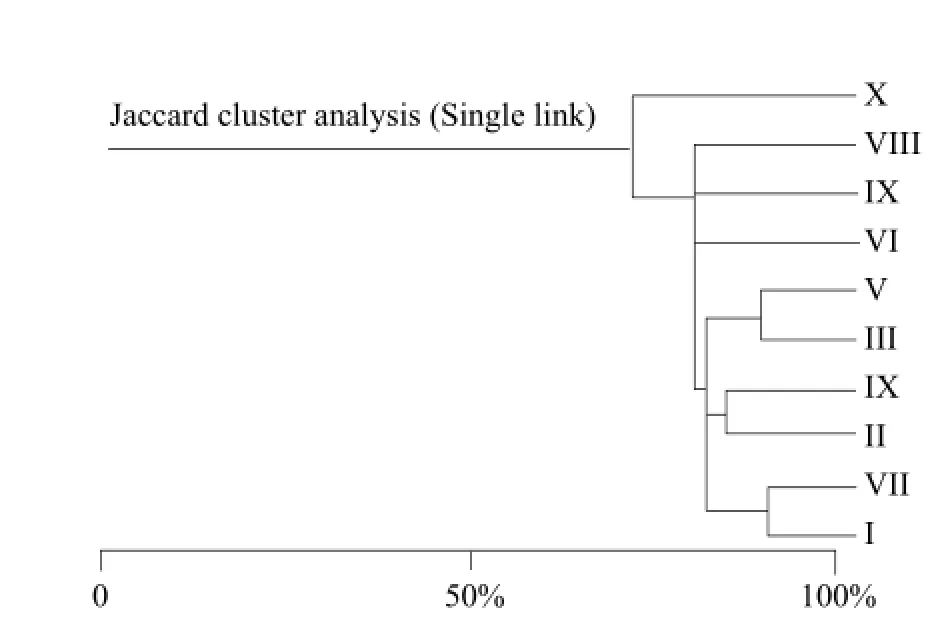
Fig. 1 Jaccard cluster analysis
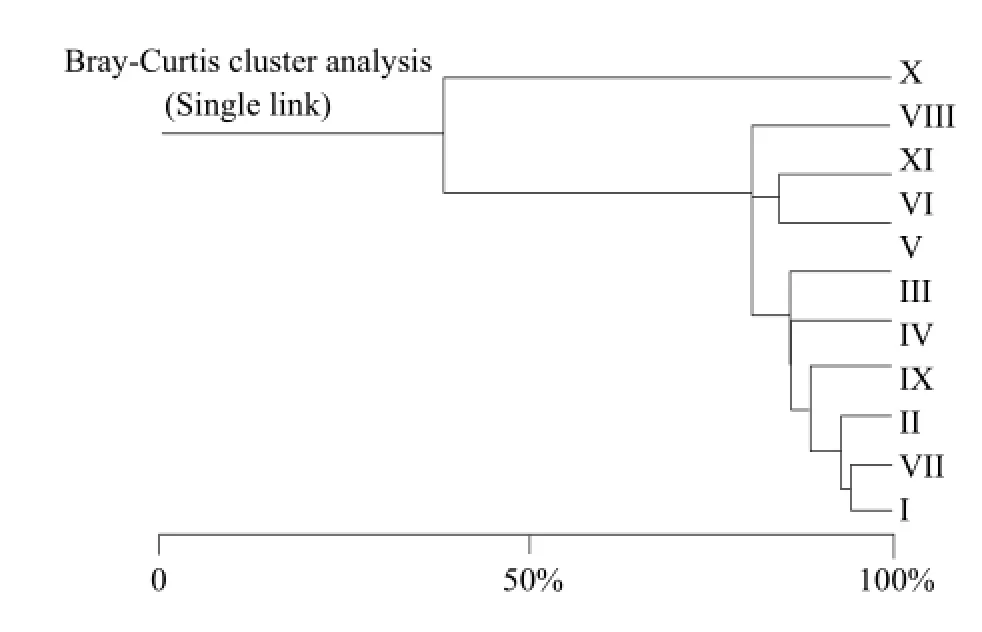
Fig. 2 Bray-Curtis cluster analysis
Discussion
Due to intensive agrotechnical measures and monocultural crops soil mesofauna' density are usually much lower (Edwards and Lofty, 1975; Rusek, 1998). The density of soil mesofauna found in various crops by Czarnecki (1989) varied from 5×103ind • m-2to 41×103ind • m-2. We found densities of soil mesofauna between 0.89×104ind • m-2and 6.36×104ind • m-2of this area in present study. Mean density of soil mesofauna (3.63×104ind • m-2) noted in this study was close to maximum densities reported by cited authors.
Soil mesofauna are ectothermic. The soil's vertical distribution is altered when the temperature changed at different seasons. In summer, the soil temperature in upper layers is higher than in lower layers, and it is one of good conditions for soil mesofauna to feeding activities. The vertical distribution and surface aggregation phenomenon are obvious during this time. In winter, the soil temperature in lower layers is higher than that in upper layers, in order to suit for the temperature changes, soil mesofauna move in lower soils. Then the inverse distribution phenomenon is more common.
Natural communities are generally characterized by having many rare species and several abundant ones (Koehler, 1999; Peter and Dana, 2012). The change of the community diversity index is correlated with number of communities and number of individuals in various groups. The more numbers of the groups in community and number of individuals in various group have, the larger the diversity index is.
Oribatid mites are considered as one of the most abundant groups within soil mesofauna in various habitats with hundreds of thousands of individuals per square meter (Coreman, 1996). On the other hand, conventionally managed crop soils are in general characterized by poor oribatid communities due to the detrimental impact of agricultural practices (Fujita and Fujiyama, 2001; Gulvik, 2007). The usual abundance of Oribatida in such habitats constitutes several hundred or at most thousands of individuals per square meter (Bedano et al., 2006; Minor and Cianciolo, 2007). However, clearly higher values of mean oribatid abundance were observed within field sites in the present study, which was probably due to the more used efficient extraction methods. In Dongying Halophytes Garden, Acari was a dominant group. The large quantity of Acari affected the rest of other species and numbers. Dominant groups made the greatest contribution to the result of the cluster analysis.
Conclusions
In Dongying Halophytes Garden in the YRD, soil mesofauna vertical distribution was mainly affected by temperature, conductivity and people's activities.
The number of soil mesofauna and density would be increased along with the salt-tolerant plants plantingyear by year. The density of dominant group made great contribution to the result of cluster analysis.
Acknowlegements
We would like to express our sincere thanks to Dr. Zhu Gengping and Liu Tengteng (Institute of Entomology, Nankai University) for reviewing the manuscript and providing valuable comments.
Bedano J C, Cantú M P, Doucet M E. 2006. Influence of three land management practices on soil mite (Archnida: Acari) densities in relation to a natural soil. Soil Ecology, 32: 293-304.
Chen P. 1983. Soil animals collection and investigation method. Chinese Journal of Ecology, 2: 46-51.
Czarnecki A. 1989. Collembola as a component of biological system of the areas under strong anthropopresion. Toruń Press, Poland. pp. 1-156. (in Polish).
Edwards C A, Lofty J R. 1975. The influence of cultivations on soil animal populations. Soil Zoology, 399-407.
Fu R S, Liu L D. 2004. Tutorial of ecology experiment. Science Press, Beijing. pp.151-162.
Fu S L. 2007. A review and perspective on soil biodiversity research. Biodiversity Science, 15(2): 109-115.
Fu S L, Zou X M, Coleman D. 2008. Highlights and perspectives of soil biology and ecology research in China. Soil Biology & Biochemistry, XXX: 1-9.
Fujita M, Fujiyama S. 2001. Comparison of soil fauna (Oribatids and Enchytraeids) between concentional and organic (tillage and notillage practices) farming crop fields in Japan. Pedosphere, 11(1): 11-20.
Gulvik M. 2007. Acari as indicators of soil biodiversity and land use monitoring. Pol Journal of Ecology, 55(3): 415- 440.
Ge B M, Kong J M, Cheng H Y, et al. 2005. Community structure of soil macrofauna in different using types of soils in autumn. Zoological Research, 26(3): 272-278.
Koehler H H. 1999. Predatory mites (Gama sina, Mesostigmata). Agric Ecosystem Environment, 74: 395-410.
Liao C H, Chen M Q. 1990. A study on the secondary succession and development of soil animal community in tropical artificial forest. Chinese Journal of Applied Ecology, 1(1): 53-59.
Minor M A, Cianciolo J M. 2007. Diversity of soil mites (Acari: Oribatida, Mesostigmata) along a gradient of land use types in New York. Soil Ecology, 35: 140-153.
Peter Luptácik, Dana Miklisová et Lubomír Ková. 2012. Diversity and community structure of soil Oribatida (Acari) in an arable field with alluvial soils. European Journal of Soil Biology, 50: 97-105.
Ponge J F, Gillet S, Dubs F. 2003. Collembolan communities asbioindicators of land use intensification. Soil Biology and Biochemistry, 35: 813-826.
Qiu J P. 1999. Earthworms and their application in environment protection I. Earthworms and their functions in ecosystem. Journal of Shanghai Agricultural College, 17(3): 227-232.
Ruiter P C, Neutel A M, Moore J C. 1998. Biodiversity in soil ecosystems the role of energy flow and community stability. Applied Soil Ecology, 10: 217-228.
Rusek J. 1998. Biodiversity of collembolan and their functional role in the ecosystem. Biodiversity and Conservation, 7: 1207-1279.
Tian J Y, Pan H J, Fu R S. 2001. Study on soil animals diversity in the Yellow River Delta. Chinese Biodiversity, 9(3): 228-236.
Wardle D A, Bardgett R D, Klironomos J N, et al. 2004. Ecological linkages between aboveground and belowground biota. Science, 304: 1629-1633.
Xie G L, Xie G W, Liu J L, et al. 2005. The seasonal variation and vertical migrating of soil mesofauna of Heze Peony Garden. Journal of Heze Teachers College, 27(2): 46-51.
Xie T Y, Fu R S, Tian J Y. 2010. Community structure of soil mesofauna and microfauna at shell islands of Yellow River Delta. Journal of Shandong Normal University, 25(6): 112-118.
Xie T Y, Xie G L, He F X, et al. 2011. Community structure of collembolans at shell island of Yellow River delta. Journal of Northeast Agricultural University, 42(9): 92-96.
Xu J, Ke X, Song J, et al. 2007. Role of collembolan in assessment of ecological risk of heavy mental contamination of soils. Acta Pedologica Sinica, 45(3): 544-549.
Yin W Y. 1992. Subtropical soil animals of China. Science Press, Beijing. pp. 4-40.
Yin W Y. 1998. Pictorial keys to soil animals of China. Science Press, Beijing. pp.70-121.
Yin W Y. 2000. The community structure and dynamics of soil animals in subtropical forest region. In: Yin W Y. Soil animals of China. Science Press, Beijing. pp. 70-77.
Q958
A
1006-8104(2014)-01-0025-06
Received 15 September 2012
Supported by the Doctoral Fund of Northeast Agricultural University (2009RC41); Postdoctoral Grants of Heilongjiang Province (LBH-Z10265) He Fu-xia (1978- ), female, lecturer, engaged in the research of biological chemistry and ecology. E-mail: hefuxia969@163.com
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Study on Relationship Between Cucumber Germplasm and Propamocarb Residue Using Subjective Rating Technique
- Cloning and Expression Analysis of Mlo Gene from Pericallis hybrida B. Nord.
- Simulation of in situ Root Decomposition of Two Barley Cultivars
- Effect of MSTN Propeptide and shRNA Co-expression Vector on Proliferation of Skeletal Muscle Satellite Cells
- Application of Protein Feed Processed by Microbial Fermentation to Dairy Cow
- Cloning and Sequence Analysis of Y-box Binding Protein Gene in Min Pig
