Effects of dietary vitamin E on muscle vitamin E and fatty acid content in Aohan fine-wool sheep
2013-09-13KunLiuSuyunGeHailingLuoDubingYueandLeyanYan
Kun Liu,Suyun Ge,Hailing Luo,Dubing Yue and Leyan Yan
Introduction
In recent years,an increase in consumer interest in the nutritional aspects of health has resulted in the development of specific health recommendations for food components,especially of dietary fat [1].The relationship between dietary fat and the incidence of lifestyle diseases,particularly cardiovascular disease and atherosclerosis,is well established.It is recommended that total fat and saturated fatty acids(SFAs)should not exceed 35%and 10%of the total dietary intake,respectively.Additionally,the ratio of polyunsaturated fatty acids (PUFAs) to SFAs (P/S)should be around 0.45,while that of n-6 to n-3 PUFAs should be<4 [2].Meat and meat products are important dietary components that provide a major source of macronutrients[3].Therefore,increasing the PUFA content and decreasing the SFA content in meat may help to improve the nutritional value of this food type to consumers.
Some authors have demonstrated that the fatty acid(FA) profile of meat can be modified by oxidative processes and that the oxidative stability of lipid fractions was associated with their FA composition [4,5].The susceptibility of unsaturated FAs (UFAs) to oxidation is related to the degree of unsaturation of FAs,because PUFAs are more prone to oxidation than monounsaturated FAs(MUFAs)[6].It was also demonstrated that lipid oxidation could reduce the content of essential PUFAs (C18:2n-6 and C18:3n-3) and long-chain PUFAs (C20:5n-3 and C22:6n-3);however,oxidation of these PUFAs may be reduced by increasing the availability of natural antioxidants that could delay or inhibit oxidation[7].
Vitamin E is a major antioxidant that protects tissues from oxidative damage.It is deposited in the cell membrane where it protects the membrane PUFAs from oxidation[8].This protective effect is carried over into the meat and can be enhanced by feeding animals with vitamin E at a level greater than that required for normal growth and reproduction.Demirel et al.reported that high dietary vitamin E supplementation provided additional protection against lipid peroxidation by increasing PUFA content and decreasing monoenoic FA content in muscle,liver,and adipose tissue [9].Morel et al.also found that dietary vitamin E could reduce lipid oxidation in pork and in processed pork products [10].It was also reported that vitamin E could stabilize PUFAs and was a major determinant of meat quality,particularly in ruminants[11].
Although the effects of dietary vitamin E on meat quality have been extensively studied in lambs [8,12,13],it is still necessary to determine the optimum tissue concentration for improving meat quality.The efficiency of turning dietary vitamin E into muscle vitamin E is influenced by several factors,including the dietary concentration,muscle type,and the type of vitamin E being used [14].For these reasons,the muscle vitamin E content is highly variable leading to inconsistencies among prior studies.
Although many researchers have investigated the effects of dietary vitamin E supplementation on meat quality in meat sheep,very few studies have examined the effects of vitamin E supplementation on muscle vitamin E content in wool sheep or evaluated the changes in FA profiles,even though wool sheep are an important source of mutton.Therefore,the objectives of the present study were:1) to examine the effects of different dietary vitamin E concentrations on muscle vitamin E content;and 2)to determine the relationship between muscle vitamin E content and FA composition in male Aohan fine-wool sheep,a common fine-wool sheep breed in China.
Materials and methods
Animals and management
Aohan fine-wool sheep,which were initially bred in the Inner Mongolia Autonomous Region of China,are well known for their high-grade fleece weight (16.2 kg),staple length (7.5–9.8 cm),fiber diameter (21.6–25 μm),and exceptional meat quality.We purchased 42 male Aohan lambs (5 mo old) with similar initial body weights from the Aohan fine-wool sheep breeding farm (Chifeng,Inner Mongolia Autonomous Region,China).The sheep were initially fed a diet with a forage/concentrate ratio of 6/4.The formulation developed according to the National Research Council (NRC) feeding standard is shown in Table 1 [15].The animals were housed individually,had free access to fresh,clean water,and were fed ad libitum.
Powdered vitamin E acetate(1 mg contains 1 IU vitamin E) was bought from Zhejiang Guobang Pharmaceutical Co.,Ltd.(Shangyu,China)and was thoroughly mixed with the food.The sheep were divided randomly into seven groups (n=6/group) and were fed diets supplemented with 0 (control),20,100,200,1,000,2,000 or 2,400 IU/sheep/d vitamin E (E0,E20,E100,E200,E1,000,E2,000,and E2,400,respectively) for 12 mo.The dose levels were 0,1,5,10,50,100,and 120 times higher than the NRC feeding standard[15]and were selected based on our previous findings[16].Dry matter intake(DMI)was recorded in the last month of feeding until the sheep were slaughtered.
Three sheep of each group were randomly selected for slaughter at the end of the feeding period and samples of the longissimus lumborum (LL) and gluteus medius(GM) muscles were collected and stored at -20°C to measure vitamin E and FA content.
All of the animal procedures were conducted with approval from the China Agricultural University Animal Care and Use Committee.
Chemical analysis
Muscle vitamin E content was measured using a commercially available vitamin E assay kit (Nanjing Jiancheng Bioengineering Institute,Nanjing,China).The principal of this kit of that,in the presence of phenanthroline,Fe3+is reduced to Fe2+by vitamin E.Phenanthroline then forms a complex with Fe2+forminga colored adduct.Vitamin E content can be calculated using the following equation:
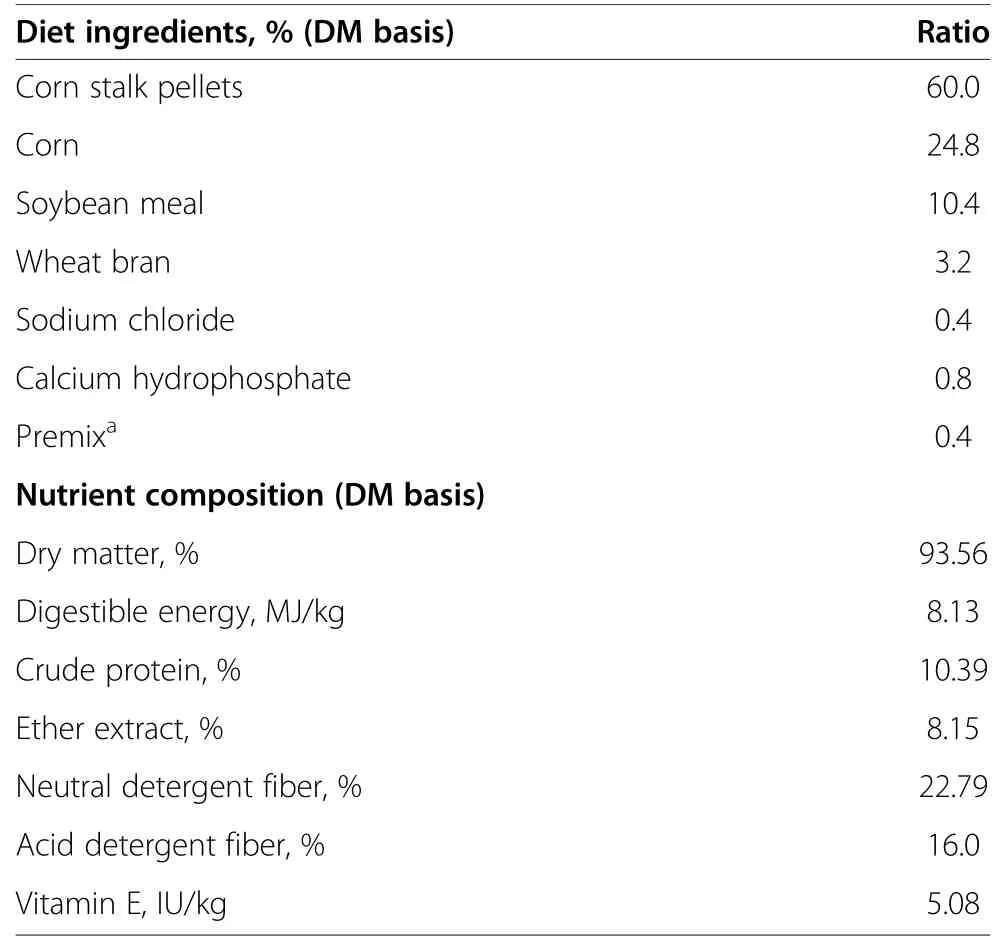
Table 1 Ingredients and nutritional composition of the experimental diet
where ODU=absorbance of the assay tube,ODS=absorbance of the standard tube,ODB=absorbance of the blank tube,CS=standard concentration,and N=dilution ratio.
Muscle FA content was analyzed by gas chromatography according to the method described previously[17].Briefly,the LL and GM were freeze-dried and 300 mg of each sample was placed into tissue culture tubes fitted with a rubber stopper and screw cap.Next,4 mL of methanol:chloroacetyl solution (v/v 10:1) and 5 mL of normal hexane solution (1 mg/mL) were added to each tube.The normal hexane solution was prepared as follows:1 g of nonadecanoic acid (Sigma,St.Louis,MO,USA) was transferred to a 1,000 mL volumetric flask and the flask was filled to a specified volume with normal hexane.The flask was covered tightly with the rubber stopper and screw cap to prevent gas leakage and was incubated in a water bath for 2 h at 80°C.After cooling to room temperature,5 mL of 7%potassium carbonate was added,the flask was thoroughly mixed and left to stand.After the formation of distinct layers in the solution,the upper layer was used for FA analysis.FA methyl esters were analyzed by gas chromatography(HP6890)using a capillary column(HP-INNOWAX [19091N-213,60.0 m × 320 μm × 0.5 μm],Agilent,Santa Clara,California,America).Gas chromatography was performed using the following conditions:the temperature was held at 220°C for 10 min and then increased to 250°C at a rate of 10°C/min;the temperature of the FID detector was set at 280°C;the split flow ratio was 20:1;the injection volume was 1 μL;the injection temperature was 250°C;and nitrogen (3.0 mL/min)was used as the flow gas.
Statistical analysis
The effects of dietary vitamin E content on muscle vitamin E and FA contents were analyzed by one-way analysis of variance followed by Duncan’s new multiple range test.We used SAS 9.1 software(SAS Institute,Cary,NC,USA)for all analyses.Results are expressed as the mean ± standard error (SE).FA content was compared between the two muscles using paired t tests.Differences at P<0.05 were considered statistically significant.
Results
Food intake and muscle vitamin E concentrations
The DMI and nutrient intake are shown in Table 2;there were no differences among the seven groups in these factors (P>0.05).The muscle vitamin E levels are presented in Table 3.Muscle vitamin E concentrations were significantly increased by dietary vitamin E supplementation.Interestingly,the increase was not dosedependent,because vitamin E levels were highest in the E200 group in the LL and GM.In the LL,vitamin E concentrations in the E200,E1,000,and E2,400 groups were significantly higher than that in the control group (E0).Vitamin E content was also significantly higher in the E200 group than in the E20 and E100 groups (P<0.05).In the GM,the vitamin E concentration was significantly higher in the E200 group than in the other groups(P<0.05) except for the E1,000 and E2,400 groups.
Muscle SFA composition
Tables 4 and 5 show the muscle SFA composition in the LL and GM,respectively.In the LL,the total SFA content was significantly lower in the vitamin E-supplemented groups than in the control group (P<0.05).In terms of individual SFAs,we found significant differences in C12:0,C14:0,C16:0,C18:0,and C20:0 FAs (P<0.05) (Table 4).Of note,the total SFA content was lowest in the E200 group,mainly because of the lower levels of C12:0,C14:0,C16:0,C18:0,and C20:0 FAs.Similarly,the ratio of C14:0+C16:0 was also significantly lower in the vitamin Esupplemented groups,except in the E2,000 group,than in the control group(P<0.05).
In the GM,the total SFA content was significantly lower in the vitamin E-supplemented groups,except for the E20 group,than in the control group (P<0.05).The total SFA content was lowest in the E200 group.Regarding individual SFAs,the C10:0 content was significantly lower in the vitamin E-supplemented groups,except in the E1,000 and E2,400 groups,than in the control group(P< 0.05).The C16:0 content was also significantly lower in each vitamin E-supplemented group than in the control group (P<0.05) (Table 5).The relative C10:0 and C16:0 contents were lower in the E200 than in the other groups.As in the LL,the ratio of C14:0+C16:0 FAs in the GM were significantly lower in the vitamin E-supplemented groups than in the control group(P<0.05),and were lowest in the E200 group.
Muscle UFA composition
The UFA profile of the LL is shown in Table 6.The total MUFA content was significantly higher in each vitamin Esupplemented group than in the control group (P<0.05),which was mainly due to the significantly higher levels of C14:1,C16:1,C18:1n9,C18:1n7,and C20:1 in the vitamin E-supplemented groups (P<0.05) (Table 6).The index(C18:0+C18:1):C16:0 was significantly increased by vitamin E supplementation (P<0.05),except in the E2,000 group,and was highest in E200 group.Unexpectedly,vitamin E supplementation did not affect the total PUFA content,or the n-6 and n-3 PUFA content (P>0.05).For individual PUFAs,only cis9 trans11-conjugated linoleicacid (c9t11-CLA) content was significantly increased by vitamin E supplementation,except in the E20 group,compared with the control group (P< 0.05).c9t11-CLA content was highest in the E200 group.Vitamin E supplementation significantly increased the MUFA:SFA ratio(P<0.05)but not the PUFA:SFA ratio(P>0.05).

Table 2 Effects of dietary vitamin E supplementation on food intake and nutrient levels in Aohan fine-wool sheep
The UFA profile of GM is presented in Table 7.Vitamin E supplementation significantly increased the total MUFA content,mainly because of changes in C18:1n9(P<0.05).The (C18:0+C18:1):C16:0 ratio was significantly higher in the E200 group than in the control group (P<0.05).However,there were no significant differences in total PUFA,individual PUFA,or in the MUFA:SFA and PUFA:SFA ratios in the GM (P>0.05).
Finally,we compared the effects of dietary vitamin E supplementation on FA deposition on FA concentration between the GM and LL.However,we found no significant differences in any of the FA parameters between the two muscles (P>0.05).
Discussion
Muscle vitamin E concentrations
Previous studies have demonstrated increases in tissue α-tocopherol concentrations following dietary vitamin E supplementation in lambs[18,19].Liu et al.fed steers with diets supplemented with 250,500 or 2,000 mg all-racα-tocopherol acetate/calf/d and reported that the mean muscle α-tocopherol concentrations were 1.39,2.27 and 4.95 μg/g,respectively,in the LL,semimembranosus,and GM [20].Lauzurica et al.reported that α-tocopherol concentrations in meat increased with increasing dietary vitamin E content[21].Kasapidou et al.also reported that the muscle α-tocopherol content increased with increasing dietary vitamin E content,and that muscle vitamin E concentrations were positively correlated with dietary vitamin E levels [8,13].In our study,muscle vitamin E content increased significantly with increasing dietary vitamin E content,although the relationship was not dose-dependent because the muscle vitamin E content in the LL and GM was greatest in the E200.Muscle vitamin E content was lower in the E1,000,E2,000,and E2,400 groups than in E200,probably because the uptake and deposition of vitamin E were saturated at E200.It has been reported that,when animal diets are supplemented with‘supranutritional’ vitamin E levels,the effects of dietary vitamin E on its tissue content diminishes [13].Slow hydrolysis was also reported as a cause of low plasma tocopherol levels in animals fed supranutritional levels of α-tocopherol acetate[22].
The duration of dietary supplementation is another factor that influences α-tocopherol deposition.In general,greater dietary vitamin E content and/or longer supplementation time are associated with higher α-tocopherol concentrations in meat [23].The muscle vitamin E content in our study was higher than that in other studies,possibly because our study was conducted over 12 mo or because we used a different animal model.Álvarez et al.reported lower semimembranosus vitamin E concentrations in younger lambs fed a diet supplemented with vitamin E for only 37 d [12].However,in the present study,muscle vitamin E levels were highly variable among sheep in the same group (Table 3).Wide variability was alsoreported by other researchers.Lynch et al.reported that α-tocopherol concentrations in beef skeletal muscle ranged from 2.50 to 6.19 μg/g in cattle given 300 mg/cow/d all-rac-α-tocopheryl acetate compared with 0.70–2.92 μg/g in the control group[24].O'Grady et al.also reported that muscle vitamin E levels ranged between 1.35 and 2.73 μg/g in the longissimus in cattle fed with 300 mg all-rac-α-tocopheryl acetate/kg feed [25].Meanwhile,Álvarez et al.reported that muscle vitamin E content differed by almost two-fold in lambs receiving the same dietary vitamin E content[12].Although there is no clear explanation for this variation,Kasapidou et al.suggested that differences in the ability of individual lambs to metabolize vitamin E might play a key role in this phenomenon [8].The small number of animals used in these studies might also contribute to the high variability in muscle vitamin E content [26].Nevertheless,greater understanding of the mechanisms controlling the uptake and metabolism of vitamin E is necessary to explain the differences observed among prior studies.
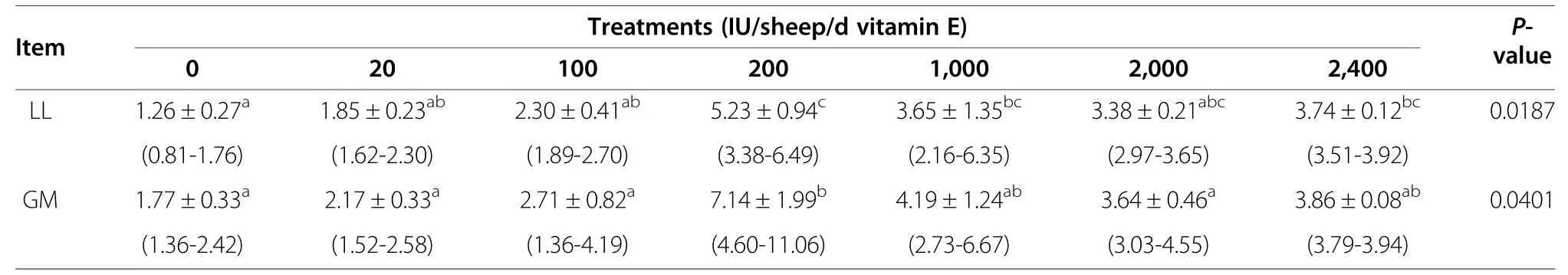
Table 3 Vitamin E content (μg/g)in the longissimus lumborum and gluteus medius at slaughter
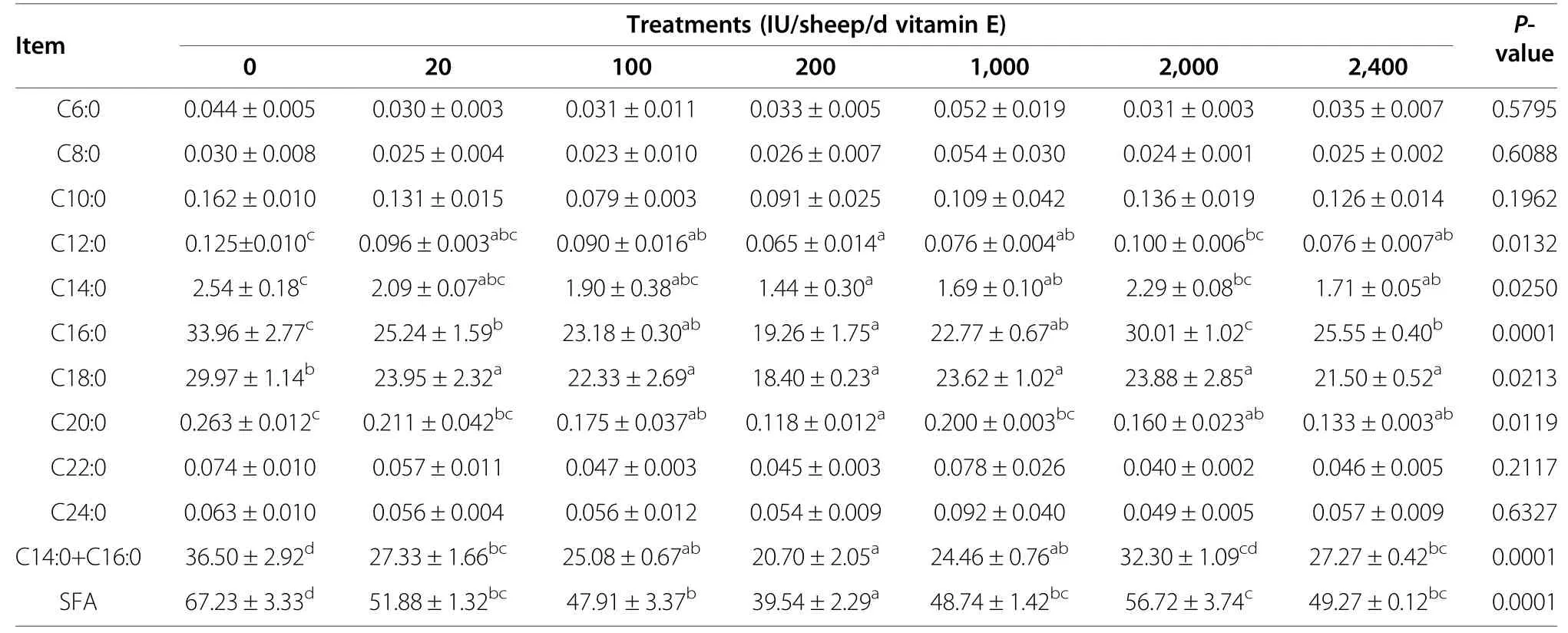
Table 4 Effects of vitamin E supplementation on saturated fatty acid content (%of total fatty acids)in the longissimus lumborum
Muscle FA profile
SFAs are implicated in the etiology of several diseases,and there is a strong positive correlation between SFAintake and the rate of coronary artery heart disease [27].Grundy and Denke also reported that high dietary levels of long-chain SFAs increase plasma cholesterol level whereas high levels of MUFAs and PUFAs do not [28].However,not all SFAs have equivalent effects.It was suggested that C14:0 and C16:0 exerted differential,dose-dependent effects on cholesterol and lipoprotein metabolism,even in animals fed low-cholesterol diets,while C18:0 did not appear to have such an effect [29].It was also reported that high concentrations of palmitic acid (C16:0) were toxic to mitochondria [30]and that the concentration of stearic acid (C18:0) was positively correlated with the odor and flavor intensity of mutton[31].In this study,the C16:0,C14:0+C16:0,and total SFA levels in the LL and GM,and C12:0,C14:0,and C18:0 levels in the LL were significantly lower in the vitamin E-supplemented groups than in the control group (P<0.05),which is consistent with the results of other studies [7,32].In our previous study,24 Boer male goats were fed diets supplemented with 0,80,320 and 880 IU/goat/d vitamin E for 5 mo,which tended to decrease the C18:0 and total SFA levels,especially in the 320 IU/goat/d group [33].However,our results did not agree with those of another study in which vitamin E supplementation (45 mg/lamb/d) significantly increased intermuscular fat stearic acid (C18:0) content but did not affect other FAs [34].Salvatori et al.also studied the effects of vitamin E supplementation on FA compositionof lamb meat and found no differences between the dietary supplementation groups [35].The reasons for these discrepancies remain unclear,and warrant further studies.
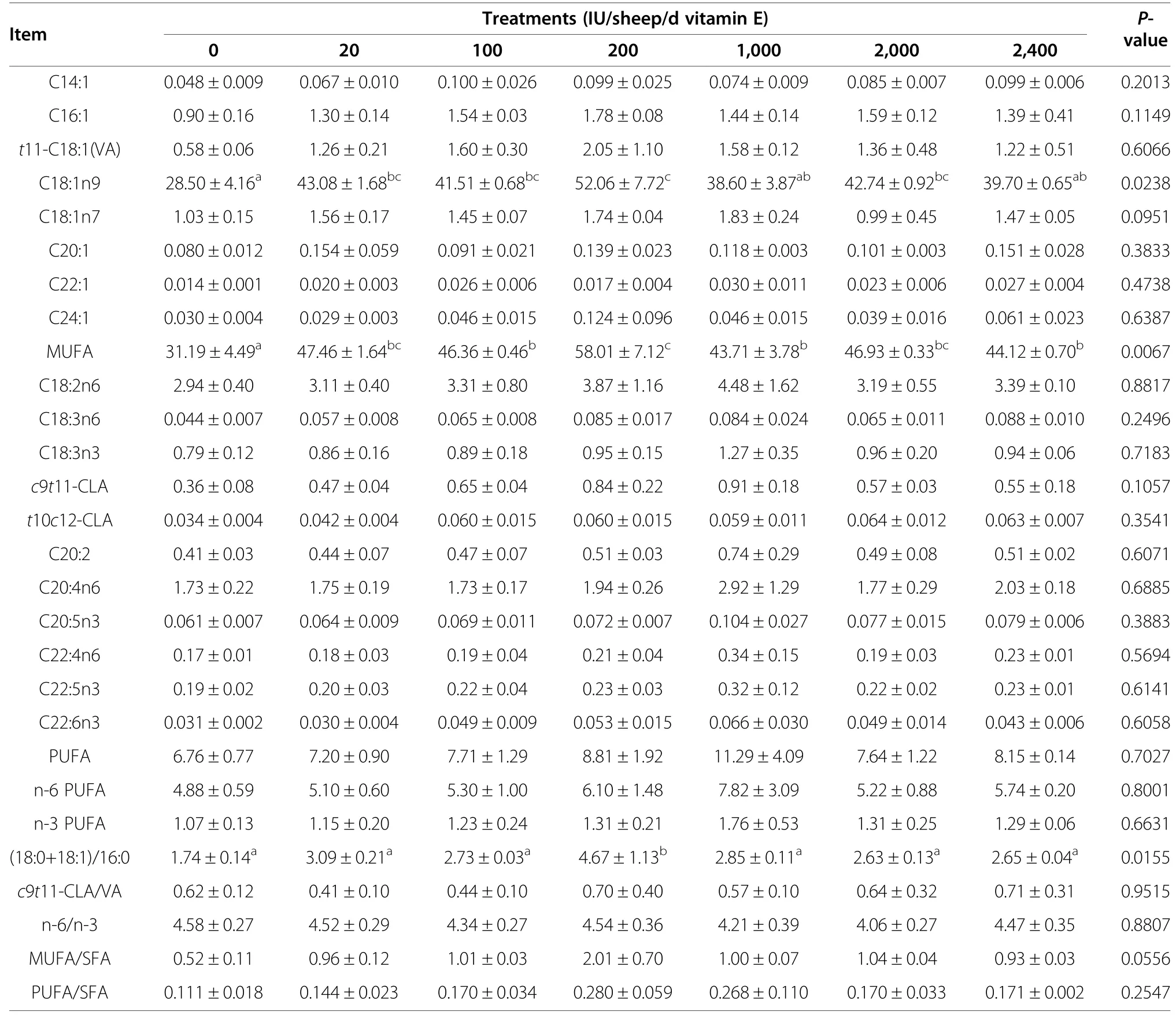
Table 7 Effects of vitamin E supplementation on unsaturated fatty acid content (%of total fatty acids) in the gluteus medius
In our study,muscle total MUFA and C18:1n9 contents were significantly higher in the vitamin E-supplemented groups than in the control group (P<0.05).The higher tissue MUFA content might be partially related to the activation of their biosynthesis in the Δ9d-catalysed reaction.Δ9d also catalyzes the tissue synthesis of c9t11-CLA from trans11-C18:1 (t11-C18:1) [36].In this study,Δ9d activity was estimated by calculating the product/substrate ratios of Δ9d-catalysed reactions (i.e.MUFA/SFA and c9t11-CLA/t11-C18:1)[37].The MUFA/SFA ratio in the LL was higher in the vitamin E-supplemented groups,indicating changes in Δ9d activity;however,we found no differences in the muscle c9t11-CLA/t11-C18:1 among the experimental groups.The vitamin E-supplemented diet significantly affected the MUFA/SFA index but not the c9t11-CLA/t11-C18:1 index,which is somewhat surprising because both indices should reflect Δ9d activity.A possible explanation for this inconsistency is that CLA formation and MUFA biosynthesis might be catalyzed by different Δ9d isoforms that are regulated by different mechanisms[36].Other animal species were reported to express more than one Δ9d isoform [38],but the number of Δ9d isoforms in ruminant tissues has not been clarified.To date,only one isoform has been identified in cattle and sheep [39].However,in a recent study using bovine tissues,two Δ9d-immunoreactive bands at close molecular weights were found,which might indicate the presence of more than one Δ9desaturase proteins [36].Total MUFA content in the GM was also higher in the vitamin E-supplemented groups because of the higher MUFA/SFA ratio,although the differences among the experimental groups were not significant (P=0.0556).As in the LL,dietary vitamin E supplementation did not affect the c9t11-CLA/t11-C18:1 index in the GM.Bonanome and Grundy reported that only C16:0 increases blood cholesterol levels,whereas C18:0 does not,and C18:1 decreases blood cholesterol levels [40].Because these molecules represent the majority of FAs,the ratio of (C18:0+C18:1):C16:0 could help to evaluate the possible clinical effects of different lipids.As shown in Tables 6 and 7,the (C18:0+C18:1):C16:0 ratio in the LL was significantly increased by vitamin E supplementation(P<0.05),and was significantly higher in the E200 group than in the control group in the GM(P<0.05).
We found no significant differences in muscle PUFA content between the vitamin E-supplemented groups and the control group(P>0.05).These findings are consistent with those reported by Kasapidou et al.who found that vitamin E levels did not affect phospholipid content in the muscle or FA composition,and that low vitamin E levels were sufficient to protect lipids from oxidation in sheep[8,13].However,in other studies,PUFA content in meat was affected by vitamin E.For example,it was reported that lambs with muscle vitamin E concentrations of 0.27 mg/g had lower PUFA content in muscle than lambs with muscle vitamin E concentrations of 0.52 mg/g[9].Álvarez et al.also reported that increasing the concentration of α-tocopherol in meat via dietary supplementation protected against PUFA oxidation [7].The differences in findings reported to date suggest to us that further studies are needed to better understand how vitamin E affects the PUFA composition in meat.Regarding individual PUFAs,only c9t11-CLA content in the LL was significantly higher in the vitamin E-supplemented groups than in the control group (P<0.05).This is consistent with the findings reported by Gabryszuk et al.who found that dietary supplementation with Se,Zn,and vitamin E improved the lipid profile and increased the concentration of CLA in the meat of Polish Merino ram-lambs [41].Chen et al.also reported that diets supplemented with soybean oil and vitamin E increased the c9t11-CLA content in lamb meat[42].
It was notable that the dietary vitamin E content of 200 IU/sheep/d seemed to be the best dose in this study because it achieved the highest muscle vitamin E content,the lowest SFA content,and the highest MUFA and c9t11-CLA contents.In our previous study,we found that semen quality,as well as the antioxidant capacity of the testicular cell membrane and mitochondria of Aohan fine-wool sheep showed the greatest improvements when the diet was supplemented with 200 IU/sheep/d vitamin E [16].These results suggest that this level of vitamin E supplementation has particularly beneficial health effects and provides the basis for future studies aimed at investigating the benefits of dietary supplementation with this antioxidant in sheep.
Conclusions
Overall,the present study showed that dietary vitamin E supplementation increases muscle vitamin E content in sheep.Dietary vitamin E also reduced SFA content and increased MUFA and c9t11-CLA contents in the muscle of Aohan fine-wool sheep.These effects of vitamin E were greatest in sheep fed a diet supplemented with 200 IU/sheep/d vitamin E.
Competing interests
The authors declare that they have no competing interests in relation to this study.
Authors’ contributions
KL carried out the statistical analysis and drafted the manuscript.SYG participated in the chemical analysis.HLL conceived the study,participated in its design and coordination,and helped draft the manuscript.DBY and LYY performed the animal experiments.All authors read and approved the final manuscript.
We are grateful to Dr.Zoltan Machaty (Purdue University,USA) for his comments on and for correcting our manuscript.We also thank Professor Yachun Wang and Dr.Congjiao Sun(China Agricultural University) for their valuable suggestions on the statistical methods.The study was financially supported by projects 200903060 and CARS-39 from China Agricultural Ministry.
Published:19 June 2013
1.Simopoulos AP:n-3 fatty acids and human health:defining strategies for public policy.Lipids 2001,36:S83–S89.
2.Department of Health:Nutritional Aspects of Cardiovascular Disease.Report on Health and Social Subjects no.46.London:H.M.Stationery Office;1994.
3.British Nutrition Foundation:Meat in the Diet London.London:British Nutrition Foundation;1999.
4.Morcuende D,Estevez M,Ruiz J,Cava R:Oxidative and lipoytic deterioration of different muscles from free-range reared Iberian pigs under refrigerated storage.Meat Sci 2003,65:1157–1164.
5.Monin G,Hortós M,Díaz I,Rock E,García-Regueiro JA:Lipolysis and lipid oxidation during chilled storage of meat from large White and Pietrain pigs.Meat Sci 2003,64:7–12.
6.Mottram DS:Flavour formation in meat and meat products:A review.Food Chem 1998,62:415–424.
7.Álvarez I,Fuente JD,Cañeque V,Lauzurica S,Pérez C,Díaz MT:Changes in the Fatty Acid Composition of M longissimus dorsi of Lamb during Storage in a High-Oxygen Modified Atmosphere at Different Levels of Dietary Vitamin E Supplementation.J Agric Food Chem 2009,57:140–146.
8.Kasapidou E,Enser M,Wood JD,Richardson RI,Wilkinson RG,Sinclair LA:Influence of vitamin E supplementation and basal diet on the vitamin E status performance and tissue fatty acid concentration in lambs.Animal 2009,3:516–526.
9.Demirel G,Wachira AM,Sinclair LA,Wilkinson RG,Wood JD,Enser M:Effects of dietary n-3 polyunsaturated fatty acids breed and dietary vitamin E on the fatty acids of lamb muscle liver and adipose tissue.Brit J Nutr 2004,91:551–565.
10.Morel PCH,Mcintosh JC,Janz JAM:Alteration of the fatty acid profile of pork by dietary manipulation.Asian-Aust J Anim Sci 2006,19:431–437.
11.Wood JD,Enser M,Fisher AV,Nute GR,Sheard PR,Richardson RI,Hughes SI,Whittington FM:Fat deposition fatty acid composition and meat quality:A review.Meat Sci 2008,78:343–358.
12.Álvarez I,Fuente JD,Díaz MT,Lauzurica S,Pérez C,Cañeque V:Estimation of α-tocopherol concentration necessary to optimise lamb meat quality stability during frozen storage in high-oxygen modified using brokenline regression analysis.Animal 2008,2:1405–1411.
13.Kasapidou E,Wood JD,Richardson RI,Sinclair LA,Wilkinson RG,Enser M:Effect of vitamin E supplementation and diet on fatty acid composition and on meat colour and lipid oxidation of lamb leg steaks displayed in modified atmosphere packs.Meat Sci 2012,90:908–916.
14.Liu Q,Lanari MC,Schaefer DM:A review of dietary vitamin E supplementation for improvement of beef quality.J Anim Sci 1995,73:3131–3140.
15.NRC:Nutrient Requirements of Sheep.Washington DC:Natl Acad Sci;1985.
16.Yue DB,Yan LY,Luo HL,Xu X,Jin XX:Effect of Vitamin E supplementation on semen quality and the testicular cell membranal and mitochondrial antioxidant abilities in Aohan fine-wool sheep.Anim Reprod Sci 2010,118:217–222.
17.Sukhija PS,Palmquist DL:Rapid method for determination of total fatty acid content and composition of feedstuffs and feces.J Agr Food Chem 1988,36:1202–1206.
18.Wulf DM,Morgan JB,Sanders SK,Tatum JD,Smith GC,Williams S:Effects of dietary supplementation of vitamin E on storage and caselife properties of lamb retail cuts.J Anim Sci 1995,73:399–405.
19.López-Bote CJ,Daza A,Soares M,Berges E:Dose–response effect of dietary vitamin E concentration on meat quality characteristics in lightweight lambs.Anim Sci 2001,73:451–457.
20.Liu Q,Scheller KK,Arp SC,Schaefer DM,Williams SN:Titration of fresh meat color stability and malondialdehyde development with Holstein steers fed vitamin E-supplemented diets.J Anim Sci 1996,74:117–126.
21.Lauzurica S,Fuente JD,Díaz MT,Álvarez I,Perez C,Cañeque V:Effect of dietary supplementation of vitamin E on characteristics of lamb meat packed under modified atmosphere.Meat Sci 2005,70:639–646.
22.Drevon CA:Absorption transport and metabolism of vitamin E.Free Radical Res Commun 1991,14:229–246.
23.Arnold RN,Scheller KK,Arp SC,Williams SN,Schaefer DM:Dietary αtocopheryl acetate enhances beef quality in Holstein and beef breed steers.J Food Sci 1993,58:28–33.
24.Lynch A,Kerry JP,O'Sullivan MG,Lawlor JB,Buckley DJ,Morrissey PA:Distribution of α-tocopherol in beef muscles following dietary αtocopheryl acetate supplementation.Meat Sci 2000,56:211–214.
25.O'Grady MN,Monahan FJ,Fallon RJ,Allen P:Effects of dietary supplementation with vitamin E and organic selenium on the oxidative stability of beef.J Anim Sci 2001,79:2827–2834.
26.Zhang DP,Zhang XY,Yu YX,Li JL,Yu ZQ,Wang DQ,Wu MH,Sheng GY,Fu JM:Intakes of omega-3 polyunsaturated fatty acids polybrominated diphenyl ethers and polychlorinated biphenyls via consumption of fish from Taihu Lake China:A risk-benefit assessment.Food Chem 2012,132:975–981.
27.Mann JI:Diet and risk of coronary heart disease and type 2 diabetes.Lancet 2002,360:783–789.
28.Grundy SM,Denke MA:Dietary influences on serum lipids.J Lipid Res 1990,31:1149–1172.
29.Banskalieva V,Sahlu T,Goetsch AL:Fatty acid composition of goat muscles and fat depots:a review.Small Ruminant Res 2000,37:255–268.
30.Judith HF:Saturated fatty acid metabolism is key link between cell division cancer senescence in cellular and whole organism aging.Age 2010,32:231–237.
31.Sanudo C,Enser ME,Campo MM,Nute GR,Maria G,Sierra I,Wood JD:Fatty acid composition and sensory characteristics of lamb carcasses from Britain and Spain.Meat Sci 2000,54:339–346.
32.Li WJ,Zhao GP,Chen JL,Zheng MQ,Wen J:Influence of dietary vitamin E supplementation on meat quality traits and gene expression related to lipid metabolism in the Beijing-you chicken.Brit Poultry Sci 2009,50:188–198.
33.Luo HL,Meng H,Zhu H,Zhang GJ:The primary mechanism of effect of vitamin E on meat quality in goat.Feed Industry 2010,z2:57–63.in Chinese.
34.Mahmut Ű:Muhammet ĺA,Mükerrem K:Effect of vitamin E supplementation to the diet of Morkaraman lambs on intramuscular and intermuscular fatty acid composition.Turk J Vet Anim Sci 2004,28:563–568.
35.Salvatori G,Pantaleo L,Cesare CD,Maiorano G,Filetti F,Oriani G:Fatty acid composition and cholesterol content of muscles as related to genotype and vitamin E treatment in crossbred lambs.Meat Sci 2004,67:45–55.
36.Vasta V,Priolo A,Scerra M,Hallett KG,Wood JD,Doran O:Δ9desaturase protein expression and fatty acid composition of longissimus dorsi muscle in lambs fed green herbage or concentrate with or without added tannins.Meat Sci 2009,82:357–364.
37.Doran O,Moule SK,Teye GA,Whittington FM,Hallett KG,Wood JD:A reduced protein diet induces stearoyl-CoA desaturase protein expression in pig muscle but not in subcutaneous adipose tissue:Relationship with intramuscular lipid formation.Brit J Nutr 2006,95:609–617.
38.Miyazaki M,Ntambi JM:Role of stearoyl-coenzyme A desaturase in lipid metabolism.Prostag Leukotr Ess 2003,68:113–121.
39.Chung M,Ha S,Jeong S,Bok J,Cho K,Baik M,Choi Y:Cloning and characterization of bovine stearoyl CoA desaturase 1 cDNA from adipose tissue.Biosci Biotech Bioch 2000,64:1526–1530.
40.Bonanome A,Grundy SM:Effect of dietary stearic acid on plasma cholesterol and lipoprotein levels.New England J Med 1988,318:1244–1247.
41.Gabryszuk M,Czauderna M,Baranowski A,Strzalkowska N,Jozwik A,Krzyewski J:The effect of diet supplementation with Se Zn and vitamin E on cholesterol CLA and fatty acid contents of meat and liver of lambs.Anim Sci Pap Rep 2007,25:25–33.
42.Chen XJ,Mao HL,Lin J,Liu JX:Effects of supplemental soybean oil and vitamin E on carcass quality and fatty acid profiles of meat in Huzhou lamb.Anim Sci 2008,58:129–135.
杂志排行
Journal of Animal Science and Biotechnology的其它文章
- Changes of hepatic biochemical parameters and proteomics in broilers with cold-induced ascites
- The effects of dietary fiber level on nutrient digestibility in growing pigs
- Potential role of N-carbamoyl glutamate in biosynthesis of arginine and its significance in production of ruminant animals
- Review on the development of genotyping methods for assessing farm animal diversity
