Efficiency of a re-usable Carius tube for determination of platinum group elements in ultramafic rocks
2014-02-20LIUYingyingQILiangZHAOZhengHUANGXiaowenandWANGYichang
LIU Yingying, QI Liang, ZHAO Zheng, HUANG Xiaowen, and WANG Yichang
1State Key Laboratory of Ore Deposit Geochemistry, Institute of Geochemistry, Chinese Academy of Sciences, Guiyang 550002, China
2University of Chinese Academy of Sciences, Beijing 100049, China
3Key Laboratory of Metallogeny and Mineral Resource Assessment, Institute of Mineral Resources, Chinese Academy of Geological Sciences, Beijing 100037, China
*Corresponding author, E-mail: qilianghku@hotmail.com
Efficiency of a re-usable Carius tube for determination of platinum group elements in ultramafic rocks
LIU Yingying1,2, QI Liang1,*, ZHAO Zheng1,3, HUANG Xiaowen1,2, and WANG Yichang1,2
1State Key Laboratory of Ore Deposit Geochemistry, Institute of Geochemistry, Chinese Academy of Sciences, Guiyang 550002, China
2University of Chinese Academy of Sciences, Beijing 100049, China
3Key Laboratory of Metallogeny and Mineral Resource Assessment, Institute of Mineral Resources, Chinese Academy of Geological Sciences, Beijing 100037, China
*Corresponding author, E-mail: qilianghku@hotmail.com
Chin.J.Geochem.(2014)33:045-052
Digestion withaqua regiain Carius tube is commonly employed for determination of PGEs in geological samples. However, silicates cannot be completely dissolved byaqua regiaand PGEs might partially remain in silicate residue. In this study, an ultramafic reference material was used to investigate the efficiencies of a new re-usable Carius tube after digestion at 220 and 240℃, and an autoclave -lined Carius tube at 260 and 280℃. The results show that about 10% of PGEs retained in the silicate residues at 220℃ and 5% still r emains even digestion temperature increases to 280℃. These results agree with previous works that increasing the digestion pressure and temperature can achieve more effective dissolution. Thus, a modified digestion procedure for determination of PGEs in ultramafic rocks by the re-usable Carius tube was proposed in this study.
PGEs; ultramafic rock; residue;aqua regia; Carius tube
1 Introduction
As valid indicators for geochemistry study of the Earth and planetary evolution, platinum group elements (PGEs),187Re-187Os and190Pt-186Os isotope systems have been extensively utilized in petromineralogy of iron meteorites (Shen et al., 1996; Smoliar et al., 1996, 1997), Ni-Cu sulfide ores (Han et al., 2006; Hu et al., 2008; Mao et al., 2003; Mathur et al., 2008; Ripley et al., 1998; Yang et al., 2008; Zhang et al., 2008; Zhong et al., 2011) and igneous mafic-ultramafic rocks (Jin et al., 2004; Lahaye et al., 2001; Puchtel et al., 1999). Accurate determination of PGEs and Re concentrations is key to the achievement of initial isotope ratios of186Os/188Os and187Os/188Os. However, ultramafic rocks contain abundant refractory minerals (e.g. chromite, olivine, etc.) which host most of the PGEs in the sample and are difficult to dissolve under relatively low temperatures. The conventional method of Carius tube digestion withaqua regiacannot ensure complete dissolution of PGEs in ultramafic rocks, resulting in inaccurate PGEs contents and initial Os ratios.
Previously reported digestion temperatures for mafic-ultramafic rocks normally range from 220-250℃ (Carius tube method)(Bennett et al., 2000; Brandon et al., 1999, 2000, 2006; Brooks et al., 1999; Chesley et al., 1999 Gao et al., 2008; Hao et al., 2011; Kent et al., 2004; Lambert et al., 1998; Li et al., 2010; Puchtel et al., 2005; Thompson et al., 2005; Tian et al., 2011; Walker et al., 1997; Zhang et al., 2008b; Zhu and Zheng, 2009) to 300℃ (high pressure asher method, HPA-S) (Barnes et al., 2009; Cucciniello et al., 2010; Dale et al., 2009; Kocks et al., 2007; Malitch et al., 2011; Melcher and Meisel, 2004; Pearson et al., 2004). Puchtel et al. (2004a, b) reported PGEs contents of a komatiite reference material (GP-13) from Carius tube-aqua regiadigestion underdifferent temperatures and time durations, which yielded poor reproducibility with no significant correlations between PGEs recovery and heating temperatures/time durations. This might relate to nugget effect of PGEs in samples. Qi and Zhou (2008) reported about 4%-15% of the PGEs remained in the silicate phases, which cannot be leached out byaqua regiaeven when digested at 300℃ using the Carius tube technique. Due to the serious nugget effect of PGEs, it is difficult to distinguish whether the result errors come from nugget effect or the uncompleted digestion. Therefore, only determination of PGEs in residues can examine whether the ultramafic samples are dissolved effectively.
Traditional Carius tube (Shirey and Walker, 1995) is fragile at relatively high temperatures, due to the inhomogeneity between its main body and neck. Moreover, both sealing Carius tubes and transferring samples into the tube are tough works. To solve these problems, a new re-usable Carius tube system has been reported recently (Qi et al., 2013). The newly designed Carius tube can be used repeatedly, easily sealed with a glass PTFE-lined stopper and easily loaded with sample powder due to the relatively large internal diameter of the neck (14 mm). This re-usable Carius tube can be used for determination of Re and Os in molybdenites and PGEs in WGB-1 (mafic rock) and UMT-1 (ultrmafic ore tailings). However, the efficiency for digesting ultramafic rocks has not been investigated yet. In this study, a Chinese ultramafic reference material, Gpt-3, was used as a sample to investigate PGEs content in silicate residues by using the re-usable Carius tube and autoclave-lined Carius tube technique at different temperatures.
2 Experimental
2.1 Instrumentation
The instrument used for PGEs analysis in this study is an ELAN DRC-e ICP-MS at the State Key Lab of Ore Deposit Geochemistry, Institute of Geochemistry, Chinese Academy of Sciences, Guiyang. Background counts for 2% HNO3solutions are normally less than 5 cps (counts per second) for PGEs. Relative standard deviations of 10 ng·mL-1PGE standard solution are typically less than 2% for raw counts. The sensitivity of the instrument was adjusted to more than 30000 cps for 1 ng·mL-1of115In, in order to achieve the desired detection limits. The instrument settings are outlined in Table 1.
2.2 Reagents and solutions
HCl was purified by double sub-boiling distillation. Water was obtained from an 18 MΩ cm grade Millipore purification system.
HNO3was firstly purified by sub-boiling distillation, and further by bubbling the clean air through the boiled reagent with H2O2to remove the volatile OsO4(Birck et al., 1997).
Spike solutions with enriched stable isotopes,193Ir,101Ru,105Pd,194Pt and190Os were prepared from pure metals (US Services Inc., Oxbow, N.J.) and then diluted to about 10-100 ng·g-1in 10% HCl solution and kept in a glass flask. Isotopic abundances of the spike are listed in Table 2. An ICP multi-element standard solution of 100 μg·mL-1Ir, Ru, Rh, Pt, Pd and Au (AccuStandard, USA) was used and diluted as needed for spike and external calibrations for the mono-isotopic element, Rh (Qi et al., 2004).
Te solution (1 mg·mL-1) was prepared by dissolving 0.25 g of TeO2in 10 mL of concentrated HCl, and diluted to 200 mL with distilled water.
A SnCl2solution (20%, w/v) was used for Te-coprecipitation and prepared by dissolving 50 g of SnCl2in 250 mL of 6 mol·L-1HCl. The Te- coprecipitation method was used to purify this solution and separate the PGEs (Qi et al., 2003).
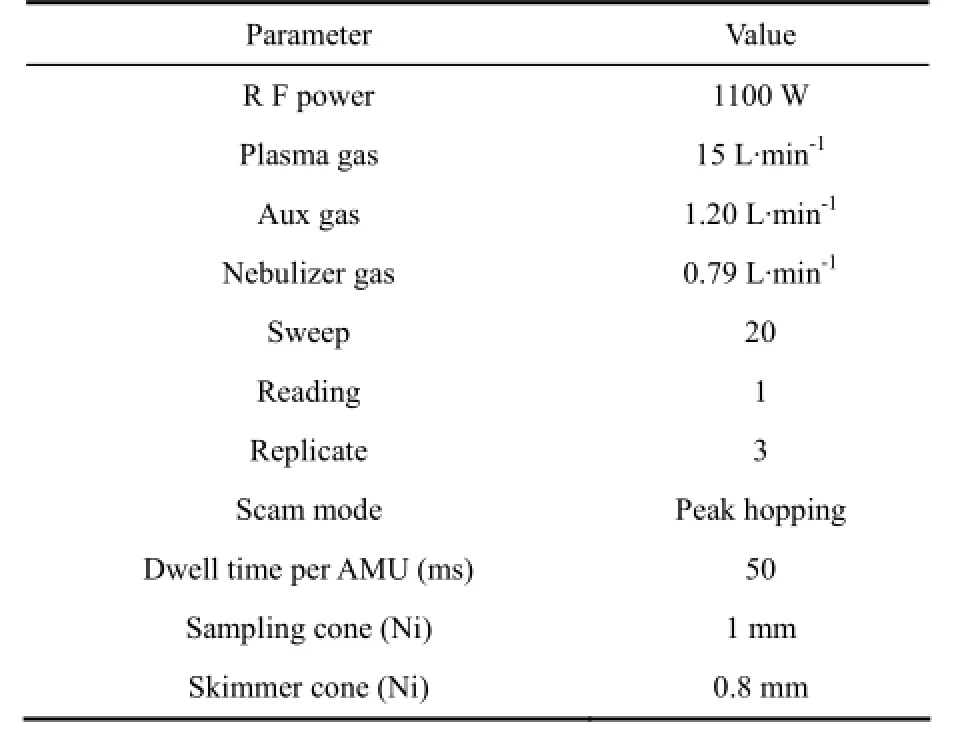
Table 1 Instrumental operating parameter
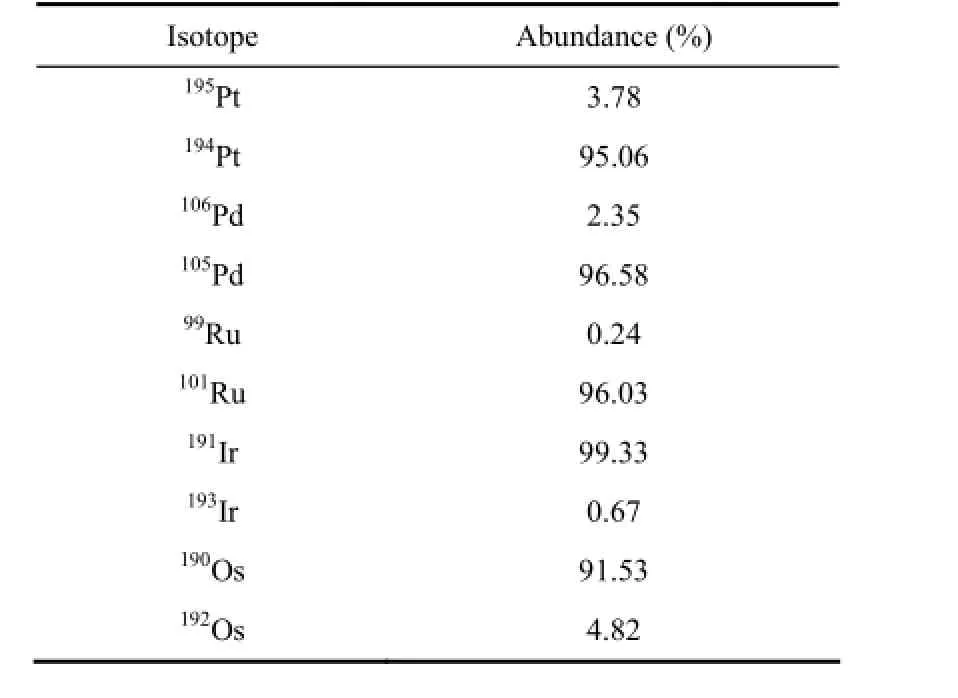
Table 2 Isotopic abundances of the spike
2.3 Laboratory ware
Carius tubes used at 220-240℃ in this study are screw top and PTFE stopper-lined re-usable Carius tubes described by Qi et al. (2013), which have an inner volume of about 200 mL. The main body has a wall thick of 3 mm, whereas both the neck and head have the same thickness of 4 mm. The custom-made sealing system includes a glass lined-PTFE stopper, a stainless steel screw cap. The tube and PTFE stopper were precleaned with 60%aqua regiaand 1% H2O2and heated to boiling for about two hours. After rinsed with water, the tubes were heated to 240℃ with 5 mL of HNO3for about two hours as sample digestion. Finally, the tube and stopper were rinsed with water and heated to 240℃ to minimize the possible Os contamination.
Because the re-usable Carius tube may explode at the temperature higher than 240℃, the Carius tubes used at 260-280℃ are conventional borosilicate glass tubes similar to those described by Shirey and Walker (1995), whereas a custom-made stainless steel high pressure autoclave was used to avoid possible explosion (Qi et al., 2007). The Carius tube has a length of 215 mm (the main body) with 21 mm inner diameter and 25 mm outer diameter, and an inner volume of about 75 mL. The tubes were also precleaned with 60%aqua regiaand then heated to 240℃ to remove possible Os.
125 mL Savillex Teflon beakers were precleaned with 60%aqua regiaand heated to boiling for about 2 hours and then rinsed with water.
Anin-situdistillation system (Qi et al., 2010, 2013) was used to distill Os from the matrix.
2.4 Analytical procedures
For digestion at 220-240℃, 3 g of Gpt-3 were accurately weighed and placed in a newly designed re-usable Carius tube (Qi et al., 2013) with 5 mL of 10 mol/L HCl and 15 mL of 16 mol/L HNO3. The tube was then sealed and placed in a stainless steel sheath, which was then heated to appropriate temperature (Table 3) in an electric oven for about 48 hours. After slowly cooling in air to room temperature, the Carius tube was further cooled in a refrigerator for 2 hours. The sample solution in the Carius tube was then transferred to a 50 mL centrifuge tube, and centrifuged at 2800 rpm for 10 minutes. The upper solution was transferred to a 125 mL Savillex Teflon beaker. The residue was rinsed with about 15 mL of 1.2 mol/L (or 6 mol/L) HCl (Table 3) for 6 times to completely remove dissolved PGEs from the residue. The resulting solution was combined with the previous solution in the 125 mL Savillex Teflon beaker. Only the first three upper solutions were combined to the beaker, because PGEs contents in the last three upper solutions can be ignored and the volume of solution can be reduced. Appropriate amounts of the enriched isotope spike solution containing193Ir,101Ru,194Pt and105Pd were accurately weighed and added into the beaker, which was then evaporated to dryness to remove HNO3for preconcentration of PGEs by Te-coprecipitation (Qi et al., 2004). Pt, Pd, Ru, and Ir were measured by isotope dilution, whilst194Pt was used as the internal standard to calculate the abundance of the mono-isotope element, Rh. These results are PGEs concentrations dissolved byaqua regia(Table 3).
After rinsed with 1.2 mol/L (or 6 mol/L) HCl for 6 times, the residue was transferred into another 125 mL Savillex Teflon beaker. 10 mL of concentrated HBr was added to prevent loss of volatile OsO4. Appropriate amounts of Ir, Ru, Pt, Pd and Os isotope spike solution were accurately weighed and added to the beaker with 10 mL of concentrated HF. Then, the beaker was placed for about 12 hours at room temperature. The solution was evaporated to dryness to remove the silicate. 10 mol/L HCl was used to remove the residual HF. Finally, the residue was dissolved by 2 mL of 10 mol/L HCl, and its solution was carefully transferred into another re-usable Carius tube directly. The tube was cooled with an ice water bath. 10 mL of 16 mol/L cooled HNO3was added into the tube and then the tube was sealed. The sealed Carius tube was placed in a stainless steel sheath, which was then sealed and heated to 240℃ in an electric oven for 24 hours. After slowly cooling in air to room temperature, the Carius tube was further cooled in a refrigerator for about 2 hours, and used forin-situdistillation of Os (Qi et al., 2010) and separation of PGEs by Te-coprecipitation as described above. These PGEs concentrations of residue are also listed in Table 3.
For digestion of sample at 260-280℃, the traditional Carius tubes coupled with custom-made stainless steel autoclaves were used to protect the tube from explosion instead of stainless steel sheath. The procedure is similar to the 220-240℃ cases described above.
3 Results and discussion
3.1 The absorption of PGEs for residue
During the experiment, we found if the sample was spiked initially, the isotope ratios of PGEs in the residue would be changed compared with the natural abundances (Table 4), even the residue was rinsed by 6 mol/L HCl for 10 times. This indicated that some of the spikes were still in the residue, and cannot be completely rinsed by 6 mol/L HCl. Therefore, the PGEs concentrations of the residue listed in Table 3 contain both the PGEs of residue and part of the PGEs ab-sorbed by residue from solution.
In order to calculate the exact PGEs concentrations absorbed by residue, Ru was used as an example to illustrate the calculation process. The total Ru content (CT) in residue contains two portions: one is the Ru concentration (CR) which did not dissolved byaqua regiain residue, another is absorbed Ru from the solution (CSo) by residue. When the samples were spiked, the spike content absorbed by residue (CS) can be calculated with the isotope dilution formula (1).
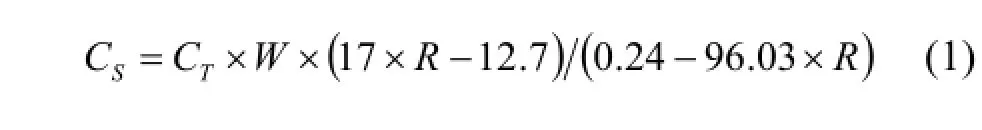
where the data of total Ru content (CT) in residue used in formula (1) is from Table 3 which is calculated by addition of spike to rinsed residue,Ris the measured ratio of99Ru/101Ru (Table 4) andWis the sample weight. Thus, the proportion of absorbed spike (RS) can be calculated by dividing the total spike added fromCS(Table 5). Because full isotopic equilibration can be achieved when highly oxidizingaqua regiais used, the proportion of absorbed nature Ru in solution by residue (RSo) has the same proportion as the absorbed spike (RS). Thus, the absorbed Ru from the solution (CSo) can be calculated as formula (2).

whereCSais the data of Ru content of the solution from Table 3. So the actual Ru content in residue can be calculated by formula (3).

Other actual PGEs concentrations in residue can be calculated by the method described above. Os content of the solution is the certified value. The results are shown in Table 6.
3.2 PGEs contents in residue
The PGEs contents in solution and residue for reference material, Gpt-3, after digestion withaqua regiaat different temperatures as described above are listed in Table 3. The PGEs concentrations of the solution show no significant correlations with the digestion temperature, which is probably due to nugget effect of PGEs. Our results are similar to those reported previously (Puchtel et al., 2004b; Palesskii et al., 2009). The total PGEs contents (residue+solution) agree well with the certified values of Gpt-3 (Table 6). However, PGEs concentrations in residues show a rough decreasing trend with the increase of digestion temperature (Table 3). The occasionally high PGEs contents at high temperatures may also caused by nugget effects of PGEs in the sample.

Table 3 PGEs concentrations in residue and solution afteraqua regiadissolution

Table 4 The change of PGEs ratios in the residue after using the spikes

Table 5 Calculated percentages of spikes absorbed by residue

Table 6 Calculated actual PGEs concentrations and the percentages of PGEs in residue
The percentages of spike absorbed by residue are listed in Table 5. Less than 0.5% of Os, Ir, Ru and Pt are absorbed by the residues, while the percentage of Pd is a little higher. Residues with relatively higher absorption percentage have relevantly higher PGEs concentrations (Tables 5 and 6).
The actual PGEs concentrations and percentages [CR/(CSa+CR)] in residue at different digestion temperatures are listed in Table 6. Most of the PGEs concentrations in residue are below 1.0 ng·g-1with less than 15% of the total contents. When digestion temperature reaches 280℃, all PGEs concentrations in residues are below 0.6 ng·g-1with less than 10% of the total contents. The elevated digestion temperature would promote the efficiency of dissolving, which is similar to the results reported previously (Reisberg and Meisel, 2002). The percentages of PGEs in residue show trends of Ir>Os>Ru and Pd>Pt. The percentages of each PGE in residue also show a roughly decreasing trend when the digestion temperature increases (Table 6). Even when the digestion temperature increases to 280℃, it is difficult to leach out all the PGEs from the residues. Therefore, the complete digestion of PGEs in ultrmafic rocks might require higher temperatures, which, however, would increase the possibility of Carius tube explosion. And for autoclave-lined Carius tube technique, it has complicated procedures. Thus a new digestion method is suggested in this study.
4 A suggested digestion method
To obtain reliable PGEs contents in ultramafic rocks, the silicate residue should be dissolved thoroughly. As discussed above, about 5% PGEs are still remained in residue for ultramafic rocks even the digestion temperature reaches to 280℃. Though the method of HNO3combined with HF digestion is effective to dissolve ultramafic rocks, Os cannot be measured by this technique (Qi et al., 2011). To simplify the analytical procedure, a suggested analytical method for determination of all PGEs in ultramafic rocks using the re-usable Carius tube at 240℃ is proposed as follow:
3 g of powdered sample and appropriate amounts of Ir, Ru, Pt, Pd and Os spikes are accurately weighed and placed in a re-usable Carius tube with 5 mL of HCl and 15 mL of HNO3. The tube is then sealed and heated in an electric oven at 240℃ for 24 hours. After cooling to room temperature, the tubes are moved to a refrigerator for about 2 hours, before opened forin-situOs distillation (Qi et al., 2010, 2013) and theconcentrated Os solution in trapping tube is still kept in a refrigerator. After distillation, the residue is separated and rinsed for 3 times by 6 M HCl as described above. All the rinsing solutions are transferred into a 125 mL Savillex Teflon beaker, whereas the residue is transferred into another 125 mL Savillex Teflon beaker. Concentrated HF and HBr are used to dissolve the silicate residue as above. After evaporating to dryness to remove HF with 10 M HCl, the residue is dissolved with 2 mL HCl and the solution is transferred to the original Carius tube, sealed again and heated at 240℃ for 12 hours to release all the residual PGEs. A second Os distillation is made as described using the original Os trapping tube. The residual solution is combined to the first 125 mL Teflon beaker and evaporated to dryness to remove HNO3with HCl. Te-coprecipitation is used to separate PGEs from matrix (Qi et al., 2004), and PGEs were then measured by ICP-MS. The analytical results for reference materials Gpt-3 are listed in Table 7. Our results agree well with the certified values. Although this suggested procedure is somewhat tedious, it can release all the PGEs from ultramafic rocks and ensure reliable results.

Table 7 Analytical results for reference materials Gpt-3 using suggested digestion techniques
5 Conclusions
The PGEs contents in residues were investigated for digestion of ultramafic rocks by reverseaqua regiain Carius tube at different temperatures. The residues contain about 2%-10% of total PGEs, even at digestion temperature of 280℃. The percentages of PGEs in residue show trends of Ir>Os>Ru and Pd>Pt. The residue can absorb a neglectable amount of the PGEs in the solution. A suggested digestion method using re-usable Carius tube was proposed for determination of PGEs in ultramafic rock.
AcknowledgementsThis research was funded by the China’s Lunar Exploration Program (TY3Q 20110029), “CAS Hundred Talents” Project from the Chinese Academy of Sciences to Prof. Qi Liang (KZCX2-YW-BR-09), and National Natural Science Foundation of China (NSFC 40973049). We would like to thank Mr. Li Tao for his painstaking works of opening and sealing the high-pressure autoclave and Mr. Li Liang for ICP-MS measurement.
Barnes S.J., Makkonen H.V., Dowling S.E., Hill R.E.T., and Peltonen P. (2009) The 1.88 Ga Kotalahti and Vammala nickel belts, Finland: Geochemistry of the mafic and ultramafic metavolcanic rocks [J].Bulletin of the Geological Society of Finland.81, 103-141.
Bennett V.C., Norman M.D., and Garcia M.O. (2000) Rhenium and platinum group element abundances correlated with mantle source components in Hawaiian picrites: Sulphides in the plume [J].Earth and Planetary Science Letters. 183, 513-526.
Birck J.L., RoyBarman M., and Capmas F. (1997) Re-Os isotopic measurements at the femtomole level in natural samples [J].Geostandards Newsletter. 21, 19-27.
Brandon A.D., Norman M.D., Walker R.J., and Morgan J.W. (1999)186Os-187Os systematics of Hawaiian picrites [J].Earth and Planetary Science Letters. 174, 25-42.
Brandon A.D., Snow J.E., Walker R.J., Morgan J.W., and Mock T.D. (2000)190Pt-186Os and187Re-187Os systematics of abyssal peridotites [J].Earth and Planetary Science Letters. 177, 319-335.
Brandon A.D., Walker R.J., and Puchtel I.S. (2006) Platinum-osmium isotope evolution of the Earth’s mantle: Constraints from chondrites and Os-rich alloys [J].Geochimica et Cosmochimica Acta.70, 2093-2103.
Brooks C.K., Keays R.R., Lambert D.D., Frick L.R., and Nielsen T.F.D. (1999) Re-Os isotope geochemistry of Tertiary picritic and basaltic magmatism of East Greenland: Constraints on plume-lithosphere interactions and the genesis of the Platinova reef, Skaergaard intrusion [J].Lithos. 47, 107-126.
Chesley J.T., Rudnick R.L., and Lee C.T. (1999) Re-Os systematics of mantle xenoliths from the East African Rift: Age, structure, and history of the Tanzanian craton [J].Geochimica et Cosmochimica Acta.63, 1203-1217.
Chu Zhuyin, Wu Fuyuan, Walker R.J., Rudnick R.L., Pitcher L. Puchtel I.S., Yang Yueheng and Wilde S.A. (2009) Destruction geodynamics of the North China craton and its Paleoproterozoic plate tectonics [J].Chinese Science Bulletin.54, 3354-3366.
Cucciniello C., Langone A., Melluso L., Morra V., Mahoney J.J., Meisel T., and Tiepolo M. (2010) U-Pb Ages, Pb-Os Isotope Ratios, and Platinum-Group Element (PGE) Composition of the West-Central Madagascar Flood Basalt Province [J].Journal of Geology.118, 523-541.
Dale C.W., Pearson D.G., Starkey N.A., Stuart F.M., Ellam R.M., Larsen L.M., Fitton J.G., and Macpherson C.G. (2009) Osmium isotopes in Baffin Island and West Greenland picrites: Implications for the187Os/188Os composition of the convecting mantle and the nature of high3He/4He mantle [J].Earth and Planetary Science Letters. 278, 267-277.
Gao Shan, Rudnick R.L., Xu Wenliang, Yuan Honglin, Liu Yongsheng, Walker R.J., Puchtel I.S., Liu Xiaomin, Huang Hua, Wang Xiaorui, and Yang Jie (2008) Recycling deep cratonic lithosphere andgeneration of intraplate magmatism in the North China Craton [J].Earth and Planetary Science Letters. 270, 41-53.
Han Chunming, Xiao Wenjiao, Zhao Guochun, Qu Wenjun, Mao Qigui, and Du Andao (2006) Re-Os isotopic analysis of the Kalatongke Cu-Ni sulfide deposit, northern Xinjiang, NW China, and its geological implication [J].Acta Petrologica Sinica.22, 163-170.
Hao Yanli, Huang Qishuai, Zhang Xiaoran, and Shi Rendeng. (2011) Re-Os isotopes of Dali picrite (Yunnan): New constraints on the formation of Emeishan Large Igneous Province [J].Acta Petrologica Sinica. 27, 2937-2946.
Hu Kebing, Yao Shuzhen, Qu Wenjun, Du Andao, and Ao Songjian (2008) Re-Os isotopic analysis of the Hulu Cu-Ni sulfide deposit magmatic ore systme, Esat Tianshan, Xinjiang, NW China [J].Acta Petrologica Sinica.24, 2359-2370.
Jin Yongbin, Zhi Xiachen, Meng Qing, Gao Tianshan, and Peng Zicheng (2004) Re-Os dating of the Raobazhai ultramafic massif in North Dabie [J].Chinese Science Bulletin.49, 508-513.
Kent A.J.R., Stolper E.M., Francis D., Woodhead J., Frei R., and Eiler J. (2004) Mantle heterogeneity during the formation of the North Atlantic Igneous Province: Constraints from trace element and Sr-Nd-Os-O isotope systematics of Baffin Island picrites [J].Geochemistry Geophysics Geosystems.5.
Kocks H., Melcher F., Meisel T., and Burgath K.P. (2007) Diverse contributing sources to chromitite petrogenesis in the Shebenik Ophiolitic Complex, Albania: Evidence from new PGE and Os isotope data [J].Mineralogy and Petrology.91, 139-170.
Lahaye Y., Barnes S.J., Frick L.R., and Lambert D.D. (2001) Re-Os isotopic study of komatiitic volcanism and magmatic sulfide formation in the southern Abitibi greenstone belt, Ontario, Canada [J].Canadian Mineralogist.39, 473-490.
Lambert D., Foster J., Frick L., Hoatson D., and Purvis A. (1998) Application of the Re-Os isotopic system to the study of Precambrian magmatic sulfide deposits of Western Australia [J].Australian Journal of Earth Sciences.45, 265-284.
Li Jie, Xu Jifeng, Suzuki K., He Bin, Xu Yigang, and Ren Zhongyuan (2010) Os, Nd and Sr isotope and trace element geochemistry of the Muli picrites: Insights into the mantle source of the Emeishan Large Igneous Province [J].Lithos. 119, 108-122.
Malitch K.N., Efimov A.A., and Badanina I.Y. (2011) Contrasting platinum-group mineral assemblages from chromitites of the Nizhny Tagil and Guli massifs (Russia): Implications for composition, sources and age [J].Doklady Earth Sciences.441, 1514-1518.
Mao Jingwen, Yang Jianming, Qu Wenjun, Du Andao, Wang Zhiliang, and Han Chunming (2003) Re-Os age of Cu-Ni ores from the Huangshandong Cu-Ni sulfide deposit in the East Tianshan Mountains and its implication for geodynamic processes [J].Acta Geologica Sinica-English Edition.77, 220-226.
Mathur R., Tornos F., and Barra F. (2008) The Aguablanca Ni-Cu deposit: A Re-Os isotope study [J].International Geology Review.50, 948-958.
Melcher F. and Meisel T. (2004) A metamorphosed early Cambrian crust-mantle transition in the Eastern Alps, Austria [J].Journal of Petrology.45, 1689-1723.
Palesskii S.V., Nikolaeva I.V., Koz’menko O.A., and Anoshin G.N. (2009) Determination of platinum-group elements and rhenium in standard geological samples by isotope dilution with mass-spectrometric ending [J].Journal of Anaytical Chemistry64, 272-276.
Pearson D.G., Irvine G.J., Ionov D.A., Boyd F.R., and Dreibus G.E. (2004) Re-Os isotope systematics and platinum group element fractionation during mantle melt extraction: A study of massif and xenolith peridotite suites [J].Chemical Geology. 208, 29-59.
Puchtel I.S., Brandon A.D., and Humayun M. (2004a) Precise Pt-Re-Os isotope systematics of the mantle from 2.7 Ga komatiites [J].Earth and Planetary Science Letters. 224, 157-174.
Puchtel I.S., Brandon A.D., Humayun M., and Walker R.J. (2005) Evidence for the early differentiation of the core from Pt-Re-Os isotope systematics of 2.8 Ga komatiites [J].Earth and Planetary Science Letters. 237, 118-134.
Puchtel I.S., Brugmann G.E., and Hofmann A.W. (1999) Precise Re-Os mineral isochron and Pb-Nd-Os isotope systematics of a mafic-ultramafic sill in the 2.0 Ga Onega plateau (Baltic Shield) [J].Earth and Planetary Science Letters. 170, 447-461.
Puchtel I.S., Humayun M., Campbell A.J., Sproule R.A., and Lesher C.M. (2004b) Platinum group element geochemistry of komatiites from the Alexo and Pyke Hill areas, Ontario, Canada [J].Geochimica et Cosmochimica Acta.68, 1361-1383.
Qi Liang, Gao Jianfeng, Zhou Meifu, Hu Jing (2013) The design of re-usable Carius tubes for the determination of Rhenium and Osmium and platinum group elements in geological samples [J].Geostandards and Geoanalytical Research. 37, 345-351.
Qi Liang and Zhou Meifu (2008) Determination of platinum-group elements in OPY-1: Comparison of results using different digestion techniques [J].Geostandards and Geoanalytical Research.32, 377-387.
Qi Liang., Gao Jianfeng, Huang Xiaowen, Hu Jing, Zhou Meifu, and Zhong Hong (2011) An improved digestion technique for determination of platinum group elements in geological samples [J].Journal of Analytical Atomic Spectrometry. 26, 1900-1904.
Qi Liang, Gregoire D.C., Zhou Meifu, and Malpas J. (2003) Determination of Pt, Pd, Ru and Ir in geological samples by ID-ICP-MS using sodium peroxide fusion and Te co-precipitation [J].Geochemical Journal.37, 557-566.
Qi Liang, Zhou Meifu, and Wang C.Y. (2004) Determination of low concentrations of platinum group elements in geological samples by ID-ICP-MS [J].Journal of Analytical Atomic Spectrometry. 19, 1335. Qi Liang, Zhou Meifu, Gao Jianfeng, and Zhao Zheng (2010) An improved Carius tube technique for determination of low concentrations of Re and Os in pyrites [J].Journal of Analytical Atomic Spectrometry. 25, 585.
Qi Liang, Zhou Meifu, Wang C.Y., and Sun Min (2007) Evaluation of a technique for determining Re and PGEs in geological samples by ICP-MS coupled with a modified Carius tube digestion [J].Geochemical Journal.41, 407.
Reisberg L. and Meisel T. (2002) The Re-Os Isotopic System: A Review of Analytical Techniques [J].Geostandards Newsletter.26, 249-267.
Ripley E.M., Lambert D.D., and Frick L.R. (1998) Re-Os, Sm-Nd, and Pb isotopic constraints on mantle and crustal contributions to magmatic sulfide mineralization in the Duluth Complex [J].Geochimica et Cosmochimica Acta.62, 3349-3365.
Shen J.J., Papanastassiou D.A., and Wasserburg G.J. (1996) Precise Re-Os determinations and systematics of iron meteorites [J].Geochimica et Cosmochimica Acta.60, 2887-2900.
Shirey S.B. and Walker R.J. (1995) Carius tube digestion for low-blank rhenium-osmium analysis [J].Analalytical Chemistry.67, 2136-2141.
Smoliar M.I., Walker R.J., and Morgan J.W. (1996) Re-Os ages of group IIA, IIIA, IVA, and IVB iron meteorites [J].Science.271, 1099-1102.
Smoliar M.I., Walker R.J., and Morgan J.W. (1997) Rhenium-osmium isochron for IA meteorites: Further refinement of the rhenium-187 decay constant [J].Meteoritics & Planetary Science.32, A122-A123.
Thompson R.N., Ottley C.J., Smith P.M., Pearson D.G., Dickin A.P., Morrison M.A., Leat P.T., and Gibson S.A. (2005) Source of the quaternary alkalic basalts, picrites and basanites of the Potrillo Volcanic Field, New Mexico, USA: Lithosphere or convecting mantle? [J].Journal of Petrology.46, 1603-1643.
Tian W., Chen B., Ireland T.R., Green D.H., Suzuki K., and Chu Z. (2011) Petrology and geochemistry of dunites, chromitites and mineral inclusions from the Gaositai Alaskan-type complex, North China Craton: Implications for mantle source characteristics [J].Lithos.127, 165-175.
Walker R.J., Morgan J.W., Beary E.S., Smoliar M.I., Czamanske G.K., and Horan M.F. (1997) Applications of the190Pt-186Os isotope system to geochemistry and cosmochemistry [J].Geochimica et Cosmochimica Acta.61, 4799-4807.
Yang Shenghong, Qu Wenjun, Tian Yulong, Chen Jiangfeng, Yang Gang, and Du Andao (2008) Origin of the inconsistent apparent Re-Os ages of the Jinchuan Ni-Cu sulfide ore deposit, China: Post-segregation diffusion of Os [J].Chemical Geology.247, 401-418.
Zhang Zhaochong, Zhi Xiachen, Chen Lei, Saunders A.D., and Reichow M.K. (2008b) Re-Os isotopic compositions of picrites from the Emeishan flood basalt province, China [J].Earth and Planetary Science Letters. 276, 30-39.
Zhang Zuoheng, Mao Jingwen, Du Andao, Pirajno F., Wang Zhiliang, Chai Fengmei, Zhang Zhaochong, and Yang Jianmin, (2008). Re-Os dating of two Cu-Ni sulfide deposits in northern Xinjiang, NW China and its geological significance [J].Journal of Asian Earth Sciences.32, 204-217.
Zhong Hong, Qi Liang, Hu Ruizhong, Zhou Meifu, Gou Tizhong, Zhu Weiguang, Liu Bingguang, and Chu Zhuyin (2011) Rhenium-osmium isotope and platinum-group elements in the Xinjie layered intrusion, SW China: Implications for source mantle composition, mantle evolution, PGE fractionation and mineralization [J].Geochimica et Cosmochimica Acta.75, 1621-1641.
10.1007/s11631-014-0658-2
Received May 5, 2013; accepted June 29, 2013
© Science Press and Institute of Geochemistry, CAS and Springer-Verlag Berlin Heidelberg 2014
杂志排行
Acta Geochimica的其它文章
- Zircon U-Pb age and Hf isotopic characteristics of the Huangtuliang monzonitic granite, North Hebei Province, China
- Petrography and geochemistry of Jumara Dome sediments, Kachchh Basin: Implications for provenance, tectonic setting and weathering intensity
- Scientific data and their release of Chang’E-1 and Chang’E-2
- Geochemistry of rare-earth elements in shallow groundwater, northeastern Guangdong Province, China
- Fluid inclusion, siliceous rock geochemistry of Shewushan lateritic gold deposit, Hubei Province, eastern China: Implication for the genesis of primary orebody
- Carbon and oxygen isotopes suggesting deep-water basin deposition associated with hydrothermal events (Shangsi Section, Northwest Sichuan Basin-South China)
