Synthesis,Crystal Structure and Antibacterial Activity of 2D Hydrogen-bonds Layered Magnesium(Ⅱ)Complex
2013-09-29TAIXiShiWANGDongFangZHAOZengBingCollegeofChemistryandChemicalEngineeringWeifangUniversityWeifangShandong606CollegeofBioengineeringWeifangUniversityWeifangShandong606DepartmenteofChemistryQinghaiNormalUniversityXining80008
TAI Xi-ShiWANG Dong-FangZHAO Zeng-Bing(College of Chemistry and Chemical Engineering,Weifang University,Weifang,Shandong 606)(College of Bioengineering,Weifang University,Weifang,Shandong 606)(Departmente of Chemistry,Qinghai Normal University,Xining 80008)
研究简报
Synthesis,Crystal Structure and Antibacterial Activity of 2D Hydrogen-bonds Layered Magnesium(Ⅱ)Complex
TAI Xi-Shi*,1WANG Dong-Fang2ZHAO Zeng-Bing3
(1College of Chemistry and Chemical Engineering,Weifang University,Weifang,Shandong 261061)(2College of Bioengineering,Weifang University,Weifang,Shandong 261061)(3Departmente of Chemistry,Qinghai Normal University,Xining 810008)
A new complex hexaaquamagnesium(Ⅱ)bis[2-(thiosemicarbazonomethyl)-benzoato],has been prepared and characterized by elemental analysis,IR spectra,molar conductivity and single-crystal X-ray diffraction.The results of crystal structure show that each Mg(Ⅱ)ion is coordinated with six oxygen atoms from water molecules and the complex formed two dimensional layered structure through intramolecule and intermolecule hydrogen bonds.The antibacterial assay of the magnesium(Ⅱ)complex and the ligand were tested using a modified version of the 2-fold serial dilution method,the results show that the complex show considerable antibacterial activity against escherichia coli,bacillus subtilis and staphylococcus white,and the ligand did not show antibacterial activity.CCDC:751423.
2-(thiosemicarbazonomethyl)-benzoato;magnesium complex;synthesis;crystal structure;antibacterial activity
Magnesium is indispensable element in biology.It is involved in several biochemical processes and is essential cofactor required for the activation of a variety of enzymes[1].And it may increase the strength of cell membranes and regulate the function of the cell walls[2-3].So magnesium plays an important role in the whole cell[4-8].Moreover,it is the metal centre of the chlorophyll which can be photosynthetic,and is related to the mechanism of some drugs[9].Thus,it is referred to the metal of life.It is significance to study on the structure and characteristic coordination of magnesium carefully for making sure about physiological and biochemical mechanisms of all lives[10].
Amino acid is an important physiological active substance.Besides bactericidal,insecticidal and anticancer biologicalactivity,amino acids directlyparticipate in biological processes,and are important to biochemistry and organic synthesis[11-14].In this paper,a new magnesium complex of amino acid was synthesized,characterized by elemental analysis,IR spectroscopy and single-crystal X-ray diffraction.The complex formed two dimensional layered structure through intramolecule and intermolecule hydrogen bonds.The antibacterial activities of the magnesium(Ⅱ)complex and the ligand were tested.
1 Experimental
1.1 Materials and measurements
The following A.R.grade chemicals were used for the preparation of the studied compound:magnesium chloride,2-carboxybenzaldehyde,thiosemicarbazide,sodium hydroxide.
The carbon,hydrogen and nitrogen content in the newly synthesized compound were analyzed on a Elementar Vario EL Ⅲ elemental analyzer.Infrared spectra (4 000~400 cm-1)was determined with KBr optics on a Nicolet AVATAR 360 FTIR spectrophotometer.The molar conductance value was determined using a DDS-11A conductivity meter with CH3OH as solvent(1 mmol·L-1solution)at 25 ℃.The crystal data was collected on a Bruker Smart-1000 CCD Area Detector.
1.2 Synthesis of the ligand
10 mmol(1.501 3 g)of 2-carboxybenzaldehyde and 20 mmol (0.8 g)of sodium hydroxide were dissolved in 100 mL of water at room temperature,and added drop by drop 10 mmol (0.911 4 g)of thiosemicarbazide by stirring at room temperature.The reaction solution was kept running for 4 h,then acidified with the solution of hydrochloric acid(1∶1,V/V)to pH=2.The white solid precipitation were collected by filtration,washed and dried under vacuum.Yield may reach up to over 65%.Elemental analysis calculated for C9H9N3O2S(%):C 48.43,H 4.04,N 18.83;found(%):C 48.56,H 3.92,N 18.96.IR(KBr,cm-1):3 408br,1756s,1 658s.
1.3 Synthesis of the complex
1.0 mmol(0.223 g)of 2-(thiosemicarbazonomethyl)-benzoic acid and 1.0 mmol (0.04 g)of sodium hydroxide were added to the 15 mL of CH3OH/H2O(2∶1,V/V)solution.After being dissolved,0.5 mmol(0.102 g)of magnesium chloride was added to the solution.The mixture was continuously stirred for 3 h at refluxing temperature.The mixture was cooled at room temperature,and was collected by filtration.By evaporation in air at room temperature,the single crystal suitable for X-raydetermination wasobtained from methanol solution after 10 d.Yield 58% .Elemental analysis calculated for C18H28MgN6O10S2(%):C 37.44,H 4.85,N 14.56;found(%):C 37.28,H 4.99,N 14.66.IR(KBr,cm-1):3402br,1755s,1656s,389m.
1.4 Antibacterial activity
The ligand and complex were dissolved in sterile water and tested against three reference strains for antibacterial activity,respectively.The antibacterial assay was performed using a modified version of the 2-fold serial dilution method[15],in which the concentration of chemical medicine decreased half as many in a sterile culture medium containing broth as the nutrient,and the strains were incubated 16 h in culture medium at constant temperature 37℃after being activated,and misce bene after being added to the test tubes of chemical medicine,then readings were taken after 24 h of incubation at constant temperature 37℃.All other testconditions were standardized.The resultant turbidities in all tubes were estimated visually,and the lowest drug concentrations were found,which is defined MIC.After 48 h of continuous incubation,the MBC were defined,too.
1.5 Crystal structure determination
A colourless block single crystal with dimensions of 0.15 mm×0.12 mm×0.06 mm was placed on a glass fiber and mounted on a Bruker Smart-1000 CCD area detector.Diffraction data were collected by φ~ω scan mode using a graphite-monochromatic Mo Kα radiation(λ=0.071 073 nm)at 273(2)K.A total of 6 596 reflections were collected in the range 1.58°~25.04°,of which 2 297 were unique(Rint=0.017 3)and 2014 were observed with I>2σ(I).The data were corrected for Lp factors.The structure was solved by direct methods and refined on F2by full-matrix leastsquares techniques with SHELXL-97[17].All non-hydrogen atoms and hydr-ogen atoms were refined anisotropically and isotropi-cally,respectively.The final R=0.0514,wR=0.1280(w=1/[σ2(Fo2)+(0.0637P)2],P=(Fo2+2Fc2)/3 and S=1.226, (Δ/σ)max=0.000).The largest peak in the final difference Fourier map is 902 e·nm-3and the minimum peak is 433 e·nm-3.Molecular graphics were drawn with the program package SHELXTL-97 crystallographic software package[18].The most relevant crystal data for complex are quoted in Table 1.
CCDC:751423

Table 1 Crystal structure parameters of the title complex
2 Results and discussion
2.1 Property of the complex
The results of elemental analyses and molar conductivity indicated that the composition of the complex as[Mg(H2O)6](TCB)2(TCB=2-(thiosemicarbazonomethyl)-benzoato),indicating that the complex conforms to 1∶2(Mg/TCB)stoichiometry.
The complex is soluble in DMF,DMSO,methanol,a little soluble in ethanol and CHCl3,insoluble in benzene,diethyl ether and cyclohexane.The molar conductance value of the complex measured in CH3OH solution (1 mmol·L-1)at 25 ℃ is 168 S·cm2·mol-1,indicating 1∶2 electrolytes and that uncoordinated TCB are in the complex[19],which is in accordance with the results of IR spectra of the complex.
2.2 IR spectra
In the infrared spectra,the ν(COOH)vibrations of the free ligand are at 1 756 cm-1.For the complex,the vibration observed at 1 755 cm-1was assigned as νas(COO-).It can be explained that the carboxylate oxygen atoms of ligands do not take part in the coordination with magnesium atoms[19].The new band at 389 cm-1is assigned to the ν(Mg-O)vibration.The band corresponding to the ν(OH)at 3 402 cm-1shows that the complex contains water molecule,which is in accordance with the result of elemental analysis.
2.3 Crystal structure of complex
Perspective view of the molecule in a unit cell and molecular packing arrangement are shown in Fig.1.SelectedbonddistancesandanglesarelistedinTable2.

Fig.1 Crystal structure of the complex
The title compound crystallizes in monoclinic system,space group P21/c.From Fig.1,it can be seen that the Mg(Ⅱ)center is six-coordinate with six oxygen atoms from the water molecules,and making up a distorted octahedral structure.The coordination atoms(O(1),O(3A),O(4),O(4A))are situated equatorial place,and the coordination atoms(O(5),O(5A))are situatedaxial place.The distances of the Mg-O bonds are in the range of 0.203 3(2)~0.209 7(3)nm,respectively.They differ only slightly and are similar to the Mg-O bond lengths reported previously[20-23].The benzene rings in the molecule do not show any unusual features,and the bond lengths and bond angles are within the range of normal values.
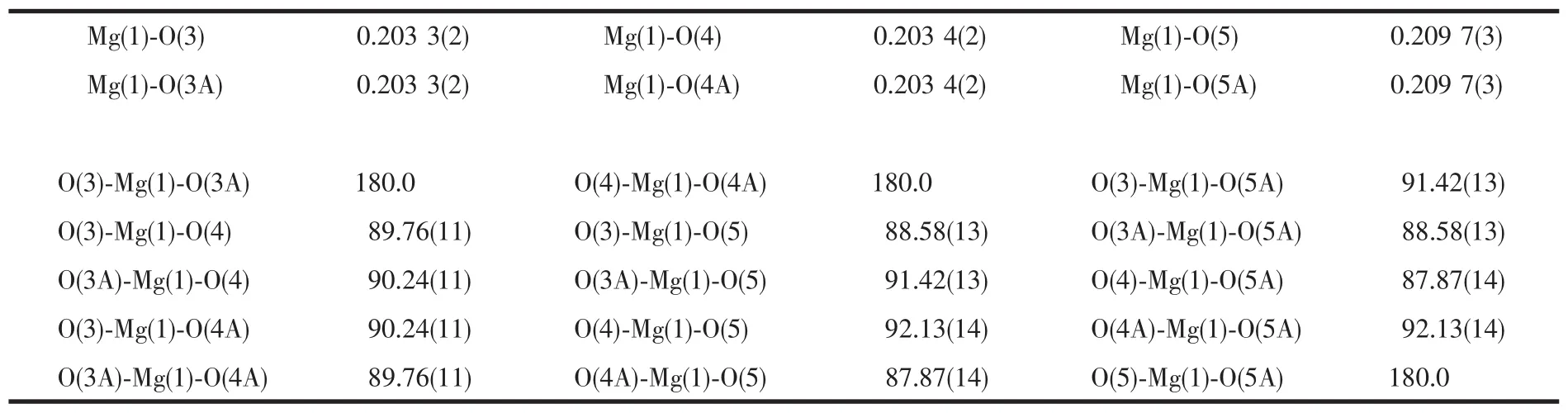
Table 2 Selected bond lengths(nm)and angles(°)of complex
Fig.2 displays the molecular forms of 2D layered structurethrough intramolecule and intermolecule hydrogen bonds,the hydrogen bonds data are listed in Table 3.Hydrogen bonds are still rich in the complex,which may be divided into three sections according to the acceptors:(1)oxygen acceptors from carboxylic acids:N3-H3B…O1,O3-H9…O2,O3-H10…O1,O4-H11…O2;(2)nitrogen acceptors from the free 2-(thiosemicarbazonomethyl)benzoato:N3-H3A…N1;(3)sulfur acceptors from the free 2-(thiosemicarbazonomethyl)benzoato:N2-H2…S1,O3-H3A…S1,O5-H13… S1.The free 2-(thiosemicarbazonomethyl)benzoato forms hydrogen bonds with the coordinated water molecules,and play a role in connecting the coordinated groups through the oxygen atoms and nitrogen atoms which lies in the two ends of them.As a result of many intramolecular and intermolecular hydrogen bonds and the π-π packing interaction,the complex forms steady two dimensional layered structure(Fig.2).
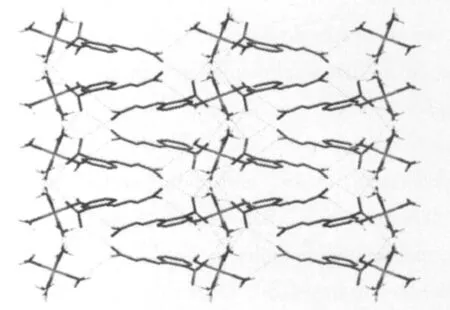
Fig.2 Two dimensional layered structure of the complex

Table 3 Hydrogen bonds data of the complex
2.4 Antibacterial activity
The antibacterial activity of the ligand and Mg(Ⅱ)complex were assayed using three positive(escherichia coli,bacillus subtilis,staphylococcus white)bacterial strains.The antibacterial results of the complex are listed in Table 4,and the results indicate that the Mg(Ⅱ)complexshowsconsiderable antibacterialactivity.Compared with the complex,the ligand did not show antibacterial activity.So the complex will provide potential applications in the broad spectrum of theantibacterial field.

Table 4 MIC and MBC of complex against three bacterial strains
3 Conclusions
According to the data and discussion above,TCB and water molecules have formed stable complex with magnesium.ObviousIR spectrum changeswere observed afterthe ligand formed complex with magnesium.The crystal data of the complex shows that the magnesium atoms were coordinated to the oxygen atoms of water molecules.The complex formed two dimensional layered structure through intramolecule and intermolecule hydrogen bonds and π-π stacking.From the antibacterial results,we can see that the Mg(Ⅱ)complex shows considerable antibacterialactivity againstescherichiacoli,bacillussubtilisandstaphylococcus white,and the ligand did not show antibacterial activity.Based on those results,a series of new magnesium complexes would be designed and synthesized to investigate further the spectral properties and antibacterial activities.
[1]Erxleben A,Schumacher D.Eur.J.Inorg.Chem.,2001,2001(12):3039-3046
[2]SU Yang(苏 扬),ZANG Shuang-Quan(藏双全),NI Chun-Lin(倪春林),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2004,20(7):845-848
[3]GAO Shan(高 山),ZHANG Zhu-Yan(张竹艳),HUO Li-Hua(霍丽华),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2005,21(5):771-774
[4]Yamniuk A P,Vogel H J.Protein Sci.,2005,14(6):1429-1436
[5]Rubin H.BioEssays.,2005,27(3):311-320
[6]Dodig S,Vlasic Z,Cepelak I,et al.J.Clin.Lab.Anal.,2009,23(1):34-40
[7]Miller K B,Caton J S,Finley J W.BioFactors.,2006,28(1):33-42
[8]TAI Xi-Shi(台夕市),XU Jun(许 军),FENG Yi-Min(冯一民),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25(3):552-555
[9]Chen Z F,Xiong R G,Zuo J L,et al.Dalton Trans.,2000,22:4013-4014
[10]YANG Zheng-Yin(杨正银),YANG Ru-Dong(杨汝栋),YU Kai-Bei(郁开北).Acta Chimica Sinica(Huaxue Xuebao),1999,57(3):236-243
[11]Chohan H Z,Arif M,Sarfraz M.Appl.Organomet.Chem.,2007,21(4):294-302
[12]Brown N J,Wu C W,Seurynck-Servoss S L,et al.Biochem.,2008,47(6):1808-1818
[13]Brady S F,Clardy J.J.Am.Chem.Soc.,2000,122(51):12903-12904
[14]Basu S,Chattopadhyay B,Ganguly A,et al.Appl.Organomet.Chem.,2009,23(12):527-534
[15]ZHENG Jun-Yong(郑均镛),WANG Guang-Bao(王光宝).Medicines and Chemical Reagents Microbiology and Assay Technology(药品微生物学及检测技术).Beijing:People′s Health Press,1989.
[16]Sheldrick G M.SHELXL-97,UniversityofGöttingen,Germany,1997.
[17]Sheldrick G M.SHELXS97,University of Göttingen,Germany,1997.
[18]Nakamoto K.Infrared and Ramen Spectra of Inorganic and Coordination Compounds.New York:John Wiley and Sons,1978.
[19]Matczak-Jon E,Kurzak B,Kafarski P,et al.J.Inorg.Biochem.,2006,100(7):1155-1166
[20]Doskocz M,Kubas K,Frckowiak A,et al.Polyhedron.,2009,28(11):2201-2205
[21]Chisholm M H,Gallucci J C,Zhen H S.Inorg.Chem.,2001,40(19):5051-5054
[22]Chisholm M H,Lin C C,Gallucci J C,et al.Dalton Trans.,2003,25(3):406-412
二维氢键层状Mg(Ⅱ)配合物的合成、晶体结构及其抗菌活性
台夕市*,1王东方2赵增兵3
(1潍坊学院化学化工学院,潍坊 261061)(2潍坊学院生物工程学院,潍坊 261061)(3青海师范大学化学系,西宁 810008)
2-羧基苯甲醛缩氨基硫脲;Mg(Ⅱ)配合物;合成;晶体结构;抗菌活性
O614.22
:A
:1001-4861(2010)08-1490-05
2010-01-04。收修改稿日期:2010-04-10。
台夕市,男,38岁,教授;研究方向:碱土金属功能配合物化学。
国家自然科学基金项目(No.20671073),山东省自然科学基金项目(No.Y2007B60)和潍坊市科技发展计划项目资助。
*通讯联系人。 E-mail:taixishi@lzu.edu.cn
猜你喜欢
杂志排行
无机化学学报的其它文章
- The Uptake and Membrane Transport of Cesium in Human Erythrocytes
- Preparation of Functionalized Graphene Sheets via Microwave-Assisted Solid-State Process and Their Electrochemical Capacitive Behaviors
- A New 2D Layer Manganese(Ⅱ)Complex Assembled by Flexible 1,4-Benzenebis(thioacetic acid)Ligand:Synthesis,Crystal Structure and Magnetic Property
- Synthesis of a New Cu(Ⅱ)Complex with in situ Generated 3-Hydroxy-2,4,6-pyridinetricarboxylate Ligand and Analysis of the Reaction Mechanism
- A New Nickel(Ⅱ)Complex Based on Novel Pyridyl-carboxylate Schiff-Base Ligand:Synthesis and Crystal Structure
- Ru掺杂SnO2半导体固溶体的电子结构研究
