N-琥珀酰低聚壳聚糖的抗氧化性能研究
2014-01-11熊小英银旭红
李 铭,熊小英,谢 晶,薛 斌,银旭红,孙 涛
上海海洋大学食品学院 上海水产品加工储藏工程研究中心,201306 上海
Introduction
Chitosan is a natural cationic polysaccharide obtained from the N-deacetylation chitin widely occurring in the nature.It has been widely applied in many fields such as food,pharmaceutics,cosmetics and chemical[1],owing to its excellent properties such as antibacterial activity,biocompatibility,nontoxicity and so on.In recent years,antioxidant activities of chitosan and its derivatives have attracted the most attention[2].The sulfated chitosan derivatives and quaternary chitosan derivatives have been reported to have a good antioxidant activity[3].But relatively little work has been reported on the antioxidant activity of acyl chitosan derivatives.Succinyl chitosan is an important acyl chitosan derivative.It has been reported that succinyl chitosan has many functional properties such as antitumour,drug carried,antibacterial activity,moisture absorption and moisture retention[4],but its antioxidant activity,especially,effect of substituting degrees of N-succinyl chitosan oligosaccharides(NSCOSs)on its antioxidant activity has not been reported.
Three NSCOSs with different substituting degrees were synthesized in order to investigate the relationship between antioxidant activity of NSCOSs and its substituting degrees in this paper.Their antioxidant activity was investigated by scavenging of superoxide radical,hydroxyl radical,DPPH radical and the determination of reducing power.
Materials and Methods
Chemicals
Chitosan oligosaccharides(COS,5000 Da)were purchased from zhejiang Golden-Shell Pharmaceuticol CO.,Ltd.Shanghai United Food Additives Co.(Shanghai,China).Luminol and DPPH were purchased from Sigma-Aldrich Chemical Co.(St.Louis,USA).All other chemicals were analytical grade reagents,supplied by Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China)
The preparation and characterization of NSCOSs
NSCOSs were synthesized following the method of Zhu[5].5 g COS was dissolved in deionized water(100 mL).Succinic anhydride(1 g in 50 mL acetone)was added by drop-wise for 30 min at room temperature,and then the reaction was kept for 4 h at 40 ℃.The resultant solution was added by drop-wise into acetione,filtered,washed with acetione repeatedly,then dried under vacuum at 60 ℃ to obtain NSCOSA.NSCOSB and NSCOSC were prepared according to the similar procedure by using 5 g and 6 g succinic anhydride,respectively.
FT-IR spectra were taken with KBr pellets on an EQUNOX55 FT-IR-Laman spectrophotometer with a revolution of 2.0 cm-1in the range of 400(4000 cm-1.The substituting degrees of NSCOSs were measured by conductometric titration according the method of Huo[6].
The degrees of substitution(DS)of NSCOSA、NSCOSB and NSCOSC were assayed by the method of Huo[6].
Superoxide anion radical scavenging activity
The superoxide radical scavenging activity was determined using chemiluminescence technology[7].The assay was carried out on a chemical luminometer(IFFMD,Xi’an,China).The chemiluminescent reaction was processed in a Na2CO3-NaHCO3(pH=10.20,0.05 M)buffer solution.Scavenging activity of the samples was evaluated according to their quenching effects on the chemiluminescence signal of the luminal-pyrogallol system.The capability of scavenging against superoxide anion using the following equation:scavenging effect(%)=(CL0-CL1)/CL0×100%,where CL0and CL1represent chemiluminescence peak areas of the blank group and test group,respectively.
The free radical produced in the system was proved to be superoxide radical tested by superoxide dismutase,catalase and mannitol.
Hydroxyl radical scavenging activity
The hydroxyl scavenging activity was determined using chemiluminescence technology.The assay was conducted on a chemical luminometer[8].The chemiluminescent reaction was processed in a KH2PO4-NaOH(pH=7.40,0.05 M)buffer solution.Scavenging activity of the samples was evaluated according to their quenching effects on the chemiluminescence signal of the system.The capability of scavenging against hydroxyl radical was calculated according to the following equation:scavenging effect(%)=(CL0-CL1)/CL0×100%,where CL0and CL1represent chemiluminescence peak areas of the blank group and test group,respectively.The free radical produced in the system was proved to be hydroxyl radical tested by superoxide dismutase,catalase and mannitol.
DPPH radical scavenging activity
The DPPH scavenging activity of the samples was measured using the modified method of Yamaguchi[9].2.0 mL of methanolic solution of DPPH(0.1 mM)was incubated with varying concentrations of test samples(2.0 mL).The reaction mixture was shaken well and incubated for 30 min at 33 ℃and the absorbance of the resulting solution was read at 517 nm against a blank.The radical scavenging activity was measured using the following equation:Scavenging effect(%)=(1_Asample/Acontrol)×100%.
Measurement of reducing power
The reducing power of all samples was determined by the method of Yen[10].Different concentrations of samples(2.0 mL)were mixed with 2.5 mL sodium phosphate buffer(pH=6.60,0.2 M)and 2.5 mL potassium ferricyanide(1% W/V).The mixtures were incubated for 20 min at 50 ℃,and then 2.5 mL trichloroacetic acid(10% W/V)was added to the mixtures,followed by centrifugation at 3000 rpm for 10 min.The supernatant was mixed with 2.5 mL distilled water and 0.5 mL ferric chloride solution(0.1% W/V)and the absorbance was measured at 700 nm.Increased absorbance of the reaction mixture indicated increased reducing power.
Statistical analysis
All analyses were performed in triplicate.Data of antioxidant evaluation were expressed as mean±standard error of the mean.SPSS 11(SPSS Inc.,Chicago,USA)was used to evaluate the significant difference at P<0.05.
Results and Discussions
Structure characterization of COS and NSCOSs
As shown in Fig.1,the FT-IR spectra of NSCOSs have the characteristic absorption bands of COS[11].A new absorption band of NSCOSs at 1400-1200 cm-1,corresponding to the carboxyl group of the succinyl,indicated that COS reacted with succinic anhydride.The increase of the amide I peak(1655 cm-1)and decrease of the amide II peak(1562 cm-1)indicated the succinyl reaction took place at the N-position[12].In addition,there are no changes in bands at 1038 cm-1and 1071 cm-1,corresponding to the stretching vibration of the primary and secondary-OH group,respectively,which suggested that the oxygen atoms in the hydroxyl group in COS were not involved in the reaction[13].
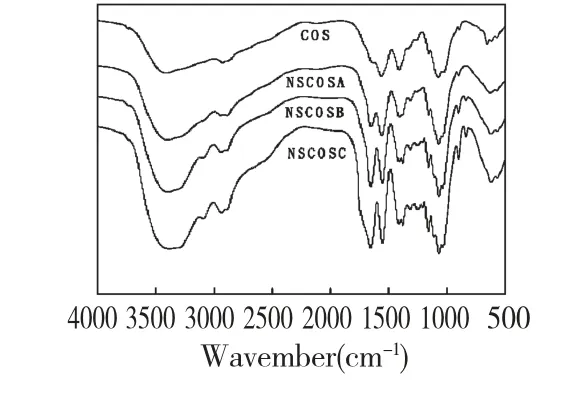
Fig.1 FTIR spectra of COS,NSCOSA,NSCOSB and NSCOSC
The substituting degrees of NSCOSA,NSCOSB and NSCOSC were to be 0.25,0.57 and 0.70,respectively.
Superoxide anion radical scavenging activity
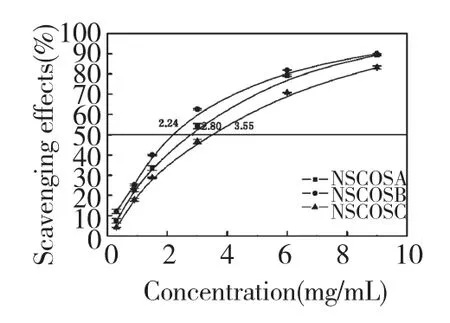
Fig.2 Scavenging effects of NSCOSA,NSCOSB and NSCOSC on superoxide anion
Superoxide anion is very harmful to cellular components as a precursor of more reactive oxidative species,such as single oxygen and hydroxyl radicals.The superoxide radical scavenging effect of NSCOSA,NSCOSB and NSCOSC is shown in figure 2.The 50% inhibition concentrations(IC50)of the NSCOSA,NSCOSB and NSCOSC were 2.80 mg/mL,2.24 mg/mL and 3.55 mg/mL,respectively.The results indicated that NSCOSB(its DS was 0.57)showed the strongest superoxide anion scavenging activity.The antioxidant activity of NSCOSs may be affected by the substituting degrees and properties of the substituting group.As an electronwithdrawing group,succinic anhydride may reduce the electron cloud density in the COS molecules chain,thereby reduce the probability of generating hydrogen bonding between hydroxyl groups and amino groups,thus the content of active amino groups and hydroxyl groups reacted with superoxide anion increased,that is the scavenging effect on superoxide anion increased when DSs increased from 0.25 to 0.57.Interestingly,while DSs further improved to 0.70,the scavenging effect of NSCOSC was weaker compared to NSCOSB.It could be related to the content of active amino groups decreased quickly because of higher substituting degree and the steric hindrance increased sharply,therefore the scavenging effect of NSCOSC decreased compared to NSCOSB.Wang also report that the antioxidant activity of COS derivatives was reduced because the amino and hydroxyl were substituted[14].Similarly,in this antioxidant evaluation system COS had better superoxide anion scavenging activity(the IC50was 1.80 mg/mL).
Hydroxyl radical scavenging activity
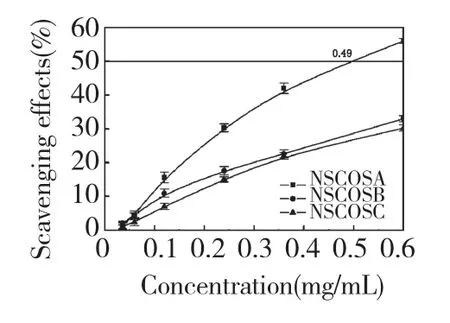
Fig.3 Scavenging effects of NSCOSA,NSCOSB and NSCOSC on hydroxyl radical
Figure 3 depicted the hydroxyl radial scavenging effect of NACOSs.Scavenging activity of hydroxyl radical increased with the increasing of concentration of the NSCOSs.The 50% inhibition concentrations(IC50)of NSCOSA was 0.49 mg/mL,however,IC50of NSCOSB and NSCOSC could not be read.The order of the NSCOSs scavenging effect on·OH was NSCOSA>NSCOSB>NSCOSC.Unlike superoxide anion,the hydroxyl radial is an extraordinary active radial with a short half-life[15],therefore the steric hindrance of reaction between the radicals and NSCOSs is an important factor influencing the hydroxyl radial scavenging activity.The hydroxyl radial scavenging effect of NACOSs decreased with the DSs increasing.Compared to NACOSs,COS had the better hydroxyl radical scavenging activity(the IC50was 0.26 mg/mL).
DPPH scavenging activity
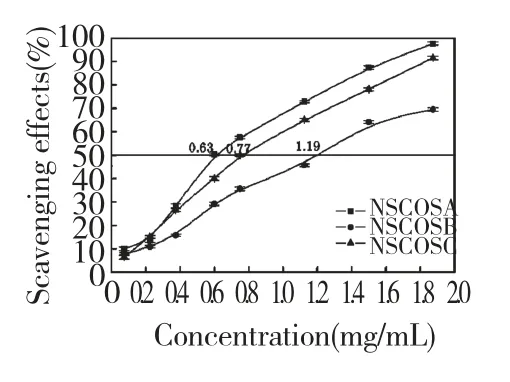
Fig.4 Scavenging effects of NSCOSA,NSCOSB and NSCOSC on DPPH radical
DPPH is one of the compounds that possessed a proton free radical with a characteristic absorption,which decreases significantly on exposure to proton radical scavenger.Further it is well accepted that the DPPH free radical scavenging by antioxidants is due to their hydrogen-donating ability[16].Figure 4 showed the DPPH radial scavenging effect of NSCOSs.Scavenging activity of DPPH radical increased with the concentration of NSCOSA,NSCOSB and NSCOSC.Moreover,IC50of NSCOSA,NSCOSB and NSCOSC were 0.63 mg/mL,1.19 mg/mL and 0.77 mg/mL,respectively.The order of the NSCOSs scavenging effect on DPPH was NSCOSA>NSCOSC>NSCOSB.Similarly,in this antioxidant evaluation system COS had the better DPPH scavenging activity than NSCOSs(the IC50of COS was 0.37 mg/mL).DPPH scavenging activity of NSCOSs is different from superoxide anion and hydroxyl radical scavenging activity.The result may be related to the substituting degrees,the properties of the substituting group and the different scavenging mechanisms against free radicals.
Reducing power
Reducing power assay has also been used to evaluate the ability of antioxidants to donate electrons.It was reported serve as a significant indicator of antioxidant activity for a compound[17].Figure 5 depicted the reducing power of NSCOSs.NSCOSs showed obvious reducing power and correlated well with increasing concentrations.At a concentration of 2.0 mg/mL,the absorbances of NSCOSA,NSCOSB and NSCOSC were 0.41,0.21,and 0.26,respectively.The reducing power order of the NSCOSs was NSCOSA>NSCOSC>NSCOSB,which showed the same regularity with the result of DPPH scavenging activity.COS also had the best reducing power in this test system,its absorbance was 0.69 at the concentration of 2.0 mg/mL.

Fig.5 Reducing power of NSCOSA,NSCOSB and NSCOSC
Conclusion
It has been reported that in different antioxidant evaluation system,the effect of substituting degree on the antioxidant activity of chitosan oligosaccharides derivatives has a greater difference.In the paper,three NSCOSs with different substituting degrees were prepared,and their antioxidant activities were evaluated.The result showed that the three NSCOSs have different antioxidant activities for superoxide radical,hydroxyl radical,DPPH and reducing power.The results may be related with the properties of substituting group,the substituting degrees,the properties of radial and the scavenging mechanisms against free radicals.
This project was supported by Foundation item:National "Twelfth Five-Year" Plan for Science & Technology Support(2011BAD24B02)and Engineering Research Center of Shanghai Committee of Science and Technology(11DZ2280300).
Reference
1 Dutta PK,Shipra T,Mehrotra G K,et al.Perspectives for chitosan based antimicrobial films in food applications.Food Chemistry,2009,114:1173-1182.
2 Xie WM,Xu PX,Liu,Q.Antioxidant activity of water-soluble chitosan derivatives.Bioorganic & Medicinal Chemistry Letters,2001,11:1699-1701.
3 Guo ZY,Xing R,G Liu S,et al.Synthesis and hydroxyl radicals scavenging activity of quaternized carboxymethyl chitosan.Carbohydrate Polymers,2008,73:173-177.
4 Yoshinori K,Hiraku O,Yoshiharu M.N-succinyl-chitosan as a drug carrier:water-insoluble and water-soluble conjugates.Biomaterials,2004,25:907-915.
5 Zhu AP,Chen T,Yuan LH,et al.Synthesis and characterization of N-succinyl-chitosan and its self-assembly of nanospheres.Carbohydrate Polymers,2006,66:274-279.
6 Huo XY,Zhang XH,Wu Q.Determination of substitution degree of N-succinyl-chitosan by conductometric titration.Journal of Beijing University of Traditional Chinese Medicine,2007,30:700-702.
7 Sun T,Xie WM,Xu PX.Superoxide anion scavenging activity of graft chitosan derivatives.Carbohydrate Polymers,2004,58:379-382.
8 Sun T,Yao Q,Zhou DX.Antioxidant activity of N-carboxymethyl chitosan oligosaccharides.Bioorganic & Medicinal Chemistry,2008,18:5774-5776.
9 Yamaguchi T,Takamura H,Matoba T,et al.HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl.Bioscience,Biotechnology,and Biochemistry,1998,62:1201-1204.
10 Yen GC,Chen HY.Antioxidant activity of various tea extracts in relation to their antimutagenicity.J Agric Food Chem,1995,43:27-32.
11 Warayuth S,Supawan T,Varawut T,et al.Synthesis and characterization of N-aryl chitosan derivatives.International Journal of Biological Macromolecules,2008,43:79-87.
12 Hirano S,Sakaguchi T,Kuramitsu K.N-Alanyl and some N-(N-aryl)glycyl derivatives of chitosan.Carbohydrate Polymers,1992,19:135-138.
13 Hirano S,Ohe Y,Ono H.Selective N-acylation of chitosan.Carbohydrate Research,1976,47:315-320.
14 Wang Q,Wang AQ.Study on the synthesis and properties of N-Succinyl hitosan.Journal of Functional Polymers,2004,17:51-54.
15 Halliwell B,Murcia MA,Chirico S.et al.Free radicals and antioxidants in food and in vivo:What they do and how they work.Critical Reviews in Food Science and Nutrition,1995,35:7-20.
16 Chen CW,Ho CT.Antioxidant properties of polyphenols extracted from green and black teas.J Food Lipids,1995,2:35-46.
17 Duh PD,Du PC,Yen GC.Action of methanolic extract of mung bean hulls as inhibitors of lipid peroxidation and nonlipid oxidative damage.Food Chem Toxicol,1999,37:1055-1061.
