Expression of cytochrome P450 2A13 in human non-small cell lung cancer and its clinical significance
2013-12-23LiSunXiaoliFan
Li Sun , Xiaoli Fan
Department of Histology and Embryology, College of Basic Medicine of Guilin Medicial University, Guilin, Guangxi 541004, China.
INTRODUCTION
Human cytochrome P450 (CYP450) enzymes are the principal enzymes involved in the metabolic activation of many compounds, such as drugs, environmental toxicants and chemical carcinogens[1]. Variations of P450 enzymes in expression or activity can contribute substantially to inter-individual differences in therapeutic efficacy or toxicant susceptibility[2]. CYP2A13 is a member of the CYP2A gene subfamily of P450s, which is predominantly expressed in the nasal mucosa and also in the lung and trachea[3,4]. CYP2A13 is highly effective in the metabolic activation of many respiratory-tract toxicants, especially 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), which is a tobacco-specific carcinogen[5-7]. Furthermore, it was found that CYP2A13 is active for the metabolism of aflatoxin B1[8]. The expression of CYP2A13 may also substantially influence the incidence of respiratory tumor. However, the precise role of CYP2A13 in carcinogenesis and tumor progression is still unclear. In the present study, we measured the expressions of CYP2A13 in lung cancer and paired normal tissues and examined their relationship with clinicopathological factors.
MATERIALS AND METHODS
Tissue samples
Thirty-five surgical specimens of adenocarcinoma and squamous cell carcinoma were used for the current study. No patients had received chemotherapy before surgery. These patients underwent surgery between 2009 and 2010 at Guilin Medical University Hospital, Guilin, Guangxi, China. Tumor and normal lung tissue samples were obtained from the resected specimens, and snap-frozen in liquid nitrogen and stored at -80°C for further analysis. The study protocol was approved by the local institutional board at the authors' affiliated institution. The use of human tissue specimens for experimental purposes was done in accordance with the established institutional and national guidelines regarding experimental use of human tissues.
Chemicals and reagents
PrimeScript RT Reagent Kit with gDNA Eraser (Perfect Real Time) and SuperReal PreMix (SYBR Green) were purchased from Takara (Dalian,Shandong, China) and Tiangen (Beijing, China), respectively. Cell lysis buffer for Western blotting assays and IP and Enhanced BCA Protein Assay Kit BCA was purvchased from Beyotime (Beijing, China).
Preparation of antibody
Rabbit polyclonal antibody against human CYP2A13 and biotinylated goat anti-rabbit IgG were obtained from Bioworld (Louis Park, MN, USA). Mouse monoclonal antibody against human β-actin goat anti-mouse IgG were purchased from Beyotime (Beijing, China).
Quantitative analysis of CYP2A13 mRNA
Total RNA (1 μg) was extracted from frozen samples by using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. The quality of the RNA samples was determined by electrophoresis through 1% agarose gels and the 18S and 28S bands were visualized under ultraviolet light. RNA samples were cleaned by using PrimeScript RT Reagent Kit with gDNA Eraser to ensure that no contaminating genomic DNA was present and then converted to cDNA (Takara). Gene expression was measured by SYBR green real-time PCR by using ABI 7500 FAST detection system applied biosystems (ABI, New York, USA). β-actin, a housekeeping gene, was used as an internal control. The primer sequences were as follows: CYP2A13-forward: 5'-ATA AGA AGG GGC AGT TTA AGA AGA G-3', reverse: 5'-ATG ATG GTG GTG AAG AAG AGA AAG-3', β-actin -forward: 5'-AGC GAG CAT CCC CCA AAG TT-3', reverse: 5'-GGG CAC GAA GGC TCA TCA TT-3'. The PCR system was performed in 20 μL buffers containing 1 μL cDNA, 0.4 μL of 50×ROX Reference Dye and 50 pmol of primer pairs (Tiangen). Thermocycling conditions consisted of a pre-incubation for 15 minutes at 95°C and 60°C for 30 seconds, followed by 40 cycles of denaturation for 10 seconds at 94°C. PCR products were separated by 2% agarose gel electrophoresis and sequenced to identify the specificity.
The experiment used comparative CT method to present relative gene expression (also known as the 2-ΔΔCtmethod). The PCR efficiency of the CYP2A13 gene must be similar to the internal control gene β-actin. The PCR efficiency of CYP2A13 and β-actin were included in the equation; therefore, the differences in the efficiency between target and internal control will be accounted for in the calculation. The equation is: 2-ΔΔCt= [CTCYP2A13- CTβ-actin]tumor- [CTCYP2A13- CTβ-actin]adjacentnormaltissue. In this way, the data may be interpreted as "the expression of the CYP2A13 gene to β-actin in the lung cancer compared with the paired normal tissue".
Western blotting assays
The proteins prepared from tissue samples were subjected to SDS-polyacrylamide gel (12%), and then transferred electrophoretically to a nitrocellulose membrane. The membrane was blocked in 5% non-fat dry milk for 1 hour at 37°C before incubation with the monoclonal anti-human CYP2A13 antibody against human and β-actin at room temperature for 2 hours. After washing with tris buffered saline, the membrane was incubated with anti-rabbit IgG and anti-mouse IgG (both conjugated with horseradish peroxidase) for 1 hour at room temperature to detect CYP2A13 and β-actin. The immunoblots were visualized by enhanced chemiluminescence (ECL) in the manufacturer's protocol.
Statistical analysis
For statistical analyses, SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, USA) was performed. The expressions of CYP2A13 between lung cancer and paired normal tissue were investigated using the paired-sample t-test and the relationship between CYP2A13 expression and clinicopathological factors were examined by using independent-sample t test. P value < 0.05 was considered significant.
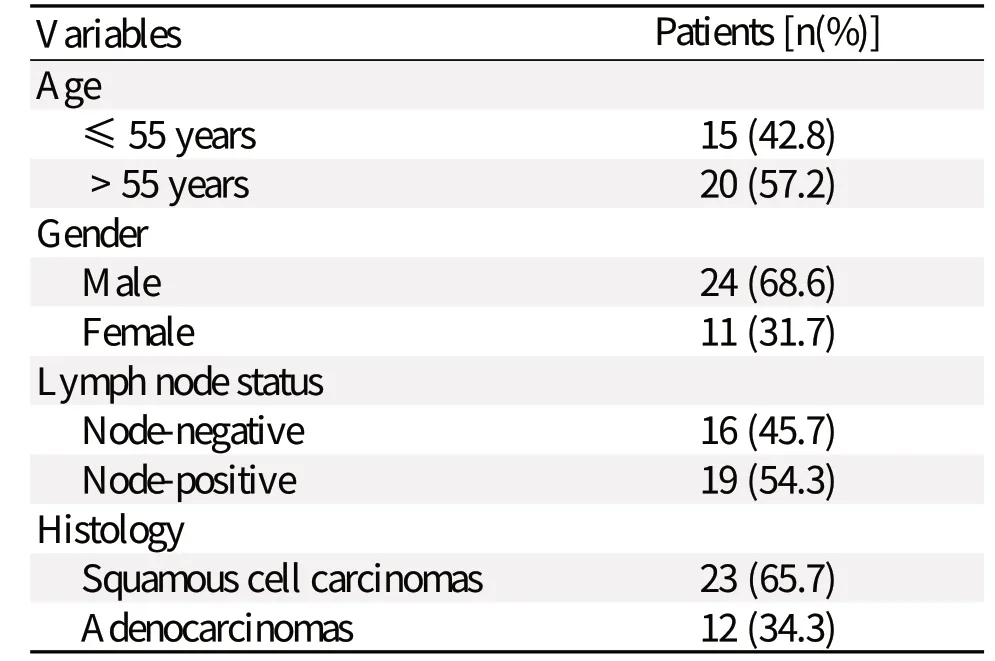
Table 1 Patient demographic and baseline characteristics
RESULTS
Patient demographic and baseline characteristics are shown in Table 1. We used H&E staining to identify different pathological types (Fig. 1). Twelve (34.3%, 12/35) patients had adenocarcinoma and 23 (65.7%) patients had squamous cell carcinoma. Nineteen (54.3%) patients had metastasis to lymph nodes.
CYP2A13 mRNA levels in adult human lung tissue samples
The CYP2A13 mRNA transcript levels were 11.92±4.08 for the tumor tissues (n = 35) and 10.18±5.88 for adjacent paired normal tissues (n = 35) (Table 2). Paired-sample t-test was used to analyze the expression levels of CYP2A13 mRNA in the 35 breast cancer samples and paired normal tissues and no significant difference was found between them (P = 0.059). The CYP2A13 mRNA transcript levels were 11.53±4.45 for the squamous cell carcinoma tissues (n = 12) and 12.31±6.76 for adjacent paired normal tissues (n = 12). The expression level of CYP2A13 mRNA transcripts between squamous cell carcinoma and matched normal tissues showed no significant difference. (P = 0.604). Furthermore, lower expression of CYP2A13 mRNA in adenocarcinoma was observed (12.12±3.95) compared with that in adjacent normal tissues (9.07±5.18) with a statistical difference between the two groups (P = 0.008), suggesting that CYP2A13 mRNA was downregulated in lung adenocarcinoma tissues compared to normal tissues.
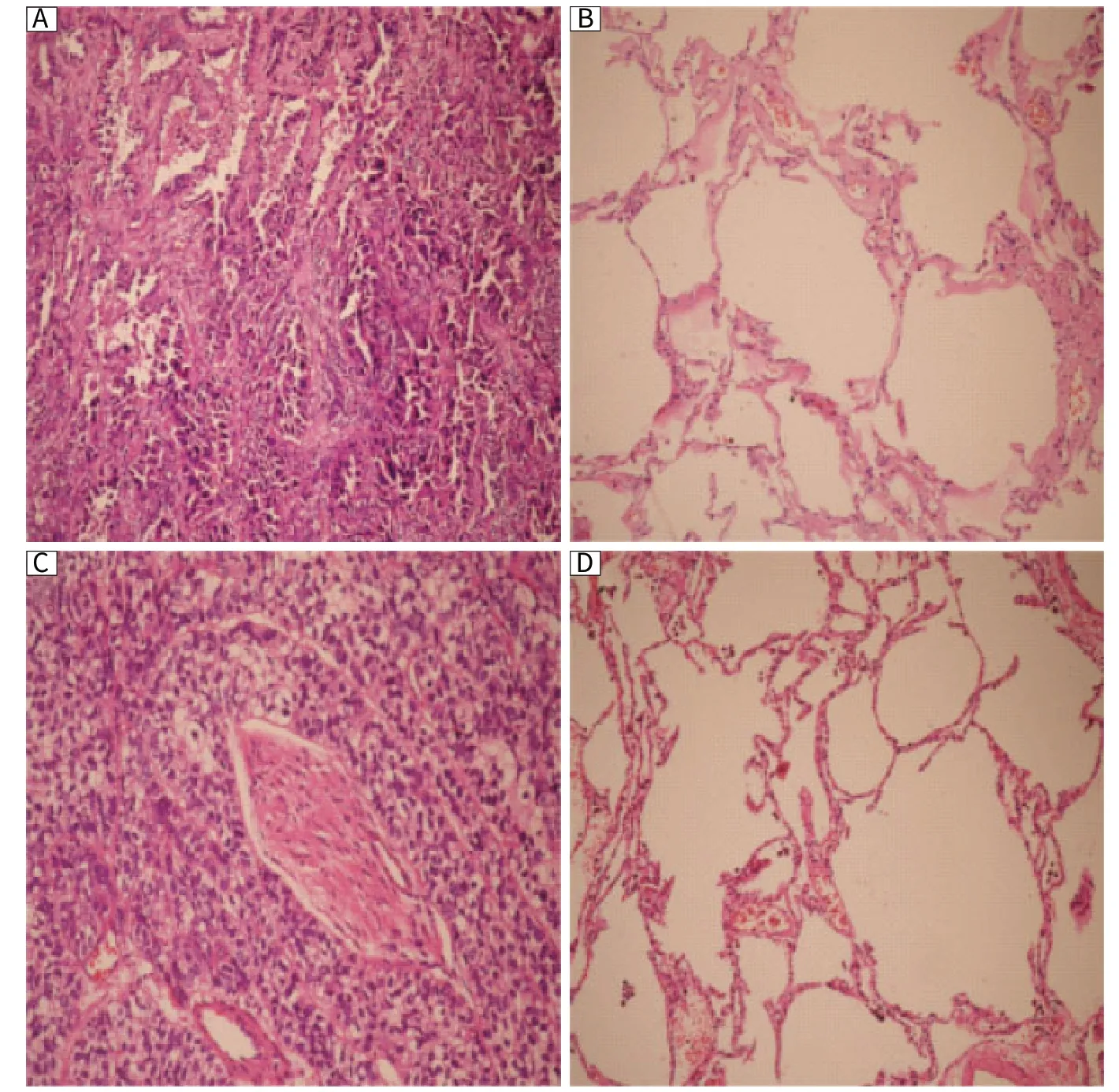
Fig. 1 H&E staining of lung cancer tissues (100×). A: adenocarcinomas. Slice shows gland forming tumor that grows into papilliferous pattern. B: paired normal tissue of adenocarcinomas; C: squamous cell carcinomas. Tumor cells show well formed keratin pearl. D: paired normal tissue of squamous cell carcinomas.
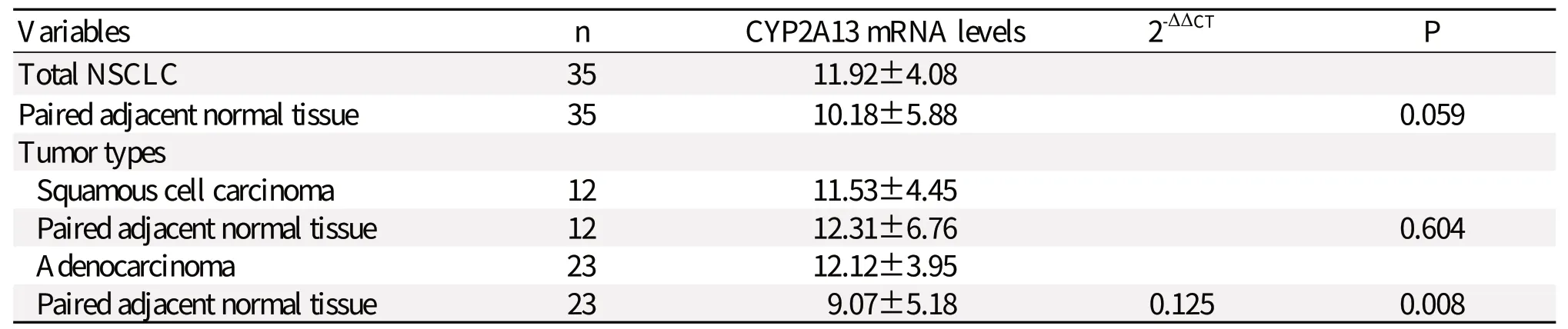
Table 2 CYP2A13 mRNA expression in non-small cell lung cancer (NSCLC) tissues and adjacent normal tissues
CYP2A13 mRNA expression and clinicopathological factors
We examined the relationship between the level of CYP2A13 mRNA and clinicopathological factors in tumors by using independent-sample t test. The expression level of CYP2A13 mRNA in tumor tissues was not associated with age, gender, histology and lymph node status of patients (Table 3).
CYP2A13 protein expression in lung tissue
The expression of CYP2A13 protein was analyzed by Western blotting assays. We randomly chose four representative cases (Fig. 2A). CYP2A13 proteins expressed in the tumor tissues were compared with those of the adjacent normal tissues. We found that CYP2A13 protein levels corresponded to its mRNA transcript levels (Fig. 2B). The expressions of CYP2A13 proteins were consistent with variations in the levels of the corresponding CYP2A13 mRNA transcripts in the same tissue samples, and the ratio of CYP2A13 protein in tumor: normal tissue almost correlated with that of mRNA (Fig. 2C).
DISCUSSION
The cytochrome P450 superfamily are the major enzymes involved in drug metabolism and bioactivation, and CYP catalyzes various environmental protoxicants and chemical procarcinogens, such as 4-aminobiphenyl, naphthalene, styrene and toluene[9,10]. There are two functional members of the human CYP2A gene subfamily: CYP2A6 and CYP2A13. CYP2A6 is an efficient human enzyme in the metabolism of coumarin and nicotine and it is mainly expressed in human liver, whereas CYP2A13 is predominantly expressed in the respiratory tract. The cDNA and protein sequences of CYP2A13 are highly similar to those of CYP2A6 with only 4.7% and 6.5% difference in the nucleotide coding sequence and amino acid sequence, respectively[11]. CYP2A13 is much more active for nicotine, cotinine, and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone metabolisms and especially alphatoxin B1. Recently, it is found that CYP2A13 catalyzes the metabolic activation of NNK through α-hydroxylation[12]. NNK and aflatoxin B1 are all potential carcinogens, and it has been reported previously that CYP2A13 was associated with lung adenocarcinomas risk[3]. For CYP2A13, variations in its expression level or metabolic capacity may significantly alter the extent of metabolic activation of NNK and other xenobiotic substrates in the respiratory tract, which may lead to altered susceptibility to tobacco-related tumorigenesis. Wang et al.[13]studied the relationship between CYP2A13 genetic polymorphisms and the susceptibility of lung cancer. The results showed that genetic polymorphisms of CYP2A13 may have a significant impact on human susceptibility to lung cancers, especially adenocarcinomas.

Table 3 Relationship between CYP2A13 mRNA expression and clinicopathological factors
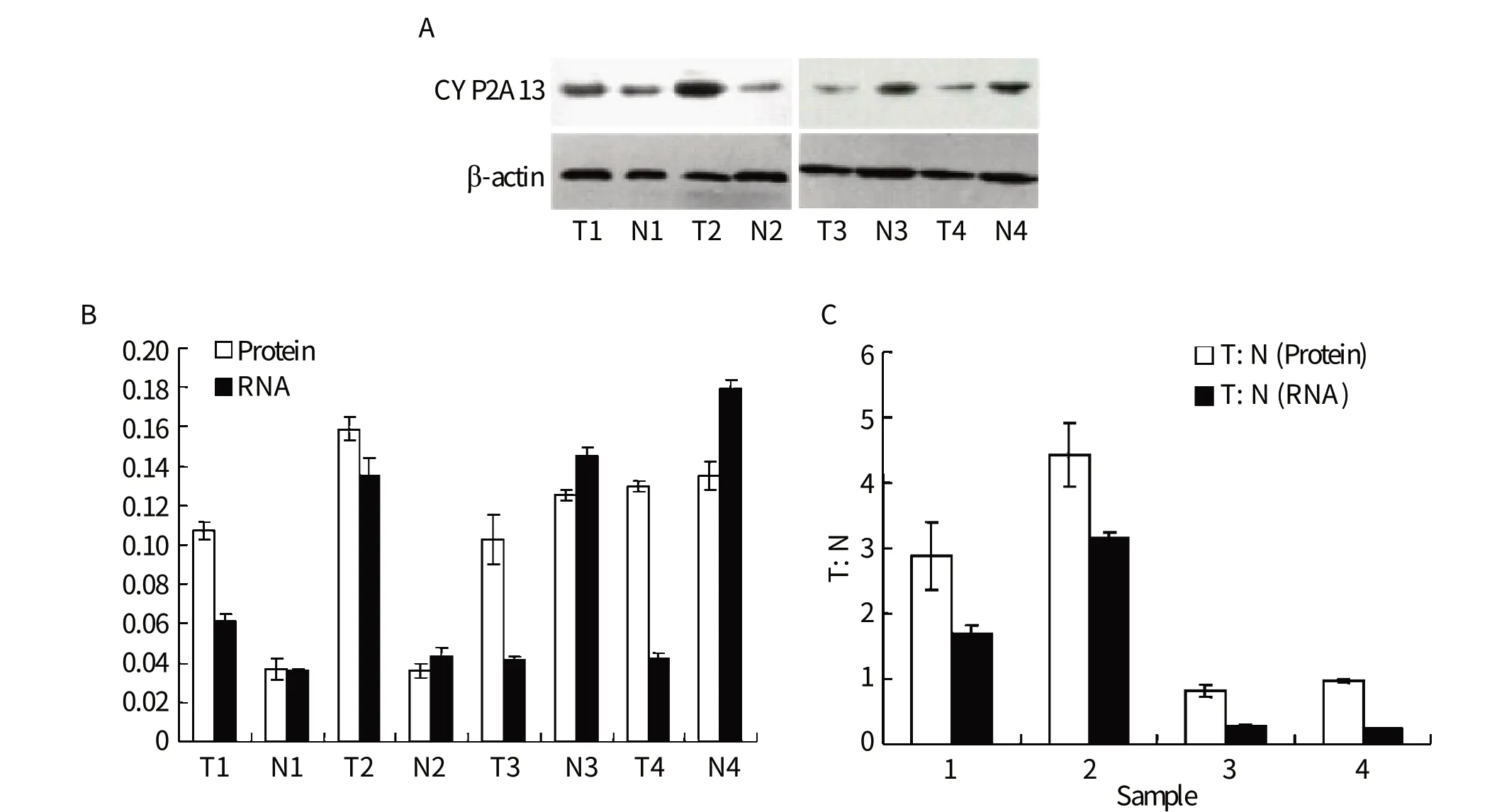
Fig. 2 Expression of CYP2A13 in lung cancer tissues. A: Western blotting for detecting the expression of CYP2A13 in the samples (T: tumor; N: adjacent normal tissue). B: The expression analysis of CYP2A13 at mRNA and protein levels. C: The comparison of CYP2A13 between tumor and pared normal tissues. The expression of the CYP2A13 proteins were consistent with variations in the levels of the corresponding CYP2A13 mRNAs in the same tissue samples.
In this study, we provided the first quantification of the amount of CYP2A13 mRNA between lung cancer tissues and its adjacent normal tissues by using quantitative RT-PCR. The expression of CYP2A13 levels in lung adenocarcinoma tissue was significantly lower than that in normal tissue. Previously, it is found that in the hepatic cancer, many P450s are downregulated, and the expression obviously decreased in tumors compared with the matched normal tissue[14]. The expression level of CYP2A13 in tumor was not associated with age, gender, histological differentiation and lymph node status of patients. Etzel et al.[15]reported that many cytochrome P450 (CYP) enzymes were involved in estrogen metabolism and regulated by estrogens. They thought that these genes may be relevant to gender-specific differences in lung cancer risk in early-onset lung cancer, where a high proportion of women were observed. A case-control study of Timofeeva et al.[16]demonstrated that CYP1B1 and CYP2A13 genotypes may be related to individual susceptibility to early-onset lung cancer in women. The expression of P450s may be markers of prognosis or influence the metabolism of many xenobiotics by P450s[17,18].
As the expression of mRNA may not truly reflect the level of protein, we detected the level of protein by using Western blotting assays. The results showed that the levels of CYP2A13 proteins varied with the levels of the corresponding CYP2A13 mRNAs in the same sample. This result is consistent with the observation of Zhang et al.[5]. So far, two research groups had used immunohistochemistry to analyze intracellular distribution of CYP2A13 and they generated specific antibody against CYP2A13. They found that CYP2A13 was present in normal trachea but not in peripheral lung tissues. Thus, it demosntrated that the expression of CYP2A13 is not uniform in the lung, which supported the negative results of immunoblotting analysis of the microsomes of the lung tissues. Zhu[19]reported that CYP2A13 protein was not detected in any large cell carcinomas (n = 3), adenocarcinomas (n = 6), squamous carcinomas (n = 3), and so on, which was opposite to the result of Fukami[20], who found that CYP2A13 was increased in NSCLC. As the reason for this discrepancy is not clear, it may partly depend on the different reactivities of the antibodies, samples or individual differences. Williams and Sandler[21]reported that its high expression in the respiratory tract and effective metabolism of NNK are consistent with the observation that most smoking-related lung cancers are bronchogenic.
CYP2A13 metabolizes many carcinogenic components of tobacco smoke, such as NNK. The mechanisms may be different between smokers and nonsmokers. The number of samples in this study is limited, and the smoking status of the study population was unknown. In summary, we showed that the expression of CYP2A13 was lower in adenocarcinoma tissues compared with paired normal tissue. It is interesting that CYP2A13 expression was downregulated in adenocarcinoma, which suggests that CYP2A13 may play an important role in the development and progression of lung adenocarcinoma.
[1] Guengerich FP. Cytochrome P450: what have we learned and what are the future issues. Drug Metab Rev 2004; 36: 159-97.
[2] Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C. Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther 2007; 116: 496-526.
[3] Su T, Bao ZP, Zhang QY, Smith TJ, Hong JY, Ding X. Human cytochrome P450 CYP2A13: Predominant expression in the respiratory tract and its high efficiency metabolic activation of a tobacco-specific carcinogen, 4-(methylnitrosamino) -1-(3-pyridyl) -1-butanone. Cancer Res 2000; 60: 5074-9.
[4] Zhu LR, Thomas PE, Lu G, Reuhl KR, Yang GY, Wang LD, et al. CYP2A13 in human respiratory tissues and lung cancers: an immunohistochemical study with a new peptide-specific antibody. Drug Metab Dispos 2006; 34: 1672-6.
[5] Zhang X, D'Agostino J, Wu H, Zhang QY, von Weymarn L, Murphy SE, et al. CYP2A13: variable expression and role in human lung microsomal metabolic activation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. J Pharmacol Exp Ther 2007; 323: 570-8.
[6] Ding X, Kaminsky LS. Human extrahepatic cytochromes P450: Function in xenobiotic metabolism and tissueselective chemical toxicity in the respiratory and gastrointestinal tracts. Annu Rev Pharmacol Toxicol 2003; 43: 149-73.
[7] Jalas JR, Hecht SS, Murphy SE. Cytochrome P450 enzymes as catalysts of metabolism of 4-(methylnitrosamino)-1-(3-pyridyl) -1-butanone, a tobacco specific carcinogen. Chem Res Toxicol 2005; 18: 95-110.
[8] He XY, Tang LL, Wang SL, Cai QS, Wang JS, Hong JY. Efficient activation of aflatoxin B-1 by cytochrome P450 2A13, an enzyme predominantly expressed in human respiratory tract. Int J Cancer 2006; 118: 2665-71.
[9] Nakajima M, Itoh M, Sakai H, Fukami T, Katoh M, Yamazaki H, et al. CYP2A13 expressed in human bladder metabolically activates 4-aminobiphenyl. Int J Cancer 2006; 119: 2520-6.
[10] Fukami T, Katoh M, Yamazaki H, Yokoi T, Nakajima M. Human cytochrome P450 2A13 efficeintly metabolizes chemicals in air pollutants: naphthalene, styrene, and toluene. Chem Res Toxicol 2008; 21: 720-5.
[11] Brian D, Smith J, Sander L, Lushington GH, Stout CD, Scott EE. Structure of the human lung cytochrome P450 2A13. Biol Chem 2007; 282: 17306-13.
[12] Chiang HC, Wang CY, Lee HL, Tsou TC. Metabolic effects of CYP2A6 and CYP2A13 on 4-(methylnitrosamino)-1-(3-pyridyl)- 1-butanone (NNK)-induced gene mutation--a mammalian cell-based mutagenesis approach. Toxicol Appl Pharmacol 2011; 253: 145-52.
[13] Wang H, Tan W, Hao B, Miao X, Zhou G, He F, et al. Substantial reduction in risk of lung adenocarcinoma associated with genetic polymorphism in CYP2A13, the most active cytochrome P450 for the metabolic activation of tobacco-specific carcinogen NNK. Cancer Res 2003; 63: 8057-61.
[14] Liu Xiufeng, Shi Ruihua, Wang Xuehao, Zhang Guoxin, Zhu Hong, Gao Hengjun, et al. Down-regulated Expression of Cytochrome P450 Related Genes Involved in Hepatocellular Carcinoma. Chinese-German J Clin Oncol 2006; 5: 159-64.
[15] Etzel CJ, Lu M, Merriman K, Liu M, Vaporciyan A, Spitz MR. An epidemiologic study of early onset lung cancer. Lung Cancer 2006; 52: 129-34.
[16] Timofeeva MN, Kropp S, Sauter W, Beckmann L, Rosenberger A, Illig T, et al. CYP450 polymorphisms as risk factors for early-onset lung cancer: gender-specific differences. Carcinogenes 2009; 30: 1161-9.
[17] Downie D, McFadyen MC, Rooney PH, Cruickshank ME, Parkin DE, Miller ID, et al. Profiling cytochrome P450 expression in ovarian cancer: identification of prognostic markers. Clin Cancer Res 2005; 11: 7369-75.
[18] Kumarakulasingham M, Rooney PH, Dundas SR, Telfer C, Melvin WT, Curran S, et al. Cytochromes P450 profile of colorectal cancer: identification of markers of prognosis. Clin Cancer Res 2005; 11: 3758-65.
[19] Zhu LR, Thomas PE, Lu G, Reuhl KR, Yang GY, Wang LD, et al. CYP2A13 in human respiratory tissues and lung cancers: an immunohistochemical study with a new peptide-specific antibody. Drug Metab Dispos 2006; 34: 1672-6.
[20] FukamiT, NakajimaM, Matsumoto I, Zen Y, Oda M, Yokoi T. Immuno- histochemical analysis of CYP2A13 in various types of human lung cancers. Cancer Sci 2010; 101: 1024-8.
[21] Williams MD, Sandler AB. The epidemiology of lung cancer. Cancer Treat Res 2001; 105: 31-52.
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Prognostic assessment of apoptotic gene polymorphisms in nonsmall cell lung cancer in Chinese
- Genetic variants in pseudogene E2F3P1 confer risk for HBV-related hepatocellular carcinoma in a Chinese population
- Liquorrhoea associated with intrapelvic meningocele resection successfully treated by conservative therapy: a case report
