多孔载体负载型Fenton催化剂降解酚类污染物的研究进展
2014-03-04徐小妹潘顺龙李健生孙秀云沈锦优韩卫清王连军
徐小妹,潘顺龙,李健生,孙秀云,沈锦优,韩卫清,王连军
(南京理工大学环境与生物工程学院,江苏 南京 210094)
Fenton催化法作为一种高级氧化技术,因其具有反应装置简单、反应条件温和、运营成本低等优势,在有机废水的处理领域备受关注[1-3]。均相催化剂对Fenton反应有很高的催化活性,但均相Fenton体系存在很多问题,如将 Fe3+还原为 Fe2+速率缓慢[4-5]、反应受pH值限制大[6-7]、含铁污泥产生量大等[8-10]。这些缺点限制了均相Fenton法的应用。自2000年以来,非均相 Fenton催化技术越来越受到国内外研究人员的重视。图1 为Web of Science中以“Heterogeneous”和“Fenton”为关键词检索到的论文数。可见,非均相Fenton催化技术快速成为近年来的热点。
非均相 Fenton催化是利用活性颗粒组分将双氧水(H2O2)转化为羟基自由基(·OH)来完成有机污染物降解的。因此,可以实现对固体催化剂的分离和重复利用。特别是多孔载体负载型非均相Fenton催化,能够将多孔载体的吸附性能和纳米催化粒子的高反应活性有效结合,正成为高级氧化技术中的重要研究方向。尽管负载型Fenton催化取得了显著进展,但该技术目前尚存在一些不足之处,如催化剂投加量大、H2O2用量大、催化剂稳定性差[11]、寿命短[12]等,在工业上的应用仍受到一定的限制。
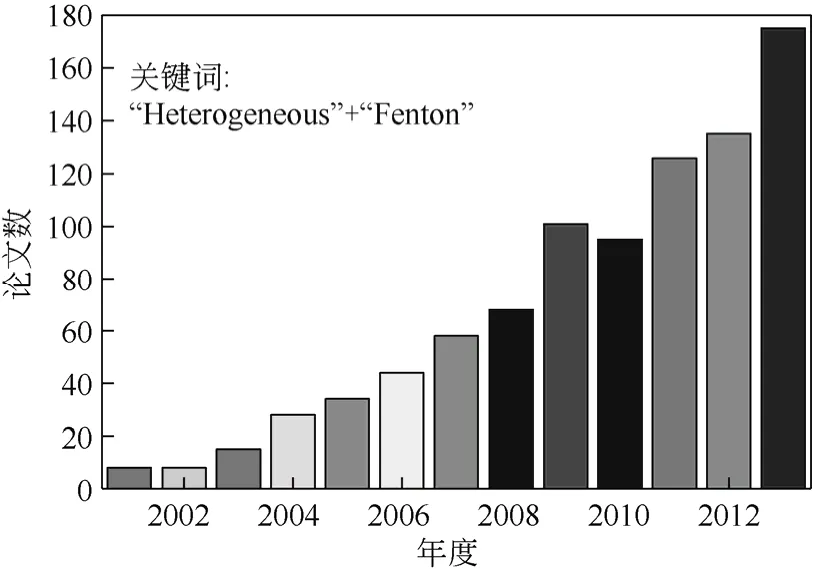
图1 Web of Science中以“Heterogeneous”和“Fenton”为关键词检索到的论文数
本文以载体种类为基线,回顾了2000年以来负载型Fenton催化剂处理酚类污染物的研究进展。重点讨论了催化剂对酚类有机物的去除效率、活性组分的溶出以及重复利用的性能,并指出了负载型Fenton催化氧化技术今后的研究方向。
1 多孔载体负载型 Fenton催化剂降解酚类
多孔载体负载型 Fenton催化剂同时具有多孔载体的吸附性能与催化剂的催化性能,更有利于降解有机物。该型催化剂降解有机物是一个十分复杂的过程,包括反应物向催化剂表面的扩散和在反应位点的吸附、反应过程中电子的转移、产物的脱附、活性位点的再生等[13]。因此,非均相Fenton反应的影响因素很多,主要有反应体系的pH值、温度、催化剂用量、H2O2浓度及污染物浓度等[7,14-17]。载体种类、晶型、粒径大小和活性组分的种类、形态[18-19]等也是重要的影响因素。本文主要阐述以黏土、沸石、介孔氧化硅、多孔碳为载体的Fenton催化剂降解酚类污染物的研究进展。
1.1 黏土
黏土是一种粒径很小(<2µm)的微孔材料,因其颗粒带有负电荷、具有与其他阳离子交换的能力,其物理吸附性和表面化学活性很好。黏土在自然界中含量丰富,且纯度高,可直接作为载体。层状黏土是最常用的Fenton催化剂载体,其结构呈片状,因铝硅酸盐与阳离子间的静电作用,有机物难以进入黏土孔道[20-21]。利用天然黏土分散在水溶液中时会发生膨胀这一性质,将粒径较大的阳离子通过离子交换引入黏土的孔道中,再经过高温煅烧,将金属阳离子转化为热稳定性的金属氧化物[22],制备出柱撑黏土。其制备过程如图2所示。柱撑黏土具有较大的层间距,比表面积和微孔率可增至原来的 4倍[22],极大地增强了其吸附和催化性能。经过煅烧,黏土与引入的阳离子之间形成共价键,有效地防止了活性组分的溶出,有利于材料的重复利用,并提高了催化剂的活性和稳定性。
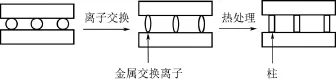
图2 柱撑黏土形成的一般步骤[23]
使用混合支撑柱,如铝与催化金属,可进一步提高催化剂的活性和稳定性。Carriazo等[24]比较了A l-Ce-Fe柱撑膨润土、Al-膨润土及天然膨润土催化H2O2分解降解苯酚的效率,催化活性依次为:Al-Ce-Fe柱撑膨润土>Al-膨润土>天然膨润土。在室温、常压下,1 h后苯酚全部被转化,4 h后TOC去除率达到 55%。铁的溶出小于 0.3 mg/L,均相Fenton反应对催化剂活性的影响基本可以忽略。但作者并未对其稳定性进行评估。Guélou等[25]报道了载铁 Al-Fe-膨润土的催化活性比载铁 A l-膨润土的高。以Al-Fe-膨润土作为载体时,在70℃下,催化剂对苯酚及 TOC的去除率分别可以达到 100%和80%,并表现出了长期的稳定性。电子自旋共振光谱表明:在载铁 A l-膨润土中,铁以独立物种或氧化物簇形式存在于黏土表面;而载铁A l-Fe-膨润土中,铁还可能存在于柱当中。催化氧化降解实验表明:以后者为催化剂载体时,体系对苯酚的催化降解效率更高。在连续流实验中,经过350 h,催化剂依然表现出高活性。铁的总溶出为5%(质量分数),且流失的铁均来自于以氧化物簇形式存在的铁。这表明仅存在于载铁A l-Fe-膨润土中的铁具有更强的活性和稳定性。这种形式的铁与铝之间产生了较强的结合力,可能是催化剂稳定性好的原因之一。Luo等[26]的报道表明,A l-Fe-柱撑黏土中的Fe和Al含量对催化剂活性也有影响。随着铁含量的增加,催化活性呈现先增大后降低的趋势。这是因为Al含量对柱撑黏土的层间距有影响。Al3+的引入增大了层间距,使得铁离子可以进入黏土;进一步增大 Al含量,Fe含量的降低导致催化剂活性降低。
载体的孔结构及比表面积[22]、活性组分的形态等均可对催化剂性能产生影响。优化制备条件可以有效地提高催化剂的活性和稳定性。Barrault等[27]将干燥的黏土粉末直接加入到含 Cu(NO3)2和A l(NO3)3的柱化液中制备Cu-A l-柱撑膨润土,并与采用传统方法制备的催化剂进行性能比较。结果表明,黏土的层间距有所增大,并具有较高的比表面积和孔容。催化降解苯酚的效率远高于传统方法制备的催化剂,且Cu2+的溶出很低,反应速率快。经过5次重复利用,催化活性并未降低,且Cu2+溶出始终低于2μg/g。Timofeeva等[28]考察了制备方法对催化剂性能的影响。采用离子交换法制备的Fe0.8A l12.2-PILC比以吸附Fe离子制备得到的催化剂表现出较高的催化活性和稳定性。在50℃,pH值 为 6.2条件下,经过3h对苯酚的去除率达到100%,溶出率低于0.01%(质量分数)。但作者没有通过重复利用实验或连续流实验进一步证明材料具有良好的稳定性和寿命长。随后,作者又研究了OH/(Fe +A l)比、柱化液的老化时间及煅烧温度对Fe-A l-蒙脱石结构及催化性能的影响[29]。结果表明OH/(Fe+A l)较低时,易形成铁物种的低聚物,并发生团聚,降低了催化性能;A l /Fe比的增大及煅烧温度的升高有利于形成独立的铁物种;老化时间的延长有助于提高材料的总表面积、总孔容和微孔容,从而可提高催化剂的性能。
虽然直接使用天然黏土作为载体方便且经济,但存在很多不确定因素[22,24],如化学组成、纯度、结晶度、同位取代的程度、可交换阳离子的类型等。目前对以柱撑黏土为载体的 Fenton催化剂的主要研究对象是制备方法、混合黏土及自制黏土等对催化性能的影响。表1列出了近年来以黏土作为Fenton催化剂载体处理酚类废水的文献报道,给出了反应条件及催化剂的活性和稳定性数据。
柱撑黏土结构的规整度不如常用的沸石等材料,但却高于活性炭[24]等多孔材料。而且天然黏土来源丰富、经济易得,以柱撑黏土为载体的Fenton催化剂高效、稳定、溶出少。因此,柱撑黏土在负载型Fenton催化剂中仍具有广泛的应用前景。但与其他多孔材料相比,其孔径和比表面积仍较小,且在很多体系中仍表现出一定的热不稳定性。因此,开发出具有更大孔径和比表面积的柱撑黏土,并进一步提高其热稳定性,将是今后柱撑黏土作为Fenton催化剂载体的重要研究方向。
1.2 沸石
沸石是一种具有笼状或渠状结构的多孔材料,比表面积可达到 400m2/g[39-40],可作为主体吸附各种分子[41],在作为Fenton催化剂载体的同时也起着吸附剂的作用,可促进催化剂对污染物的去除。沸石具有开放的结构,可与多种金属阳离子进行交换,阳离子交换能力达到几mg/kg[40],因此金属/金属氧化物-沸石复合物的制备较容易。沸石的孔径与反应物尺寸相似[42],是一种理想的Fenton催化剂载体。在Fenton反应的应用中,常被用作载体的沸石有ZSM-5(MFI)[43-45]、HY-5(FAU)[46]、X型沸石[47-48]等。
Fe/ZSM-5是催化H2O2分解降解酚类污染物的高效催化剂,可将苯酚完全去除[49]。Ovejero等[50]采用不同的方法制备含铁沸石,用于催化 H2O2分解降解苯酚。催化剂均表现出很高的活性,苯酚基本可被完全去除,TOC去除率达到了50%~80%。材料重复使用后,催化活性仅略有降低;体系中铁溶出率低。沸石Y[46,51]、沸石X[47]也被认为是一种高效、稳定的负载型 F enton催化剂载体。Zrnčeric等[46]采用离子交换法制备Cu/Y-5型Fenton催化剂用于苯酚降解。当H2O2/苯酚摩尔比为14时,苯酚被完全去除需要150m in;当H2O2浓度加倍时,缩短至40m in。体系中Cu2+溶出低,经过5次利用后催化活性并没有明显降低。Valkaj等[52]考查了在不同煅烧温度下制备的 C u/13X系列催化剂的性能,其在80℃、低H2O2浓度条件下对苯酚的去除率为100%,TOC去除率达到了 5 2.2%~54%。但 C u2+溶出高,且作者未对催化剂进行重复利用或进行连续流实验,对其稳定性作出实际评估。表2给出了近年来以沸石作为 Fenton催化剂载体处理酚类废水的文献报道。
沸石具有很强的离子交换能力,用于制备金属/金属氧化物的复合材料所需条件简单,其本身具有的特性有利于小分子有机物的去除。沸石具有较大的比表面积及多孔性可使活性组分颗粒均匀分布,防止纳米催化剂的团聚,还能够实现对催化剂的回收。沸石合成技术的不断进步以及介孔材料等的出现进一步拓展了其应用范围。总之,沸石在负载型Fenton催化剂中的应用将越来越广。但沸石分子筛的择形选择性同时导致了分子在沸石孔道中的扩散速率较慢,限制了反应的发生速率,可通过优化催化剂的设计提升其催化性能。
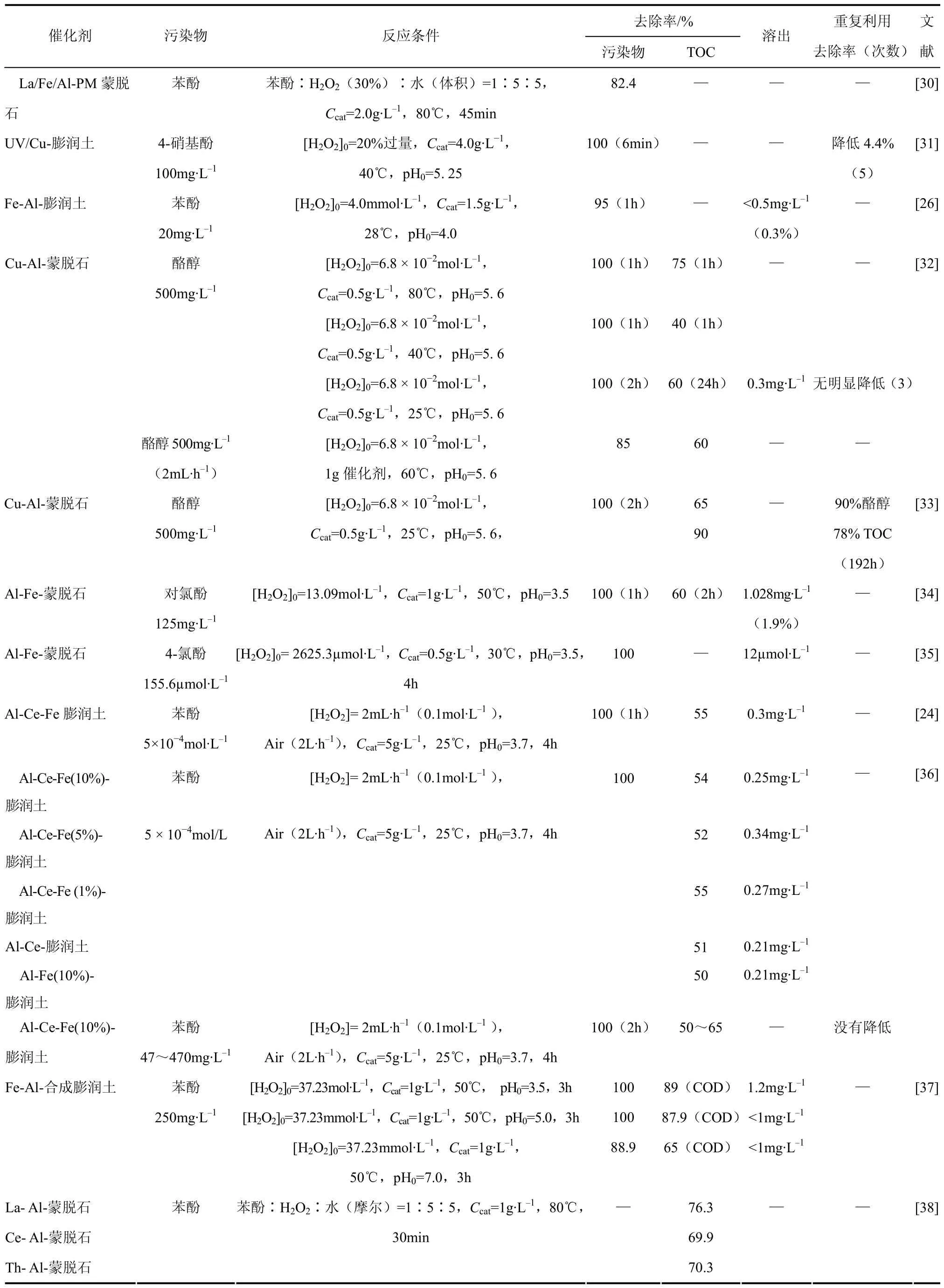
表1 黏土基Fenton催化剂处理酚类污染物
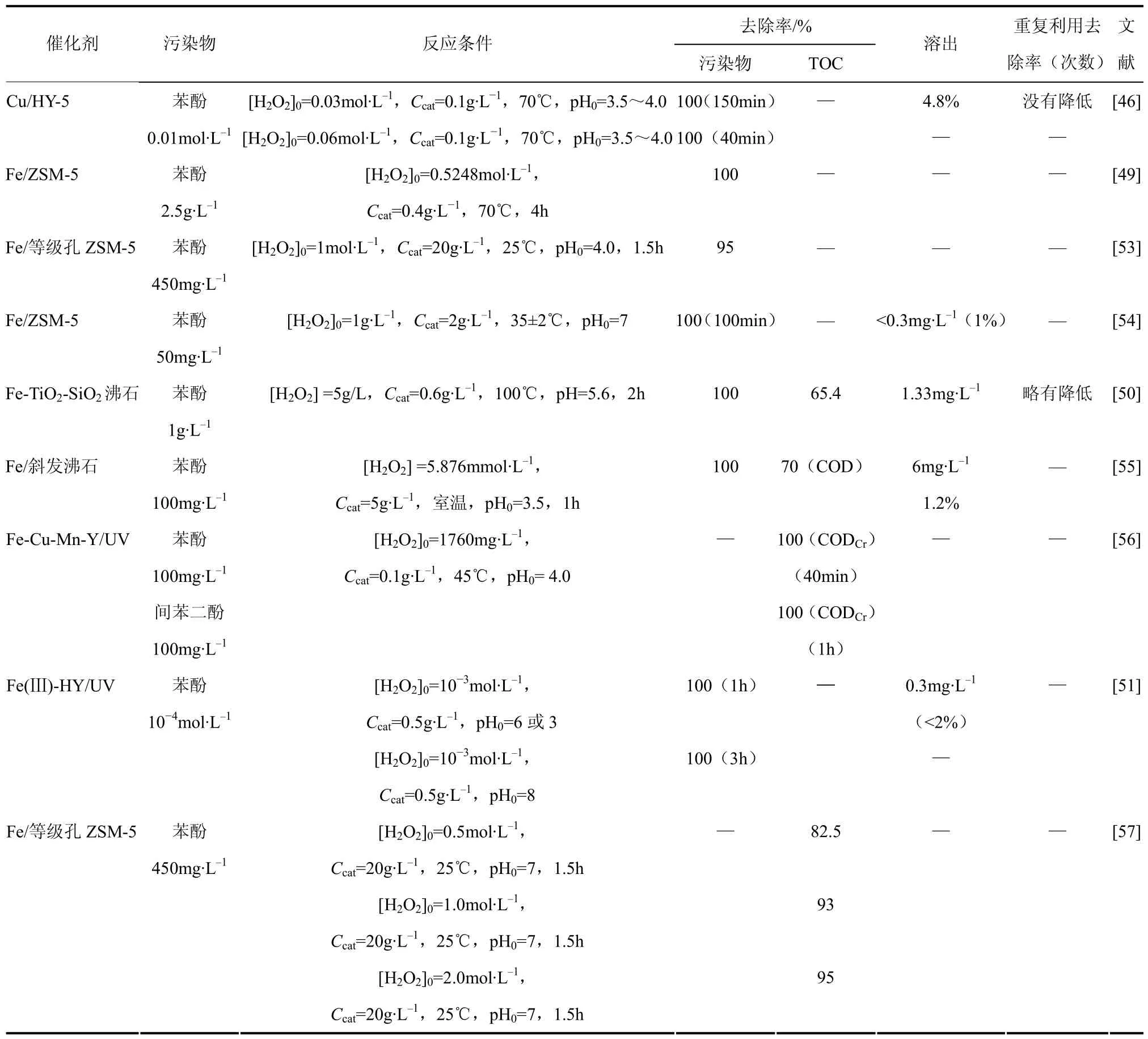
表2 沸石基Fenton催化剂处理酚类污染物
1.3 介孔氧化硅
介孔氧化硅的孔道结构有序,具有较大的比表面积和孔容,可分别达到1000m2/g和1.0cm3/g[40],孔径为2~30nm,并在一定范围内可调。与沸石及黏土等微孔材料相比,介孔材料将可去除有机物的大小扩展至介孔范围。1992年,Mobil公司首次合成了MCM-41[58],虽然几乎所有的金属都可以进入孔道与MCM-41形成金属-MCM-41复合物,但其水热稳定性较差,限制了其应用[59]。与 MCM-41相比,Zhao课题组[60]合成的SBA-15具有较大的孔径、良好的力学性能和水热稳定性,使介孔氧化硅在负载型Fenton催化剂中得到更好的应用。
Martínez等[61]采用固定床反应器,以 Fe2O3/ SBA-15催化H2O2分解降解苯酚。在pH值为5.5时,苯酚及TOC去除率分别可达到100%和66%。经过连续34h的反应,仅TOC去除率有所下降,铁的溶出率仅为1.3%,稳定性较好。Botas[6]等得到同样的结论,并研究了制备方法和载体种类对催化剂性能的影响。结果表明:直接法制备的催化剂比浸渍法稳定性更高;Fe2O3/SBA-15比 Fe2O3/无定形SiO2具有更高的催化活性和稳定性。Melero等[62]也报道了 Fe2O3/SBA-15在处理苯酚废水时表现出很高的活性和稳定性。pH值为7时,仍保持其活性。作者通过控制反应体系的pH值,可以降低活性组分溶出,增强其稳定性。
介孔氧化硅作为载体在 Fenton催化剂中的应用已取得一定的进展。表3给出了近年来以介孔氧化硅为 Fenton催化剂载体处理酚类污染物的文献
报道,列出了反应条件及材料的催化性能数据。
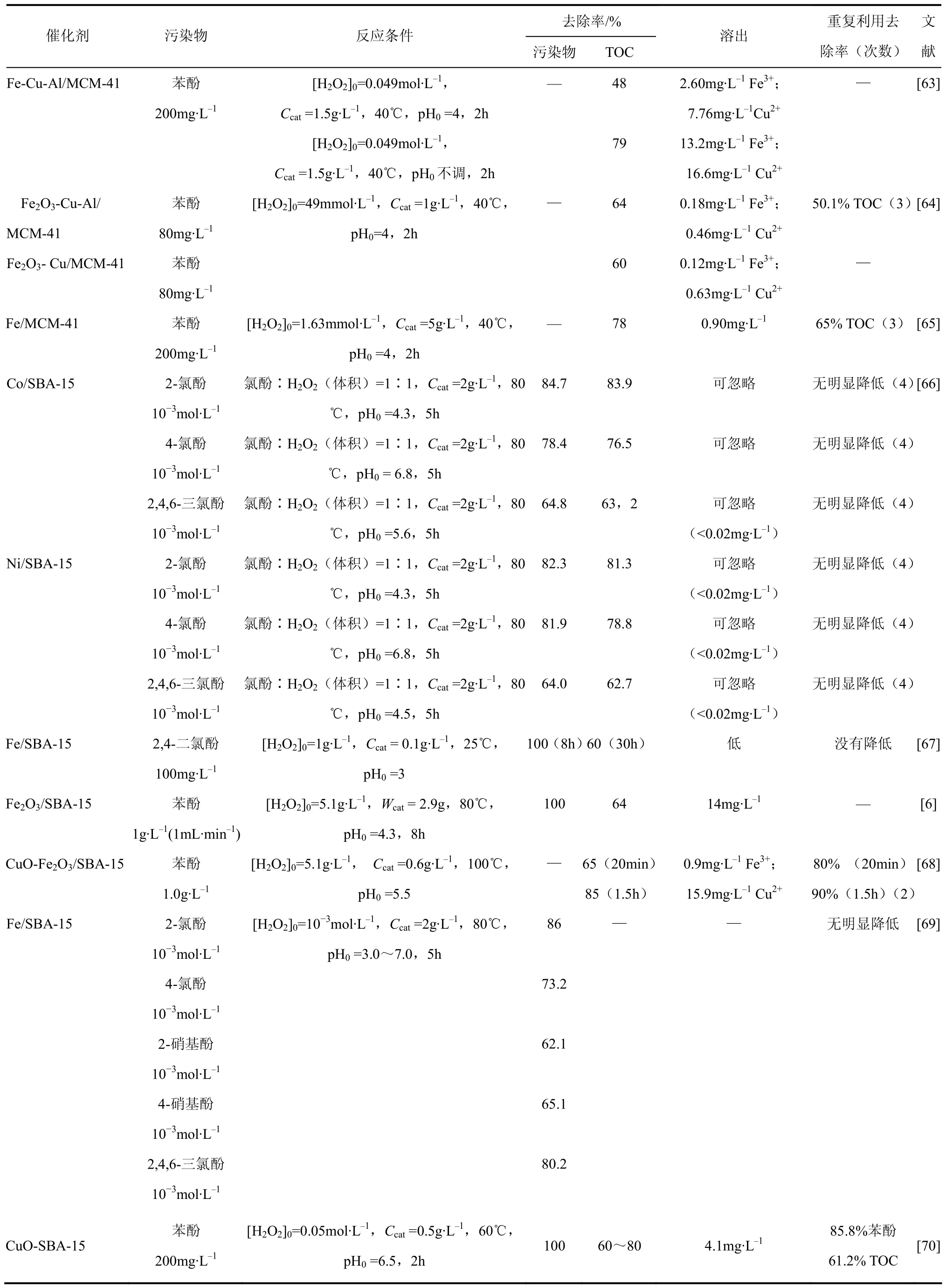
表3 介孔氧化硅基Fenton催化剂处理酚类污染物
介孔氧化硅在去除不能进入微孔材料孔道的大分子污染物上具有明显的优势。可调的孔径使其能选择去除特定的污染物。在制备方法取得长足进步下,作为负载型Fenton催化剂的金属/金属氧化物-介孔氧化硅复合物必然发挥着重要的作用。另外,结构的有序性使得介孔氧化硅易进行表面改性[71],拓展了其应用范围。改善M 41S型介孔氧化硅的水热稳定性,加强对其他介孔氧化硅的改性和修饰研究将进一步促进介孔氧化硅在负载型 Fenton催化剂中的应用。
1.4 多孔碳
多孔碳作为一种催化剂载体,在废水处理中应用广泛。活性炭具有比表面积大、吸附性能好等优点,是水处理中常用的 Fenton催化剂载体。Zazo等[1]分别以活性炭(AC)、硅、氧化铝、沸石、树脂及柱撑黏土为载体制备Fenton催化剂,处理苯酚废水。以活性炭为载体时,催化剂表现出了优异的性能,其催化效果可与以其他材料为载体的Fenton催化剂相媲美。在长期连续实验中,经过20~25h,对TOC的去除率仍达到80%。但由于活性炭与金属间作用力较弱[72],活性组分易溶出,不利于材料的重复利用,因此通过改善制备方法,增强活性组分与碳基体之间的作用力,得到稳定性好的Fenton催化剂,是今后的一个重要研究方向。其他的碳材料如碳纳米管[73]、碳纤维[74]、碳凝胶[75]、介孔碳[76]等,在Fenton反应中的应用日益受到关注。
有序介孔碳由介孔氧化硅发展而来,衍生出了CMK、ZCS、MCF等系列介孔碳材料。但介孔碳发展较晚,在负载型 Fenton催化剂中的应用还比较少。Kong等[76]制备MnO-有序介孔碳,在60℃,1g/L MnO-C、1g/Lh2O2、pH=5.8条件下对100mg/L苯酚的去除率达到95%,且催化剂在pH值为3~9范围内均表现出了高活性。重复使用材料发现,经过20次循环利用,催化剂活性并未明显降低,且活性组分溶出低,表现出较强的稳定性。锰离子溶出仅为0.29mg/g。由此可见,有序介孔碳是一种良好的Fenton催化剂载体,在负载型Fenton催化剂中的应用具有很大前景,也将是研究者们的又一研究重点。表4给出了近年来以多孔碳作为Fenton催化剂载体处理酚类有机物的文献报道。

表4 多孔碳基Fenton催化剂处理酚类污染物
多孔碳比表面积较大,具有很好的吸附性及良好的水热稳定性,在废水处理中的应用广泛,是Fenton催化剂的良好载体。有序介孔碳在近年来得到快速发展,且在负载型Fenton催化剂的应用中表现出较好的性能,这将是研究者们关注的一个研究热点。
2 结 语
Fenton氧化法是降解有机污染物,尤其是低浓度有机污染物的一种重要方法。非均相Fenton催化氧化技术可以有效解决均相 Fenton催化体系中面临的受pH值限制大、含铁污泥产生量大、Fe3+还原为Fe2+速率缓慢等问题。负载型Fenton催化剂在有机物,尤其是难生物降解的有机物去除中有着举足轻重的地位。具有高催化活性和长期稳定性的催化剂是非均相Fenton催化氧化技术研究中的热点。因此,今后负载型Fenton催化剂的研究方向主要集中在以下几个方面。
(1)提高催化剂的稳定性。在材料制备过程中添加某种金属或其他物质,增强活性组分颗粒与载体间的作用力,也可选择合适的载体,使活性组分更好的固定。
(2)提高催化剂在中性条件下的活性。可通过改变反应的外在条件,如使用紫外、超声、可见光等手段,提高体系产生自由基的效率,增强其活性。
(3)延长催化剂的使用寿命。制备孔径较大的复合材料,如有序介孔材料,将可去除污染物的分子大小扩大至介孔范围,可在一定程度上避免碳沉积引起孔道堵塞;通过在材料表面嫁接官能团,使材料具有选择性吸附能力,可防止短链脂肪酸类物质的吸附而造成催化剂的中毒。
(4)开发新型多孔材料载体。通过选用性能更好的新型催化剂载体,提高催化剂的活性,增强活性组分与载体间的作用力,提高其稳定性。
[1] Zazo J,Casas J,Mohedano A,et al.Catalytic wet peroxide oxidation of phenol w ith a Fe/active carbon catalyst[J].Applied Catalysis B:Environmental,2006,65(3):261-268.
[2] Esplugas S,Gimenez J,Contreras S,et al.Comparison of different advanced oxidation processes for phenol degradation[J].Water Research,2002,36(4):1034-1042.
[3] Rey A,Faraldos M,Casas J A,et al.Catalytic wet peroxide oxidation of phenol over Fe/AC catalysts:Influence of iron precursor and activated carbon surface[J].Applied Catalysis B:Environmental,2009,86(1-2):69-77.
[4] Kwan W P,Voelker B M.Rates of hydroxyl radical generation and organic compound oxidation in m ineral-catalyzed Fenton-like systems[J].Environmental Science and Technology,2003,37(6):1150-1158.
[5] Nogueira R F P,Trovó A G,da Silva M R A,et al.Fundamentos e aplicações ambientais dos processos Fenton e foto-Fenton[J].Química Nova,2007,30(2):400.
[6] Botas J A,Melero J A,Martínez F,et al.Assessment of Fe2O3/SiO2catalysts for the continuous treatment of phenol aqueous solutions in a fixed bed reactor[J].Catalysis Today,2010,149(3-4):334-340.
[7] Melero J A,Martínez F,Botas J A,et al.Heterogeneous catalytic wet peroxide oxidation systems for the treatment of an industrial pharmaceutical wastewater[J].Water Research,2009,43(16):4010-4018.
[8] Xu L,Wang J.A heterogeneous Fenton-like system w ith nanoparticulate zero-valent iron for removal of 4-chloro-3-methyl phenol[J].Journal of Hazardous Materials,2011,186(1):256-264.
[9] Martínez F,Calleja G,Melero J A,et al.Iron species incorporated over different silica supports for the heterogeneous photo-Fenton oxidation of phenol[J].Applied Catalysis B:Environmental,2007,70(1-4):452-460.
[10] Valdés-Solís T,Valle-Vigón P,Álvarez S,et al.Manganese ferrite nanoparticles synthesized through a nanocasting route as a highly active Fenton catalyst[J].Catalysis Communications,2007,8(12):2037-2042.
[11] Muthuvel I,Swam inathan M.Hetro-Fenton m ineralization of AY 17 by novel Fe3+/fire clay composite using UV light[J].International Journal of Environmental Science and Technology,2013,1(4):9-13.
[12] Li B,Dong Y,Ding Z.Photoassisted degradation of CI Reactive Red 195 using an Fe(III)-grafted polytetrafluoroethylene fibre complex as a novel heterogeneous Fenton catalyst over a w ide pH range[J].Coloration Technology,2013,129(6):403-411.
[13] 王彦斌,赵红颖,赵国华,等.基于铁化合物的异相 Fenton 催化氧化技术的发展[J].化学进展,2013,25(8):1246-1259.
[14] Khieu D Q,Quang D T,Lam T D,et al.Fe-MCM-41 w ith highly ordered mesoporous structure and high Fe content:Synthesis and application in heterogeneous catalytic wet oxidation of phenol[J].Journal of Inclusion Phenomena and Macrocyclic Chemistry,2009,65(1-2):73-81.
[15] Ramirez J,Maldonado-Hódar F,Pérez-Cadenas A,et al.Azo-dye orange II degradation by heterogeneous Fenton-like reaction using carbon-Fe catalysts[J].Applied Catalysis B:Environmental,2007,75(3):312-323.
[16] Devi L G,Rajashekhar K E,Raju K S A,et al.Kinetic modeling based on the non-linear regression analysis for the degradation of Alizarin Red S by advanced photo Fenton process using zero valent metallic iron as the catalyst[J].Journal of Molecular Catalysis A:Chemical,2009,314(1-2):88-94.
[17] 张乃东,郑威.Fenton 法在水处理中的发展趋势[J].化工进展,2001,20(12):1-3.
[18] Contreras S,Yalfani M S,Medina F,et al.Effect of support and second metal in catalytic in-situ generation of hydrogen peroxide by Pd-supported catalysts:Application in the removal of organic pollutants by means of the Fenton process[J].Water Science & Technology,2011,63(9):2017.
[19] 何莼,奚红霞,张娇,等.沸石和活性炭为载体的Fe3+和Cu2+型催化剂催化氧化苯酚的比较[J].离子交换与吸附,2003,19(4):289-296.
[20] Vaccari A.Preparation and catalytic properties of cationic and anionic clays[J].Catalysis Today,1998,41(1):53-71.
[21] Rives V,Angeles Ulibarri M a.Layered double hydroxides (LDH)intercalated w ith metal coordination compounds and oxometalates[J].Coordination Chemistry Reviews,1999,181(1):61-120.
[22] Herney-Ramírez J,Madeira L M.Use of pillared clay-based catalysts for wastewater treatment through Fenton-like processes[M].Springer:Pillared Clays and Related Catalysts,2010:129-165.
[23] Navalon S,A lvaro M,Garcia H.Heterogeneous Fenton catalysts based on clays,silicas and zeolites[J].Applied Catalysis B:Environmental,2010,99(1-2):1-26.
[24] Carriazo J,Molina R,Moreno S.A study on Al and Al–Ce–Fe pillaring species and their catalytic potential as they are supported on a bentonite[J].Appl.Catal.A:Gen.,2008,334(1):168-172.
[25] Guélou E,Barrault J,Fournier J,et al.Active iron species in the catalytic wet peroxide oxidation of phenol over pillared clays containing iron[J].Applied Catalysis B:Environmental,2003,44(1):1-8.
[26] Luo M,Bowden D,Brimblecombe P.Catalytic property of Fe-A l pillared clay for Fenton oxidation of phenol by H2O2[J].Applied Catalysis B:Environmental,2009,85(3):201-206.
[27] Barrault J,Bouchoule C,Echachoui K,et al.Catalytic wet peroxide oxidation (CWPO) of phenol over m ixed (AlCu)-pillared clays[J].Applied Catalysis B:Environmental,1998,15(3):269-274.
[28] Timofeeva M,Khankhasaeva S T,Badmaeva S,et al.Synthesis,characterization and catalytic application for wet oxidation of phenol of iron-containing clays[J].Applied Catalysis B:Environmental,2005,59(3):243-248.
[29] Timofeeva M,Khankhasaeva S T,Chesalov Y A,et al.Synthesis of Fe,Al-pillared clays starting from the Al,Fe-polymeric precursor:Effect of synthesis parameters on textural and catalytic properties[J].Applied Catalysis B:Environmental,2009,88(1):127-134.
[30] Kurian M,Babu R.Iron alum inium m ixed pillared montmorillonite and the rare earth exchanged analogues as efficient catalysts for phenol oxidation[J].Journal of Environmental Chemical Engineering,2013,1(1-2):86-91.
[31] Ayodele O,Hameed B.Synthesis of copper pillared bentonite ferrioxalate catalyst for degradation of 4-nitrophenol in visible light assisted Fenton process[J].Journal of Industrial and Engineering Chemistry,2013,19(3):966-974.
[32] Achma R B,Ghorbel A,Dafinov A,et al.Copper-supported pillared clay catalysts for the wet hydrogen peroxide catalytic oxidation of model pollutant tyrosol[J].Appl.Catal.A:Gen.,2008,349(1):20-28.
[33] Ben Achma R,Ghorbel A,Dafinov A,et al.Synthesis of stable Cu-supported pillared clays for wet tyrosol oxidation w ith H2O2[J].Journal of Physics and Chemistry of Solids,2012,73(12):1524-1529.
[34] Catrinescu C,Arsene D,Teodosiu C.Catalytic wet hydrogen peroxide oxidation of para-chlorophenol over Al/Fe pillared clays(AlFePILCs) prepared from different host clays[J].Applied Catalysis B:Environmental,2011,101(3):451-460.
[35] Catrinescu C,Arsene D,Apopei P,et al.Degradation of 4-chlorophenol from wastewater through heterogeneous Fenton and photo-Fenton process,catalyzed by Al–Fe PILC[J].Applied Clay Science,2012,58:96-101.
[36] Carriazo J,Guélou E,Barrault J,et al.Catalytic wet peroxide oxidation of phenol by pillared clays containing Al-Ce-Fe[J].Water Research,2005,39(16):3891-3899.
[37] Catrinescu C,Teodosiu C,Macoveanu M,et al.Catalytic wet peroxide oxidation of phenol over Fe-exchanged pillared beidellite[J].Water Research,2003,37(5):1154-1160.
[38] Kurian M,Eldhose A,Thasleenaki R M.M ild temperature oxidation of phenol over rare earth exchanged[J].International Journal of Environmental Research,2012,6(3):669-676.
[39] Auerbach S M,Carrado K A,Dutta P K.Handbook of zeolite science and technology[M].Boca Raton:CRC Press,2003.
[40] Hartmann M,Kullmann S,Keller H.Wastewater treatment w ith heterogeneous Fenton-type catalysts based on porous materials[J].Journal of Materials Chemistry,2010,20(41):9002-9017.
[41] Aravindhan R,Fathima N N,Rao J R,et al.Wet oxidation of acid brown dye by hydrogen peroxide using heterogeneous catalyst Mn-salen-Y zeolite:A potential catalyst[J].Journal of Hazardous Materials,2006,138(1):152-159.
[42] Tekbaş M,Yatmaz H C,Bektaş N.Heterogeneous photo-Fenton oxidation of reactive azo dye solutions using iron exchanged zeolite as a catalyst[J].Microporous and Mesoporous Materials,2008,115(3):594-602.
[43] Valkaj K M,Katovic A,Zrncević S.Investigation of the catalytic wet peroxide oxidation of phenol over different types of Cu/ZSM-5 catalyst[J].Journal of Hazardous Materials,2007,144(3):663.
[44] Centi G,Perathoner S,Torre T,et al.Catalytic wet oxidation w ith H2O2of carboxylic acids on homogeneous and heterogeneous Fenton-type catalysts[J].Catalysis Today,2000,55(1):61-69.
[45] Kuznetsova E,Savinov E,Vostrikova L,et al.Heterogeneous catalysis in the Fenton-type system FeZSM-5/H2O2[J].Applied Catalysis B:Environmental,2004,51(3):165-170.
[46] Zrnčevic S,Gomzi Z.CWPO:An environmental solution for pollutant removal from wastewater[J].Industrial & Engineering Chemistry Research,2005,44(16):6110-6114.
[47] Shukla P,Wang S,Singh K,et al.Cobalt exchanged zeolites for heterogeneous catalytic oxidation of phenol in the presence of peroxymonosulphate[J].Applied Catalysis B:Environmental,2010,99(1):163-169.
[48] He F,Shen X,Lei L.Photochem ically enhanced degradation of phenol using heterogeneous Fenton-type catalysts[J].Journal of Environmental Sciences,2003,15(3):351-355.
[49] Phu N H,Hoa T T K,Tan N V,et al.Characterization and activity of Fe-ZSM-5 catalysts for the total oxidation of phenol in aqueous solutions[J].Applied Catalysis B:Environmental,2001,34(4):267-275.
[50] Ovejero G,Sotelo J L,Martínez F,et al.Wet peroxide oxidation of phenolic solutions over different iron-containing zeolitic materials[J].Industrial & Engineering Chemistry Research,2001,40(18):3921-3928.
[51] Noorjahan M,Durga Kumari V,Subrahmanyam M,et al.Immobilized Fe(Ⅲ)-HY:An efficient and stable photo-Fenton catalyst[J].Applied Catalysis B:Environmental,2005,57(4):291-298.
[52] Valkaj K M,Katović A,Zrncevic S.Catalytic properties of Cu/13X zeolite based catalyst in catalytic wet peroxide oxidation of phenol[J].Industrial & Engineering Chemistry Research,2011,50(8):4390-4397.
[53] Sashkina K,Labko V,Rudina N,et al.Hierarchical zeolite FeZSM-5 as a heterogeneous Fenton-type catalyst[J].Journal of Catalysis,2013,299:44-52.
[54] Gonzalez-Olmos R,Martin M J,Georgi A,et al.Fe-zeolites as heterogeneous catalysts in solar Fenton-like reactions at neutral pH[J].Applied Catalysis B:Environmental,2012,125:51-58.
[55] Bayat M,Sohrabi M,Royaee S J.Degradation of phenol by heterogeneous Fenton reaction using Fe/clinoptilolite[J].Journal of Industrial and Engineering Chemistry,2012,18(3):957-962.
[56] Zheng Zhanwang,Lei Lecheng,Xu Shengjuan,et al.Heterogeneous UV/Fenton catalytic degradation of wastewater containing phenol w ith Fe-Cu-Mn-Y catalyst[J].Journal of Zhejiang University Science,2004,5(2):206-211.
[57] Sashkina K A,Semeikina V S,Labko V S,et al.Template method for the synthesis of a heterogeneous fenton catalyst based on the hierarchical zeolite FeZSM-5[J].Kinetics and Catalysis,2013,54(5):638-643.
[58] Kresge C,Leonow icz M,Roth W,et al.Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism[J].Nature,1992,359(6397):710-712.
[59] Armengol E,Corma A,Fornés V,et al.Cu2+-phthalocyanine and Co2+-perfluorophthalocyanine incorporated inside Y faujasite and mesoporous MCM-41 as heterogeneous catalysts for the oxidation of cyclohexane[J].Appl.Catal.A:Gen.,1999,181(2):305-312.
[60] Zhao D,Huo Q,Feng J,et al.Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered,hydrothermally stable,mesoporous silica structures[J].Journal of the American Chemical Society,1998,120(24):6024-6036.
[61] Martínez F,Melero J A,Botas J Á,et al.Treatment of phenolic effluents by catalytic wet hydrogen peroxide oxidation over Fe2O3/SBA-15 extruded catalyst in a fixed-bed reactor[J].Industrial & Engineering Chemistry Research,2007,46(13):4396-4405.
[62] Melero J,Calleja G,Martínez F,et al.Nanocomposite Fe2O3/SBA-15:An efficient and stable catalyst for the catalytic wet peroxidation of phenolic aqueous solutions[J].Chemical Engineering Journal,2007,131(1):245-256.
[63] Xia M,Long M,Yang Y,et al.A highly active bimetallic oxides catalyst supported on Al-containing MCM-41 for Fenton oxidation of phenol solution[J].Applied Catalysis B:Environmental,2011,110:118-125.
[64] Ling Y,Long M,Hu P,et al.Magnetically separable core–shell structural γ-Fe2O3@Cu/A l-MCM-41 nanocomposite and its performance in heterogeneous Fenton catalysis[J].Journal of Hazardous Materials,2014,264:195-202.
[65] Xia M,Chen C,Long M,et al.Magnetically separable mesoporous silica nanocomposite and its application in Fenton catalysis[J].Microporous and Mesoporous Materials,2011,145(1-3):217-223.
[66] Mayani S V,Mayani V J,Kim S W.Decomposition of 2-chlorophenol,4-chlorophenol and 2,4,6-trichlorophenol by catalytic oxidation over cobalt and nickel impregnated SBA-15[J].The Canadian Journal of Chemical Engineering,2013,91(7):1270-1280.
[67] Shukla P,Wang S,Sun H,et al.Adsorption and heterogeneousadvanced oxidation of phenolic contaminants using Fe loaded mesoporous SBA-15 and H2O2[J].Chemical Engineering Journal,2010,164(1):255-260.
[68] Melero J A,Calleja G,Martínez F,et al.Nanocomposite of crystalline Fe2O3and CuO particles and mesostructured SBA-15 silica as an active catalyst for wet peroxide oxidation processes[J].Catalysis Communications,2006,7(7):478-483.
[69] Mayani S V,Mayani V J,Kim S-W.Catalytic oxidation of phenol analogues in aqueous medium over Fe/SBA-15[J].Bulletin of the Korean Chemical Society,2012,33(9):3009-3016.
[70] Zhong X,Barbier J,Duprez D,et al.Modulating the copper oxide morphology and accessibility by using m icro-/mesoporous SBA-15 structures as host support:Effect on the activity for the CWPO of phenol reaction[J].Applied Catalysis B:Environmental,2012,121-122:123-134.
[71] Sousa A,Souza K,Reis S,et al.Positron annihilation study of pore size in ordered SBA-15[J].Journal of Non-Crystalline Solids,2008,354(42):4800-4805.
[72] Navalon S,Dhakshinamoorthy A,Alvaro M,et al.Heterogeneous Fenton catalysts based on activated carbon and related materials[J].Chem.Sus.Chem.,2011,4(12):1712-1730.
[73] Vu H,Gonçalves F,Philippe R,et al.Bimetallic catalysis on carbon nanotubes for the selective hydrogenation of cinnamaldehyde[J].Journal of Catalysis,2006,240(1):18-22.
[74] Lu W,Chen W,Li N,et al.Oxidative removal of 4-nitrophenol using activated carbon fiber and hydrogen peroxide to enhance reactivity of metallophthalocyanine[J].Applied Catalysis B:Environmental,2009,87(3):146-151.
[75] Duarte F,Maldonado-Hódar F,Pérez-Cadenas A,et al.Fenton-like degradation of azo-dye Orange II catalyzed by transition metals on carbon aerogels[J].Applied Catalysis B:Environmental,2009,85(3):139-147.
[76] Kong L,Wei W,Zhao Q,et al.Active coordinatively unsaturated manganese monoxide-containing mesoporous carbon catalyst in wet peroxide oxidation[J].ACS Catalysis,2012,2(12):2577-2586.
[77] Chun J,Lee H,Lee S H,et al.Magnetite/mesocellular carbon foam as a magnetically recoverable fenton catalyst for removal of phenol and arsenic[J].Chemosphere,2012,89(10):1230-1237.
[78] Martínez F,Pariente M I,Botas J Á,et al.Influence of preoxidizing treatments on the preparation of iron-containing activated carbons for catalytic wet peroxide oxidation of phenol[J].Journal of Chemical Technology & Biotechnology,2012,87(7):880-886.
[79] Liou R M,Chen S H.CuO impregnated activated carbon for catalytic wet peroxide oxidation of phenol[J].Journal of Hazardous Materials,2009,172(1):498-506.
[80] Messele S A,Stüber F,Bengoa C,et al.Phenol degradation by heterogeneous Fenton-like reaction using Fe supported over activated carbon[J].Procedia Engineering,2012,42:1373-1377.
[81] Liao Q,Sun J,Gao L.Degradation of phenol by heterogeneous Fenton reaction using multi-walled carbon nanotube supported Fe2O3catalysts[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2009,345(1):95-100.
