Cu/Co/Fe 水滑石衍生的复合氧化物催化高氯酸铵热分解的研究
2013-08-20刘洪博黄志勇郭冰之矫庆泽
刘洪博 黄志勇 郭冰之,2 矫庆泽*,,2
(1 北京理工大学珠海学院化工与材料学院,珠海 519088)
(2 北京理工大学化工与环境学院,北京 100081)
Ammonium perchlorate (AP) is the most commonly used oxidizer in composite solid propellants. Thermal decomposition characteristics of AP are highly relevant to the combustion behavior of the propellants.Transition metal oxides (TMO) such as CuO, Co3O4,Fe2O3, MnO2, Cr2O3have shown good activity for thermal decomposition of AP, and their mixtures(Mixed oxides, MOs) exhibit even better catalytic property than the single oxide[1-5]. Several methods have been employed to prepare MOs, such as solid state reaction[6], homogeneous precipitation[7], sol-gel method[8], citric acid complexing approach[9], autocombustion[10]and layered double hydroxides (LDHs)derived MOs[11]. LDHs are a family of lamellar solids,whose structure can be represented by the general formula of [M2+(1-x)M3+x(OH)2]x+·An-x/n·zH2O, where M2+and M3+are di- and trivalent cations, respectively, the value of x is equal to the molar ratio of nM3+/(nM2++nM3+)and An-is an anion[12]. LDHs derived MOs have attracted increasing attention due to their large surface area, small crystallite size and stability against sintering. Cu/Co and Cu/Fe-MOs derived from Cu/Co and Cu/Fe-LDHs, respectively, were used as good catalysts for thermal decomposition of AP in our previous work[13-14]. With the addition of Cu/Co-MOs,thermal decomposition temperature of AP can be lowered by 113 ℃. However, Cu/Co and Cu/Fe-MOs are not easy to form due to the “Jahn-Teller” effect of Cu, the only M2+in the layer. Furthermore, Co is prevented from consideration in practical application because of its high price. In the present work, Fe was introduced into Cu/Co-LDHs on the base of our previous work in order to take advantage of synergistic effect between different metals. Cu/Co/Fe-LDHs were prepared by co-precipitation reaction without hydrothermal treatment. Cu/Co/Fe-MOs were then obtained by calcination of Cu/Co/Fe-LDHs precursors.The catalytic property of Cu/Co/Fe-MOs for improving thermal decomposition of AP was investigated. The catalytic mechanism was also discussed.
1 Experimental
1.1 Synthesis of Cu/Co/Fe-LDHs and Cu/Co/Fe-MOs
A series of Cu/Co/Fe-LDHs with Cu/Co/Fe molar ratio of 1 ∶1 ∶1, 2 ∶4 ∶3, 1 ∶3 ∶2, 2 ∶8 ∶5 and 1 ∶5 ∶3,respectively, (assigned as Cu1Co1Fe1, Cu2Co4Fe3,Cu1Co3Fe2, Cu2Co8Fe5and Cu1Co5Fe3-LDHs, respectively) were prepared using a co-precipitation method[10].An aqueous solution of Cu(NO3)2·3H2O/Co(NO3)2·6H2O/Fe(NO3)3·9H2O was added into an aqueous solution of Na2CO3/NaOH under vigorous stirring. The pH value was adjusted to 9 and the resulting slurry was then aged at 80 ℃for 24 h. The final precipitate was filtered off and thoroughly washed with distilled water before being dried overnight at 80 ℃in an oven. The Cu/Co/Fe-MOs catalysts were obtained by calcination of as-obtained Cu/Co/Fe-LDHs precursors in muffle furnace with a temperature controller. The sample was heated at a constant heating rate of 3 ℃·min-1up to 400, 500, 600, 700 and 800 ℃, respectively, maintained at the assigned temperature for 3 h and then cooled down to ambient temperature.
1.2 Characterizations
Elemental chemical analysis for Cu, Co and Fe were performed by inductively coupled plasma emission (ICP), on a JY, model ULTIMA, using solutions prepared by dissolving the sample in 0.1 mol·L-1HNO3.Powder X-ray diffraction(XRD)patterns were recorded using a PANalytical X′Pert PRO MPD diffractometer, Cu Kα (λ=0.154 06 nm) radiation operating with 40 kV and 40 mA. The receiving slit size is 12.75 mm. A scan rate of 0.04°·s-1was used to record the patterns in the 2θ range of 5° ~80° .Transmission electron microscope (TEM) was taken on a Hitach-800 electron microscope, working at 100 kV.Low temperature N2-adsorption measurements were taken in a Quantachrome NOVA 1200e Surface Area& Pore Size Analyzer. Prior to analysis, the samples were outgassed in vacuum at 120 ℃. BET method was used to obtain the specific surface area.
1.3 Catalytic performance
To test the catalytic activity of Cu/Co/Fe-MOs in the thermal decomposition of AP, both the AP and appropriate amount of Cu/Co/Fe-MOs were dispersed in absolute ethanol by grinding to achieve a uniform dispersion. These samples were then dried in vacuum oven at 80 ℃for 1 h. Finally, the powder mixtures were characterized by differential thermal analysis(DTA). The test was carried out on a Beijing Optical Instrument Factory WCR-1100, in a N2atmosphere with a flow of 20 mL·min-1over the range of 50~500℃(heating rate of 20 ℃·min-1) to investigate thermal decomposition behavior of AP. The samples were also tested by thermal gravimetric analyzer coupled with an online mass spectrometer (TG-MS, PerkinElmer Diamond, model Thermostar TM), operated under flowing argon (50 mL min-1) with a heating rate of 10℃·min-1over 50~500 ℃.
2 Results and discussion
2.1 Composition of Cu/Co/Fe-LDHs
Elemental chemical analysis data for metals in Cu/Co/Fe-LDHs precursors are listed in Table 1. The results of Cu/Co/Fe molar ratio in the solid are close to the values used in the reaction stoichiometries,which means the precipitation of Cu, Co and Fe is efficient.
2.2 Structure of Cu/Co/Fe-LDHs and Cu/Co/Fe-MOs
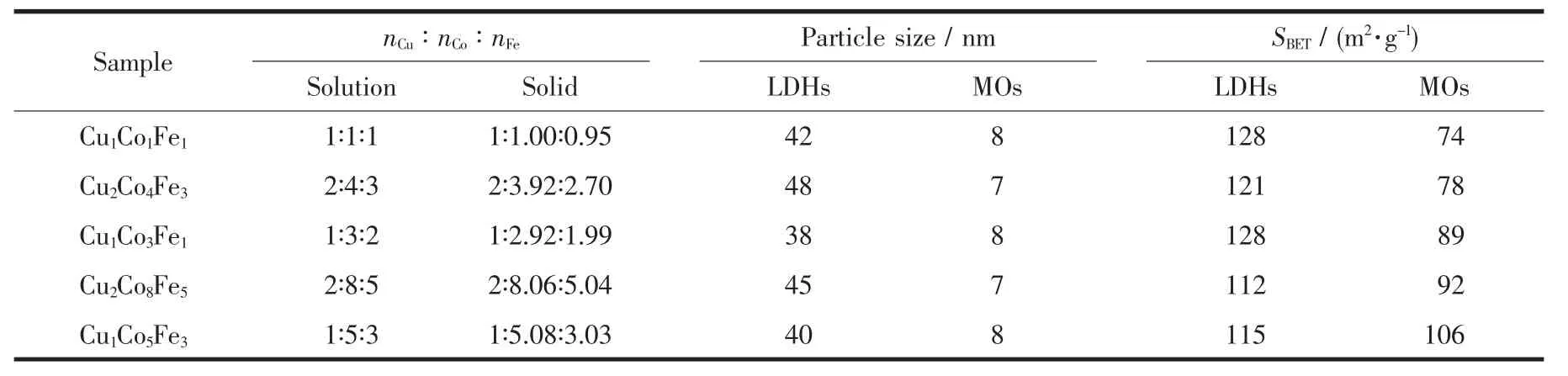
Table 1 Selected data for Cu/Co/Fe-LDHs and their derived Cu/Co/Fe-MOs
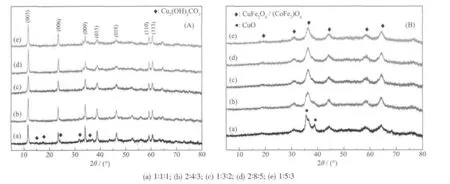
Fig.1 XRD Patterns for Cu/Co/Fe-LDHs (A) and Cu/Co/Fe-MOs (B) prepared with differeent molar ratios
The XRD patterns for Cu/Co/Fe-LDHs precursors with different molar ratios of Cu/Co/Fe are shown in Fig.1A. In each case, the XRD patterns exhibit the characteristic reflections of LDHs materials. Sharp,intense peaks at low diffraction angles (peaks close to 2θ= 11.7°, 23.5°, 34.1°) are ascribed to diffraction by basal plane of (003), (006) and (009), respectively,indicating relatively well-formed crystalline layered structures with rhombohedral symmetry (PDF: 41-1428)[12]. No other phases are observed for all Cu/Co/Fe-LDHs samples except a little Cu2(OH)2CO3impurity for Cu1Co1Fe1-LDHs sample. This can be attributed to the “Jahn-Teller” effect caused by high content of Cu,which leads to a difficulty for Cu to be introduced into the layers. On the other hand the board diffraction peaks at 38° and 47° corresponding to the (015) and(018) crystal planes are characteristic of LDHs. The well defined (110) and (113) diffraction peaks at 59.9°and 61.2° reveal a reasonable dispersion of metal ions in the hydroxide layers. Remarkable changes are observed for Cu/Co/Fe-LDHs samples after calcination at 500 ℃as shown in Fig.1B. CuFe2O4(PDF: 77-0010) and (CoFe2)O4(PDF: 79-1744) spinel phase with board peaks at 2θ=19°, 31°, 36°, 43°, 57° and 63° are observed for all the samples. This drastic change in the diffract patterns reveals that thermal decomposition of the LDHs takes place via a highly disordered structure. And a little CuO phase is seen for the Cu1Co1Fe1-MOs sample, which can be ascribed to the existence of Cu2(OH)2CO3impurity in the Cu1Co1Fe1-LDHs precursor.
BET surface areas and grain sizes (calculated by Scherrer formula using XRD data) of Cu/Co/Fe-LDHs and Cu/Co/Fe-MOs catalysts are also listed in Table 1. The BET results show that Cu/Co/Fe-LDHs and Cu/Co/Fe-MOs catalysts have specific surface area in the range of 110 ~130 m2·g-1and 70 ~110 m2·g-1,respectively. Grain size is around 40~50 nm and 10 nm, respectively. Higher specific surface area and smaller grain size are obtained than the previous Cu/Co and Cu/Fe-MOs[13-14].
2.3 Morphology of Cu/Co/Fe-LDHs and Cu/Co/Fe-MOs
The TEM images of Cu2Co8Fe5-LDHs precursors and Cu2Co8Fe5-MOs catalysts are shown in Fig.2. The micrographs of the Cu2Co8Fe5-LDHs precursors show homogenous regular flakes with particle size of 40~50 nm. Cu2Co8Fe5-MOs obtained by calcination at 500 ℃exhibits well dispersed particles with particle size of 20 ~30 nm. These particles are smaller than those obtained by auto-combustion and other methods[7,10].
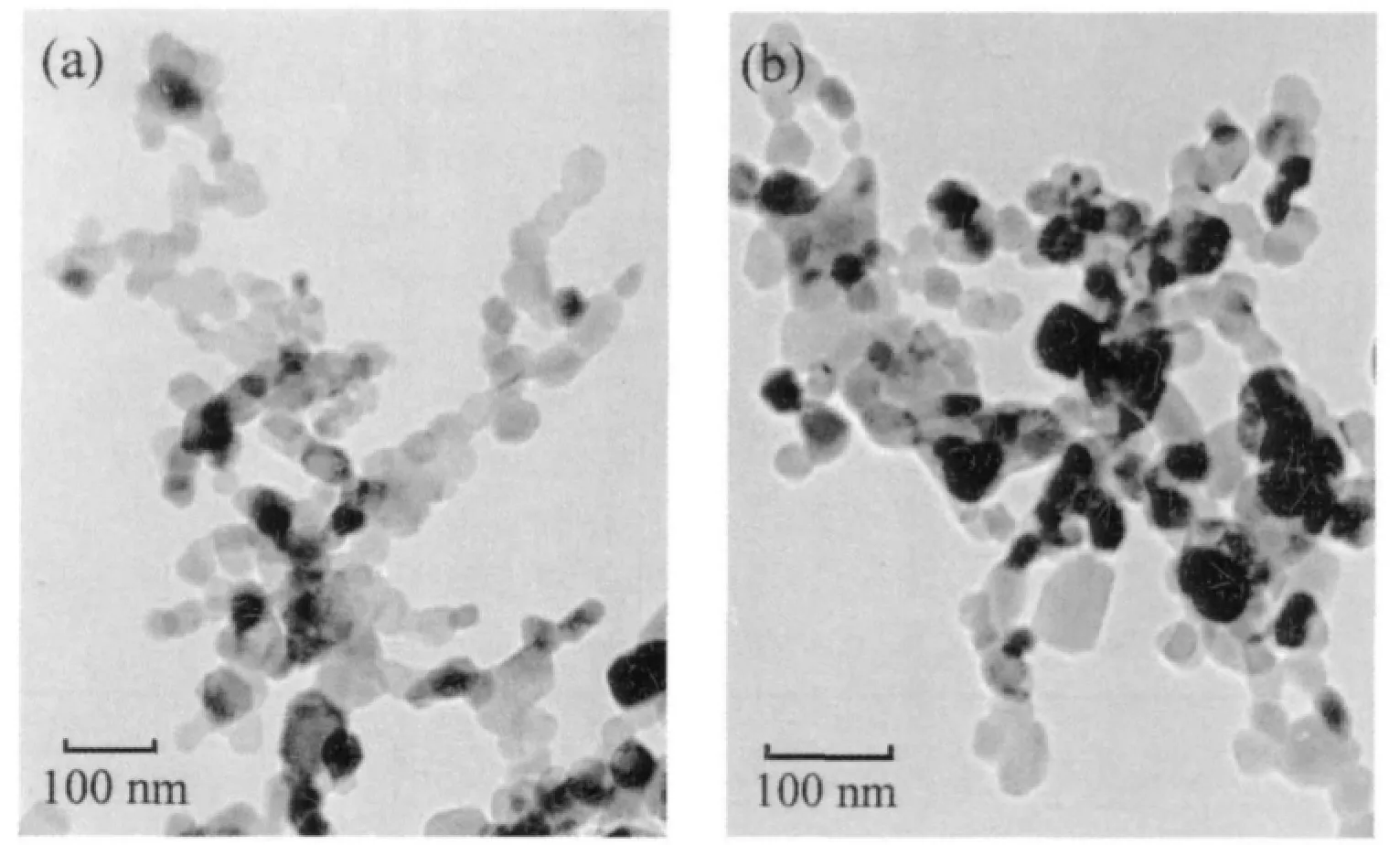
Fig.2 TEM photos forCu2Co8Fe5-LDHs (a) andCu2Co8Fe5-MOs (b)
2.4 Catalytic performance on thermal decomposition of AP
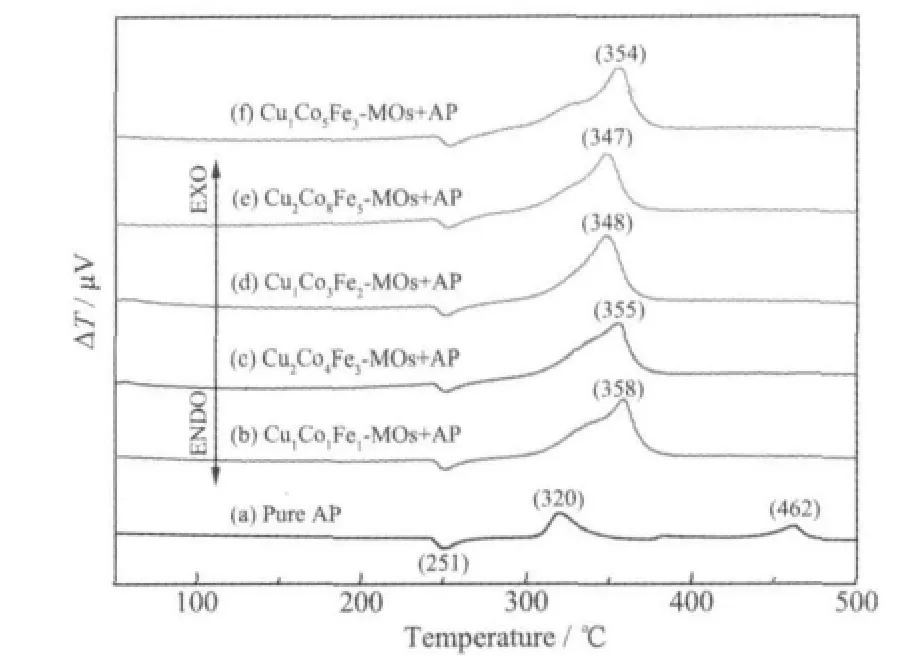
Fig.3 DTA curves for thermal decomposition of AP
The catalytic thermal decomposition of pure AP and AP with Cu/Co/Fe-MOs (4wt%) in different Cu/Co/Fe molar ratios are shown in Fig.3. It can be observed that the addition of Cu/Co/Fe-MOs in AP leads to a significant reduction of the ending decomposition temperature of AP. Thermal decomposition of pure AP has apparently three peaks similar to the previous results[15].With the addition of Cu/Co/Fe-MOs,the same peak at 251 ℃ appear in all cases,indicating that the Cu/Co/Fe-MOs have little effect on the crystallographic transition temperature of AP.However, dramatic changes in the exothermic peak of AP decomposition are observed in a relatively high temperature region after adding Cu/Co/Fe-MOs catalysts. The first exothermic peak disappears for all cases and the high temperature decomposition peaks shift to lower temperature region. The thermal decomposition temperature of AP is lowered by 104~115 ℃. Cu/Co/Fe-MOs show better catalytic activity than Cu/Co and Cu/Fe-MOs reported by our previous work[13-14].
DTA curves for thermal decomposition of AP with Cu2Co8Fe5-MOs prepared at different calcination temperatures are shown in Fig.4. For 400 and 500 ℃calcined sample, only one exothermic peak is observed at 323 and 348 ℃, respectively. With the increase of calcination temperature, another peak appears and becomes more and more obvious.Furthermore, the temperature of the second exothermic peak shifts to high temperature region. It indicates that the catalytic activity of Cu/Co/Fe-MOs in thermal decomposition of AP depends on their calcination temperature. Samples calcined at lower temperature show higher catalytic activity than those calcined at higher temperature.
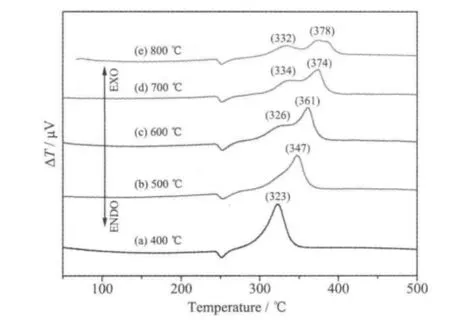
Fig.4 DTA curves for thermal decomposition of AP catalyzed by Cu2Co8Fe5-MOs with different calcination temperatures
As shown in Fig.5, the decomposition temperature of AP decreases from 352 to 316 ℃when the catalyst amount is increased from 1wt% to 10wt%. It is clear that the catalytic activity of Cu/Co/Fe-MOs depends on the percentage of Cu/Co/Fe-MOs catalysts in AP. Higher amount of Cu/Co/Fe-MOs favors the further decrease of the thermal decomposition temperature of AP. These results are the same as what we obtained before[13-14]but different from those obtained by Liu et al.[10]who reported the opposite results. The reason leading to this difference is the different catalytic mechanism for the two catalysts. Liu′ s catalyst promotes the transfer of electrons, which makes the activation energy of thermal decomposition of AP reduced, so the decomposition temperature of AP decreases and the decomposition rate of AP accelerates. When the content of the catalyst exceeds certain value, the positive holes and electrons are partially annihilated, so the catalytic activity decreases. While for our catalysts, the improvement of thermal decomposition of AP is achieved via the superoxide ion (O2-) on the surface of the catalysts.The higher amount of the catalyst, the more O2-on the surface, thus the higher catalytic activity.

Fig.5 Relationship between decomposition temperature of AP and Cu2Co8Fe5-MOs catalyst amount
2.5 Catalytic mechanism
TG-MS results are listed in Table 2. The results show that the products of thermal decomposition of pure AP are H2O, NH3, O2, N2O, NO, NO2, HCl, and a small amount of Cl2, NOCl, ClO, ClO2and ClO3(which are the intermediate products). In the presence of Cu/Co/Fe-MOs, the products are almost the same as those of pure AP. However, the intensity of N2O, NO,NO2ions are much higher than pure AP, which indicates that the NH3produced by the decomposition of AP is more completely oxidized. These results are similar to the previous results obtained by Yu[16].According to our experimental results and literature data[16-19], the catalytic mechanism of Cu/Co/Fe-MOs is suggested. The schematic presentation of catalytic thermal decomposition of AP is shown in Fig.6. The thermal decomposition of AP is possibly preceded by the following main reactions:
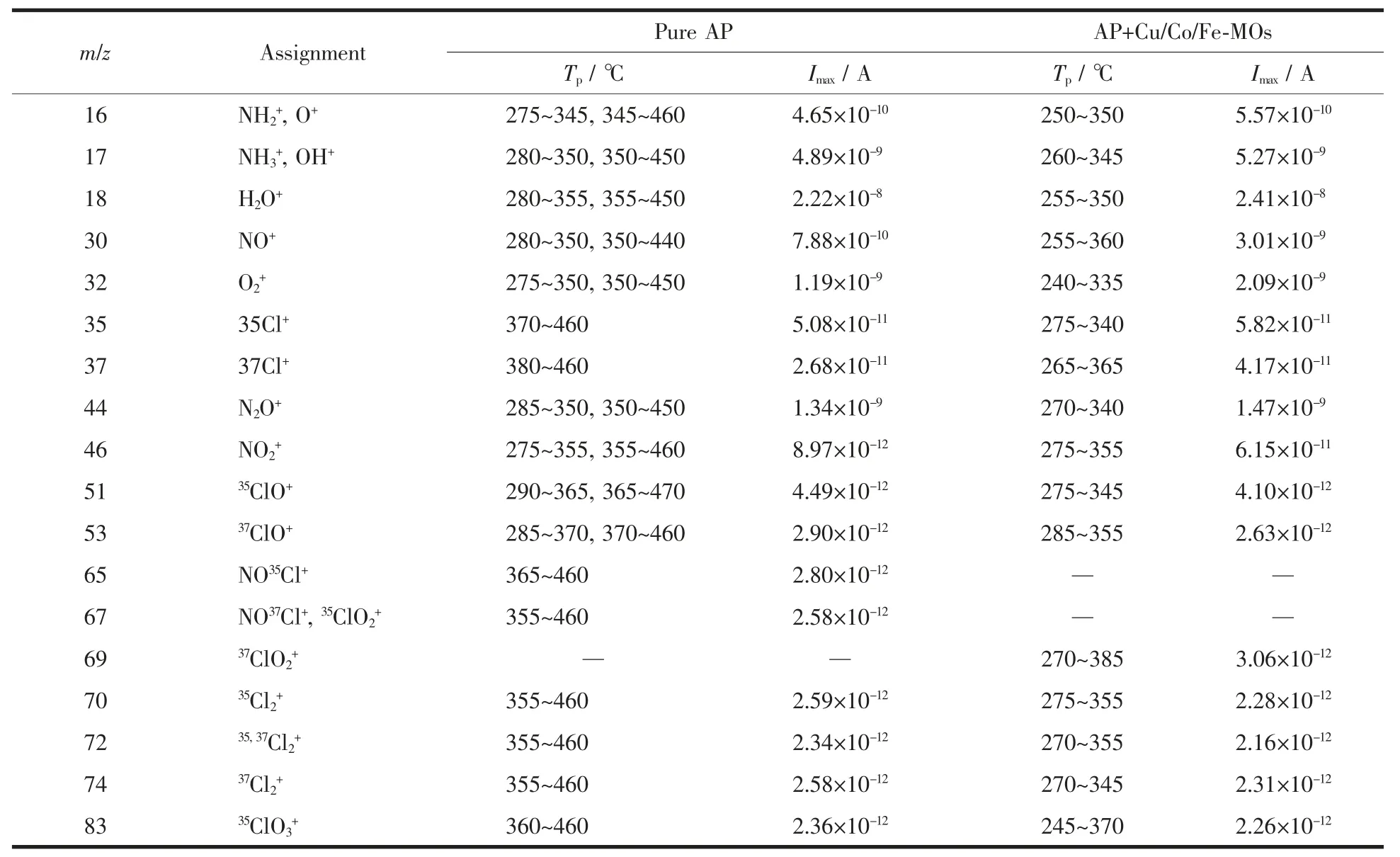
Table 2 Assignment and intensity maxima of the mass spectroscopic ions during the thermal decomposition of AP
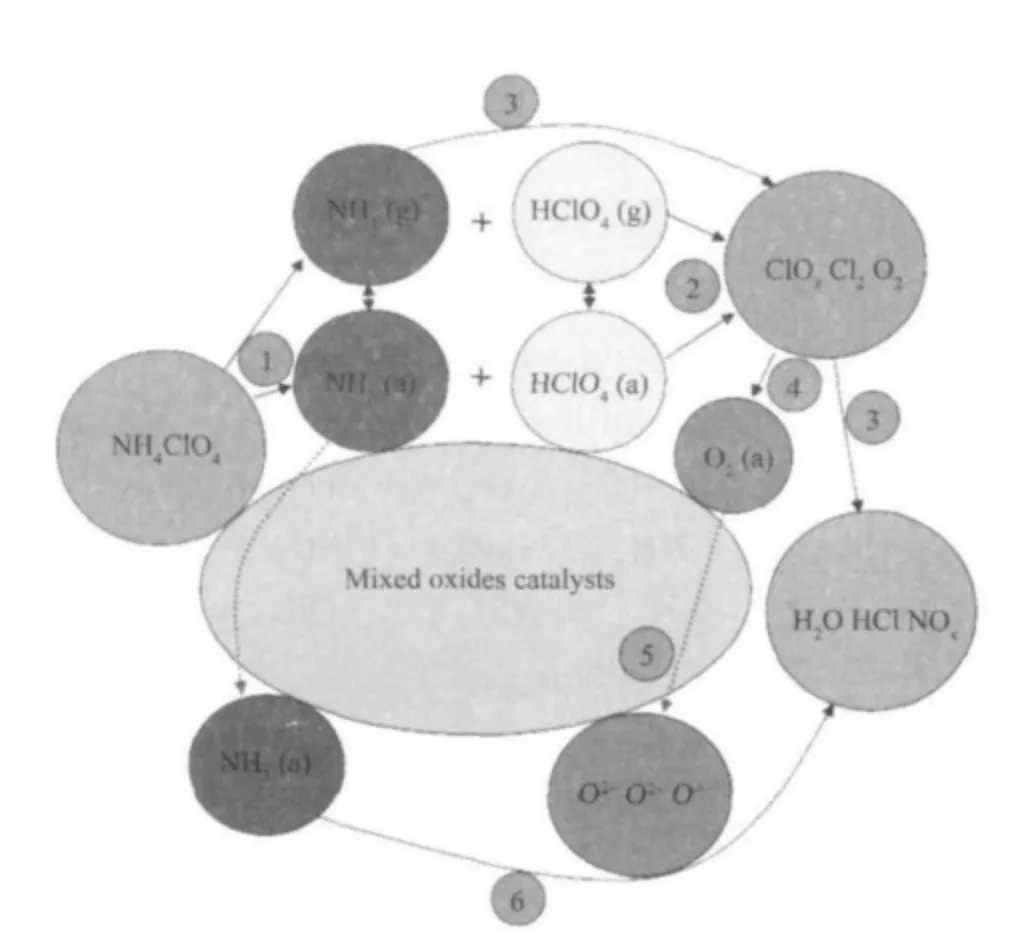
Fig.6 Schematic presentation of catalytic decomposition process of AP by Cu/Co/Fe-Mos
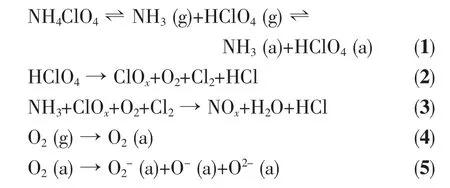
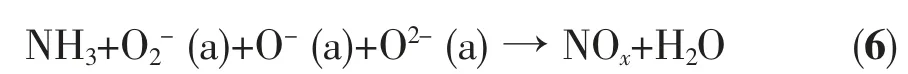
Note: 1. (a): adsorbed; 2. ClOxrefers to ClO-, ClO2-, ClO3-and ClO4-. The relative amount of the four species is uncertain in the present study; 3. NOxrefers to NO, NO2and N2O. The relative amount of the three species can not be defined clearly in this study.
The mechanism of catalytic action is based on the presence of superoxide ion (O2-) on the surface of Cu/Co/Fe-MOs. During the thermal decomposition of AP, the O2-ions, which are formed from the oxygen adsorbed on the surface of Cu/Co/Fe-MOs, are proton traps, and they can simplify thermal decomposition of AP.
3 Conclusions
In summary, Cu/Co/Fe-MOs as new catalysts for thermal decomposition of AP were prepared by calcination of Cu/Co/Fe-LDHs precursors. The Cu/Co/Fe-MOs catalysts exhibit CuFe2O4and CoFe2O4phase with high specific surface areas and they have homogenous particles with particle size around 25 nm.Cu/Co/Fe-MOs catalysts show excellent catalytic performance on thermal decomposition of AP. The decomposition temperature of AP is lowered by 139℃with 4wt% of 400 ℃calcined Cu/Co/Fe-MOs. The improvement in thermal decomposition of AP is achieved via the superoxide ion (O2-) on the surface of Cu/Co/Fe-MOs.
[1] Kapoor I P S, Srivastava P, Singh G. Propell. Explos.Pyrotech., 2009,34(4):351-356
[2] YU Zong-Xue(余宗学), LU Lu-De(陆路德), YANG Xu-Jie(杨 绪 杰), et al. Chinese J. Inorg. Chem.(Wuji Huaxue Xuebao), 2010,26(12):2155-2159
[3] Li L P, Sun X F, Qiu X Q, et al. Inorg. Chem., 2008,47(19):8839-8846
[4] Zhang L L, Zhang W G, Zhong H, et al. Mater. Chem. Phys.,2011,125(3):322-325
[5] Ebrahim A G, Behrouz S, Ali K, et al. Powder Technol.,2012,217:330-339
[6] Said A A,Al-Qasmi R.Thermochim.Acta,1996,275(1):83-91
[7] Singh G, Kapoor I P S, Dubey S. Propell. Explos. Pyrotech.,2009,34(1):72-77
[8] Srivastava P, Kapoor I P S, Singh G. J. Alloys Compd.,2009,485(1-2):88-92
[9] Li W, Cheng H. Solid State Sci., 2007,9(8):750-755
[10]Liu T, Wang L S, Yang P, et al. Mater. Lett., 2008,62(24):4056-4058
[11]Hansen H C B, Koch C B. Appl. Clay Sci., 1995,10(1-2):5-19
[12]Cavani F, Trifiro F, Vaccari A. Catal. Today, 1991,11(2):173-301
[13]Liu H B, Jiao Q Z, Zhao Y, et al. J. Alloys Compd., 2010,496(1-2):317-323
[14]Liu H B, Jiao Q Z, Zhao Y, et al. Mater. Lett., 2010,64(15):1698-1700
[15]Boldyev V V. Thermochim. Acta, 2006,443(1):1-36
[16]Yu Z X, Sun Y, Wei W, et al. J. Therm. Anal. Calorim.,2009,97(3):903-909
[17]Zhao F Q, Chen P, Li S W. Thermochim. Acta, 2004,416(1-2):75-78
[18]Chen L J, Li L P, Li G S. J. Alloys Compd., 2008,464(1-2):532-536
[19]YU Zong-Xue(余宗学), JIANG Xiao-Hong(江晓红), LU Lu-De(陆 路 德), et al. Chinese J. Inorg. Chem.(Wuji Huaxue Xuebao), 2009,25(10):1747-1752
