Ammonium Ion Adsorption and Settleability Improvement Achieved in a Synthetic Zeolite-Amended Activated Sludge*
2013-07-31EmiliaOtalLuVilchesYolandaLunaRodrigoPobleteJuanGarcMayaandConstantinoFernndezPereira
Emilia Otal**, Luís F. Vilches, Yolanda Luna, Rodrigo Poblete, Juan M. García-Maya and Constantino Fernández-Pereira
Department of Chemical and Environmental Engineering, School of Engineering, University of Seville, Camino de los Descubrimientos s/n, 41092 Seville, Spain
Ammonium Ion Adsorption and Settleability Improvement Achieved in a Synthetic Zeolite-Amended Activated Sludge*
Emilia Otal**, Luís F. Vilches, Yolanda Luna, Rodrigo Poblete, Juan M. García-Maya and Constantino Fernández-Pereira
Department of Chemical and Environmental Engineering, School of Engineering, University of Seville, Camino de los Descubrimientos s/n, 41092 Seville, Spain
Municipal wastewater treatment plants typically exhibit two classic problems: high ammonium concentration in water after conventional biological treatment and, in some cases, poor activated sludge sediment ability. Potential solutions to these problems were investigated by adding a synthetic zeolite obtained from coal fly ash to different steps of activated sludge treatment. The experimental results for ammonium removal fit well with the theoretical adsorption isotherms of the Freundlich model with a maximum adsorption capacity of 13.72 mg·g−1. Utilization of this kind of zeolite to improve activated sludge sediment ability is studied for the first time in this work. It is found that the addition of the zeolite (1 g·L−1) to an activated sludge with settling problems significantly enhances its sediment ability and compact ability. This is confirmed by the sludge volume index (SVI), which was reduced from 163 ml·g−1to 70 ml·g−1, the V60value, which was reduced from 894 ml·L−1to 427 ml·L−1, and the zeta potential (ζ), which was reduced from −19.81 mV to −14.29 mV. The results indicate that the addition of this synthetic zeolite to activated sludge, as an additional waste management practice, has a positive impact on both ammonium removal and sludge settleability.
low-cost sorbent, nitrogen, settleability, zeolite adsorption, zeta potential
1INTRODUCTION
Wastewater discharge from municipal wastewater treatment plants (WWTP) usually contains high concentrations of nitrogen and phosphorous that may lead to eutrophication, cause depletion of dissolved oxygen and can be potentially toxic for aquatic life. To prevent these problems, discharge concentration limits that are safe have been adopted in several countries, but achieving these low levels may be a challenge for some WWTP. The reason why secondary effluents from conventional WWTP have high nitrogen and phosphorus concentrations is well known, since the carbon-tonitrogen and carbon-to-phosphorus ratios are higher in urban wastewater than in the equilibrated metabolism of biomass. To achieve an acceptable level of nutrients (nitrogen and phosphorus) in the secondary effluents of urban wastewater, numerous advanced methods (chemical and biological) have been developed.
An additional problem in WWTP is poor operation due to the low settling characteristics of the activated sludge. One reason for this is its charged colloidal structure (commonly in negative charge), which inhibits particle aggregation. In most cases, coagulation and flocculation of these colloidal suspensions allow breaking of the colloidal structure and neutralization of the particle zeta potential, thus improving the settleability of the sludge. Moreover, sludge density depends mainly on the ratio of organic to inorganic material, as the inorganic fraction increases sludge density. The addition of inorganic ballasting agents in batch and/or continuous operation as a strategy to solve the problem has been tested [1-10], and it is concluded that the addition of mineral material improves activated sludge settling ability [3-10] and that sludge dewatering can be enhanced by adding the mineral material saturated with some cations (Ca or Na) [7].
For ammonium removal, natural zeolites, which are hydrated aluminium-silicate minerals with cation exchange capacity, have been widely used [11-14]. The material can be added at different stages of the wastewater treatment process or used in packed beds. An alternative to natural zeolites are the synthetic zeolites obtained from fly ash, a by-product of coal power plants, which have been used for sustainability purposes in a number of applications, mainly because of their ion exchange and adsorption capacities. The zeolites are synthesized using the hydrothermal [15-22] or the fusion methods [23, 24]. The synthetic zeolite that has been investigated in this work (CV-Z) is mainly composed of NaP1 and low levels of analcime and chabazite zeolites as well as the fly ash remaining after the hydrothermal alkaline activation. The synthetic zeolite has a cation exchange capacity of 2.7 meq·g−1, equivalent to 60% zeolite content [25, 26]. CV-Z can be considered a low cost adsorbent [27] when comparing the cost effectiveness of the treatment using the synthetic zeolite to a commercial adsorbent (zeolite type A). This material has previously been tested for the removal of contaminants in industrial wastewaters. Those studies show that the use of CV-Z does not significantly improve COD removal efficiency, but it is a promising material for nitrogen and phosphorus removal [25, 27, 28].
The aim of this research is to investigate the ammonium adsorption capacity of CV-Z in urban wastewater, which is a multi-component aqueous solution and some competitive adsorption problems could occur [29]. Another objective of this study is to examine the effect of CV-Z on the activated sludge settling and dewatering performance of the WWTP, which has not been studied, and to assess the feasibility of its application on activated sludge effluents to simultaneously solve or alleviate both problems.
2EXPERIMENTAL
2.1Adsorption studies
Urban wastewater was collected from the effluent of the primary settling tank of a WWTP in Seville (Spain). The sample was stored at 4 °C until use. CV-Z was obtained from the hydrothermal alkaline activation of coal fly ash from the Narcea Power Plant (Spain).
To elucidate the ammonium adsorption capacity of CV-Z, batch experiments at room temperature (20 °C) were performed in 200 ml volume flasks, using solutions that were prepared by dissolving 10, 25 and 50 mg·L−1of ammonium, as (NH4)2HPO4, in wastewater. For each ammonium concentration, the influence of the adsorbent loading on the ammonium removal was investigated using different CV-Z concentrations, from 1 to 4 mg·L−1. Control tests were carried out for all ammonium concentrations using blanks without CV-Z. The flasks were then mechanically shaken for 20 min at 150 r·min−1. All tests were performed in duplicate. Ammonium concentrations in the filtered samples were determined using an ammonium selective electrode (Crison Instruments).
To determine the4NH+adsorption capacity of the synthetic zeolite, the Freundlich and Langmuir models were used. The amounts of4NH+adsorbed onto CV-Z were calculated from the difference between the initial and the remaining ammonium concentrations in the solution. The amount of4NH+adsorbed per gram of synthetic zeolite X/m (mg·g−1) was given by (C0−Ce)V/m, where X is the mass of ammoniumadsorbed onto CV-Z (mg), m is the mass of CV-Z (g), C0is the initial ammonium concentration (mg·L−1), Ceis the equilibrium liquid phase ammonium concentration (mg·L−1), and V is the volume of solution (L).
The Freundlich model shows that the ratio of ammonium adsorbed to the adsorbent is a function of the equilibrium liquid phase solute concentration.

where K (L·g−1) and n represent the Freundlich isotherm constants. The Freundlich coefficient K represents an indicator of adsorption capacity; 1/n indicates the adsorption intensity, while its reciprocal n represents the affinity factor for the Freundlich model.
The Langmuir isotherm is another useful and simple isotherm for describing both physical and chemical adsorption. It is a two parameter model.
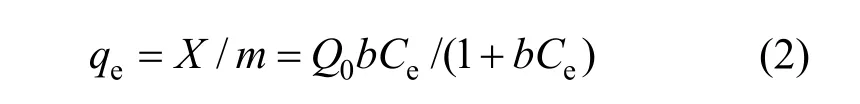
where Q0is the Langmuir monomolecular layer capacity (mg·g−1) and b is the Langmuir isotherm constant.
2.2Settling tests
Two different sludge samples, collected from the same WWTP mentioned above, with different sedimentation qualities were used for the experiments. Batch settling tests were performed to investigate the effect of CV-Z on sludge properties, such as sediment ability, monitored as settling sludge volume after 60 min (V60) and SVI. Sludge was used within the first hour after collection. Settling tests were carried out in a 500 ml settling vessel at room temperature (20 °C). The synthetic zeolite was added to the sludge as a dry powder at concentrations of 1, 2, and 3 g·L−1. For the blank tests, no zeolite was utilized. The suspensions formed with CV-Z and activated sludge were stirred in a Jar-Test (Selecta) for 15 min at 100 r·min−1and then settled for 90 min. Total suspended solids (TSS), V60and SVI were established according to the Standard Methods for the Examination of Water and Wastewater [30]. Total nitrogen (TN) content was determined using a Shimadzu TOC-V CPH/TOC-V CPN analyzer, with a 5.0% coefficient of variation. Samples were filtered through a 0.45 μm cellulose acetate filter before TN analysis. All the analyses were conducted in duplicate, from which the average was calculated. Solutions of CV-Z in distilled water or activated sludge were centrifuged at 4000 r·min−1for 10 min and the supernatant was taken and used for the experimental ζ measurements. pH was adjusted with 0.1 mol·L−1H2SO4or NaOH solutions. The equipment used, Zetaphoremeter IV (ZetaCompact CAD), has a microprocessor unit which determines the electrophoretic mobility of particles, each value being the mean of ten acquisition data. The ζ values were calculated from the electrophoretic mobility values, with the aid of the O’Brien conversion theory.
3RESULTS AND DISCUSSION
3.1Ammonium ion adsorption of the synthetic zeolite
The influence of adsorbent loading on ammonium removal has been investigated using different concentrations of (NH4)2HPO4dissolved in wastewater, ranged from 10 to 50 g·L−1. For different initial ammonium concentrations C0, the effect of the adsorbent dose on the variation of ammonium concentration at equilibrium Ceis shown in Fig. 1. As reported by other authors [31], the ammonium adsorption efficiency increases with the increase in the adsorbent dose for a constant ammonium concentration. The effect of the adsorbent concentration is the strongest when the initial ammonium concentration is the highest: at C0of 18.5 mg·L−1,Ceslightly decreased from 12.2 mg·L−1to 7.5 mg·L−1as the adsorbent dose increased from1 g·L−1to 4 g·L−1, while it decreased more evidently, from 48.6 mg·L−1to 27.3 mg·L−1, at C0of 62.3 mg·L−1.
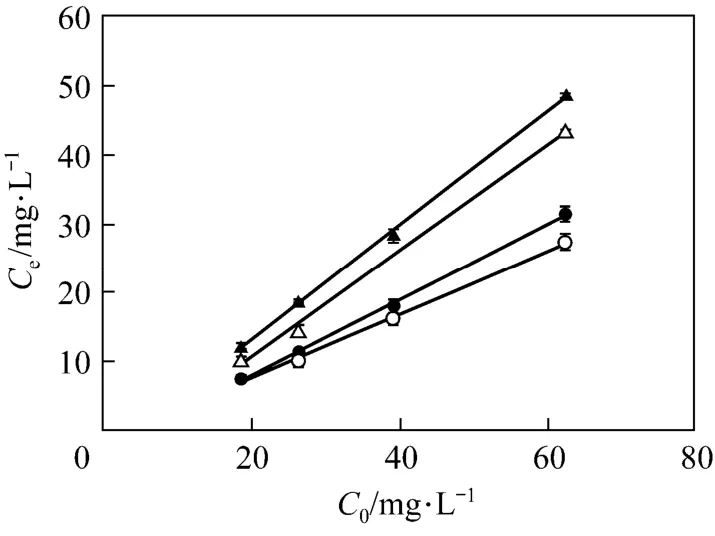
Figure 1Variation of equilibrium ammonium concentrations (Ce) in relation to the initial ammonium (C0) and adsorbent concentrations (error bars: standard deviations)▲ 1 g·L−1CV-Z; △ 2 g·L−1CV-Z; ● 3 g·L−1CV-Z; ○ 4 g·L−1CV-Z
Data of ammonium adsorption to CV-Z were analyzed with the Freundlich and Langmuir models. Freundlich equilibrium isotherms at 20 °C for CV-Z range of 1 to 4 g·L−1and the coefficients calculated from the isotherms are shown in Fig. 2. This model fits experimental data adequately, giving R2values higher than 0.95. As the concentration of zeolite increases, 1/n (intensity of the reaction) increases. Under all the experimental conditions, n values are greater than unity (from 1.1416 to 1.8252), indicating that the ammonium ion is favourably adsorbed to the sorbent surface. It has been reported by other authors that n values between 1 and 10 represent beneficial adsorption [32-35]. As can be seen, the K coefficient decreases with an increase in CV-Z concentration. The maximum ammonium adsorption capacity of the zeolite (qe) is observed to be 13.7 mg·g−1in the 50 mg·L−1of (NH4)2HPO4solution, for a CV-Z concentration of 1 g·L−1[Fig. 2 (a)].
Langmuir equilibrium isotherms at 20 °C for CV-Z range of 1 g·L−1to 4 g·L−1solution are presented in Fig. 3. The linear form of the Langmuir isotherm fits experimental data adequately for 1, 2, and 3 g·L−1of CV-Z, giving R2values above 0.95. However, the R2value for 4 g·L−1is very poor (0.57). Langmuir constants (Q0and b) are calculated by least squares analysis. The maximum monomolecular layer capacity, Q0=40.8 mg·g−1, is obtained with the highest zeolite concentration [Fig. 3 (d)]. However, the data from this condition fit the Langmuir model very poorly, and the estimated values are always found to be higher than the experimental ones.
3.2Settling tests
The effects of CV-Z concentration on the sludge settling properties were studied at three zeolite doses. During the settling tests, two samples of activated sludge with different settleability were evaluated: an activated sludge with “standard” settleability (AS1) and a sludge coming from the same WWTP but collected after an industrial discharge episode (AS2). Primary data of settleability, nitrogen removal and final pH after the zeolite treatments are shown in Table 1, and settleability curves are shown in Fig. 4. pH values of both types of sludge were increased due to the higher CV-Z dose, as CV-Z contained the remaining NaOH used in the zeolite synthesis.
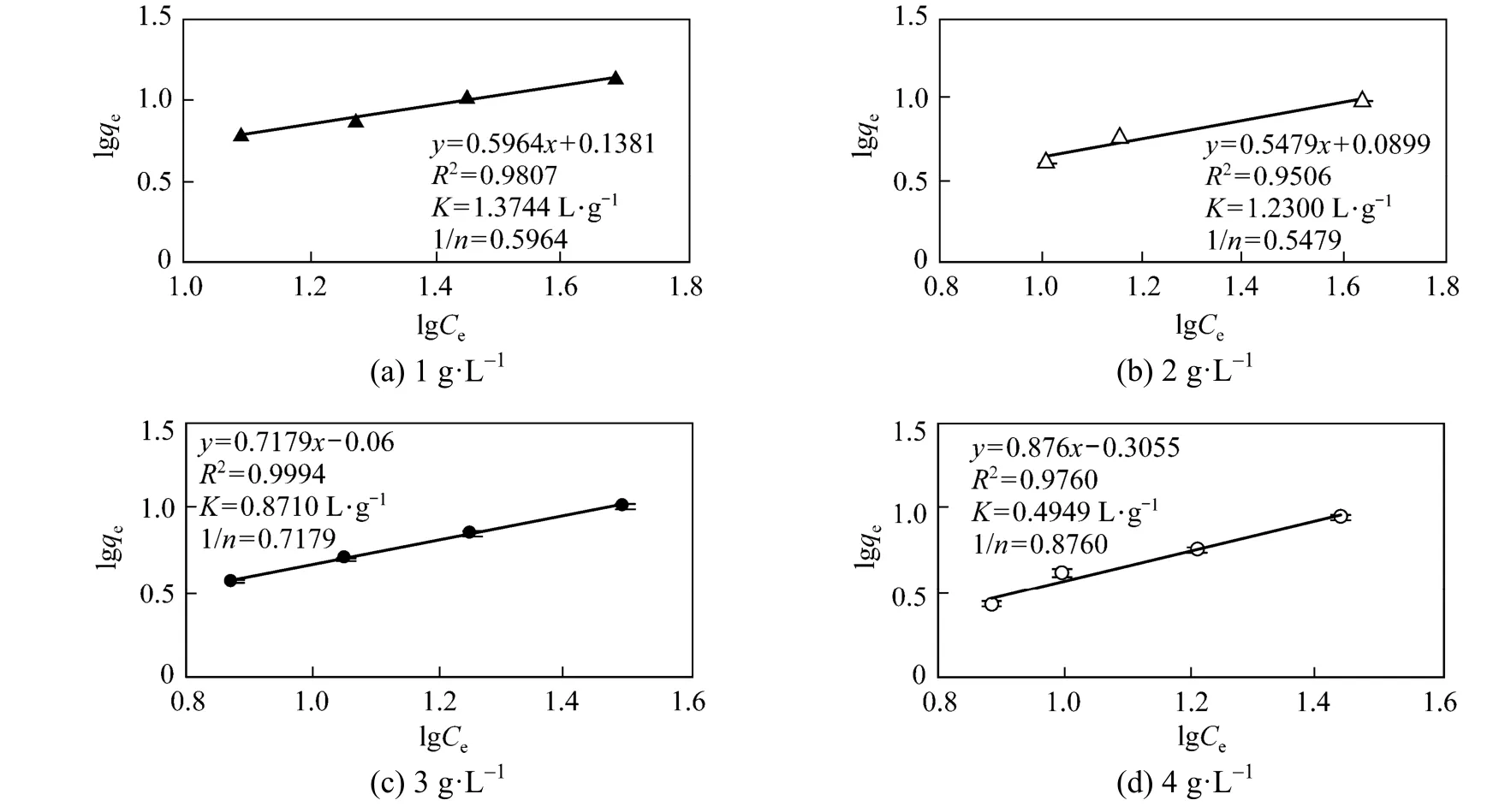
Figure 2Freundlich adsorption isotherm curves and adjustable parameters for different CV-Z concentrations at 20 °C (error bars: standard deviations)
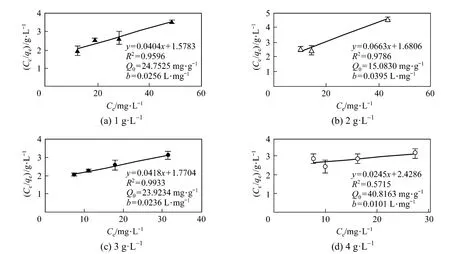
Figure 3Langmuir adsorption isotherm curves and adjustable parameters for different CV-Z concentrations at 20 °C (error bars: standard deviations)
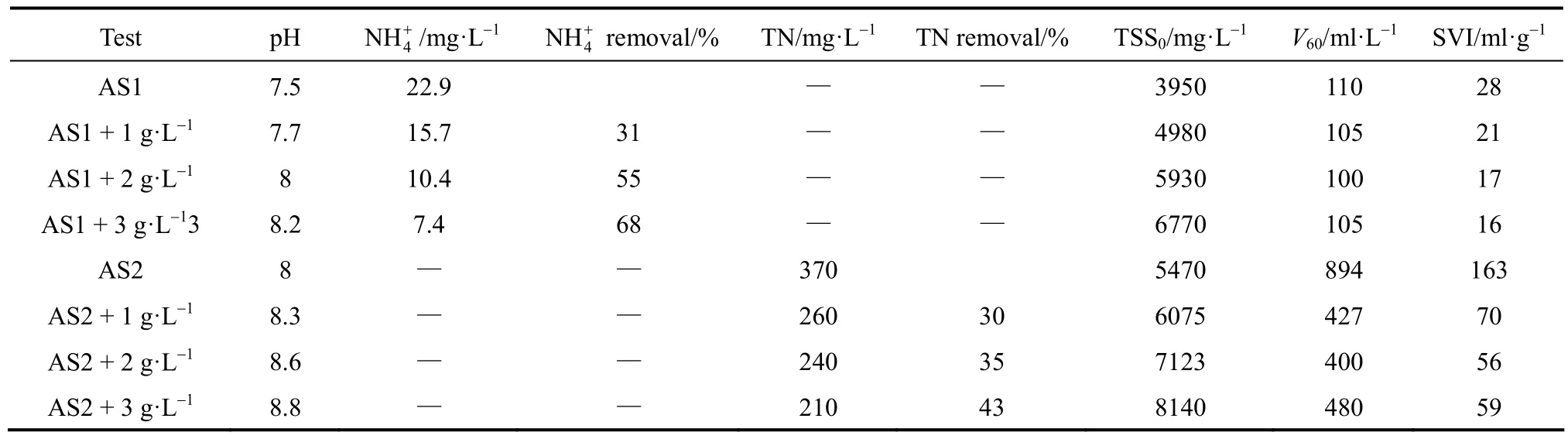
Table 1Settling test parameters for aerobic sludge (AS1 and AS2) using three CV-Z zeolite loadings
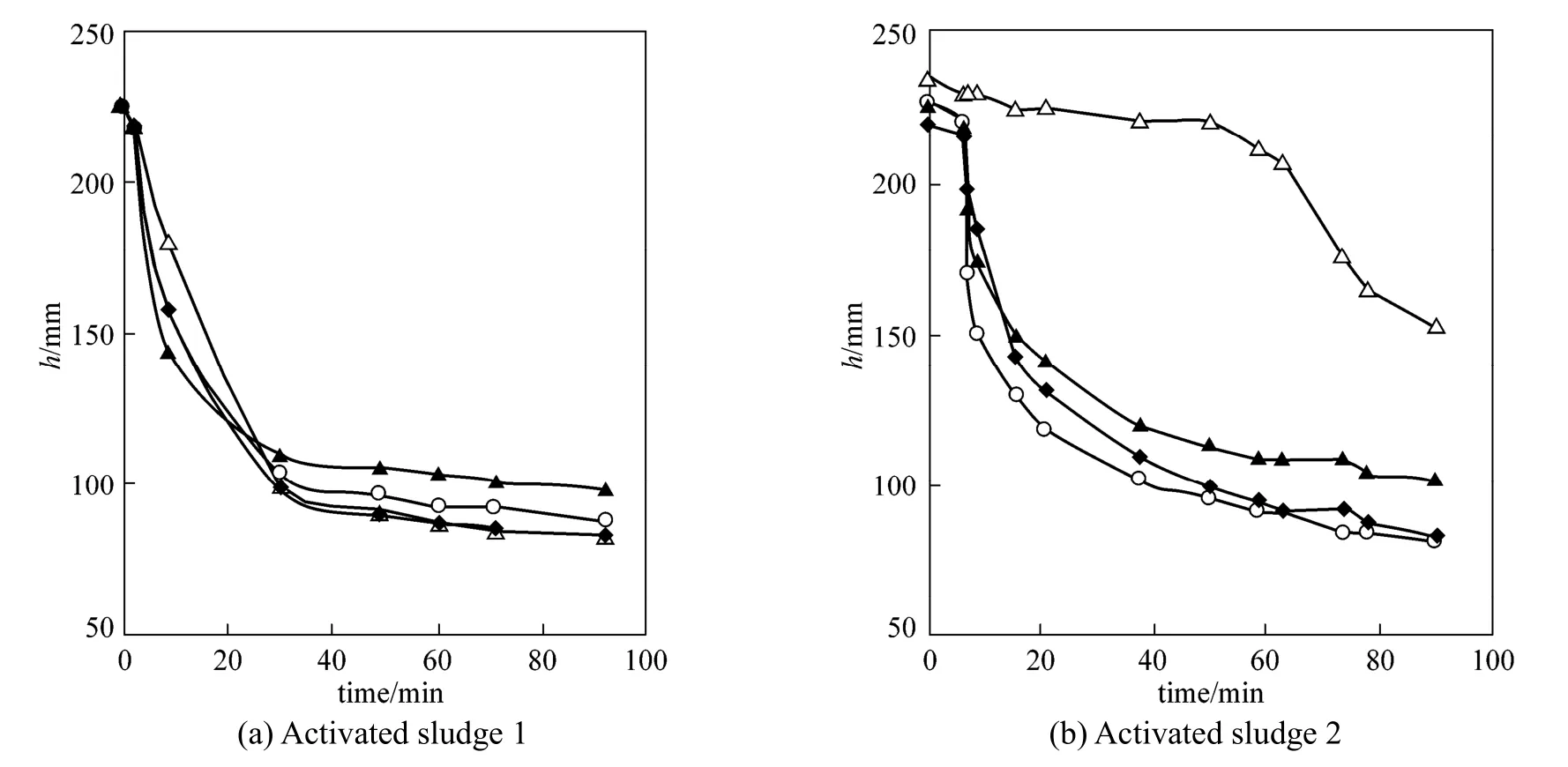
Figure 4Height vs. time of activated sludge in settling experiments (data: mean values of three measurements; standard deviations: below 3 mm)
Figure 4 (a) shows the standard settling curves obtained for the AS1 sludge. As previously reported [36], the settling curves show the four stages of induction, constant rate, falling rate and compression periods. The AS1 sludge settles properly by itself and settleability raw data (Table 1) indicate that the sludge settles to 1/10 of the original volume in 60 min. The addition of zeolite does not improve the settleability [Table 1, Fig. 4 (a)]. In order to test zeolite performance in the AS1 sludge, ammonium removal was also tested. Increasing the CV-Z dose improves the ammonium removal significantly, reaching up to 70%. This removal efficiency is very similar to that obtained in treatments of the amended wastewater (Fig. 1).
The AS2 sludge [Fig. 4 (b)] shows different behavior since settleability shows a 60 min lag period (the height of the sludge on the vessel only decreased 10% during the first 60 min) followed by a constant rate stage until 78 min, and finally by the falling rate and compression periods. Nevertheless, the sludge height after 90 min was still very high (152.7 mm), indicating its poor settleability. In the case of the AS2 sludge, the treatment with zeolite had an obvious effect on the sludge settleability. As shown in Table 1, CV-Z flocculation was effective regarding SVI reduction as the CV-Z loading was increased. This was most significant at a zeolite dose of 1 g·L−1, which showed 57% SVI reduction. However, when the concentration increased two-fold, the SVI additionally reduced was just 9%, and no further reduction was found with a higher CV-Z dose. The improvement in settling observed for the zeolite amended AS2 is attributed to the fact that the volatile fraction diminishes by adding this inorganic material and also to the ballasting effect produced by CV-Z on the floc formed. Therefore, addition of synthetic zeolite may be an appropriate treatment to favor settleability of activated sludge.
In agreement with this, Fig. 4 shows that the lag phase disappeared even at the lowest zeolite dose tested, and no obvious improvement was observed with higher CV-Z doses. The sludge became compact, settled rapidly, and showed very similar settling kinetics to that of AS1. Therefore, attempts to operate at higher zeolite concentrations to improve efficiency are useless.
In addition, total nitrogen in the water effluent was tested. Total nitrogen is generally the parameter to be controlled in the WWTP effluents since all nitrogen species contribute to the occurrence of eutrophication of surface waters. As shown in Table 1, in spite of the high nitrogen content of the waste water, CV-Z amendment of the sludge also achieved significant nitrogen reduction, yielding a maximum efficiency of 43%.
Similar concentrations of different ballasting agents have been used to obtain substantial settleability effects [1-10]. As previously discussed [4], although the amount of sorbent used may seem too high to make the process economically satisfactory in continuous operation, the added inorganic ballasting agent may be recycled along with the return sludge to the aeration tank.
The chemical composition and colloidal structure of solid particles in activated sludge may inhibit sludge settling ability and dewater ability, as in the case of AS2. To enhance sludge settling efficiency it is important to use additives that produce charge destabilization and favor coagulation of the flocs. The ζ potential is a parameter that provides information about the particle charge. When the sludge charge is negative (the most common situation), the particles repel each other due to electrostatic interactions, this being one of the reasons for poor settling of the sludge [37-40]. Therefore, it is necessary to reduce the negative charges in an attempt to reach the neutral charge by increasing the ζ value.
Comparison of the ζ values of CV-Z suspensions in distilled water and activated sludge at different pH values is shown in Fig. 5. In both cases, the ζ potential measured gives lower values at basic pH. The ζ pattern for the CV-Z suspensions shows a C-type curve. On the other hand, the sludge curve shows that ζ changes very little in the pH range of 4-8. With the AS2 treated with CV-Z (Fig. 6), the pH of the samples increased due to the CV-Z alkalinity, varying from 8 to 8.8. Results reflect the effect of CV-Z addition on ζ, which changes from −20 mV to −7 mV with the increase of the CV-Z dose. These data support the theory that the use of CV-Z as a ballasting agent improves the sludge settling mechanism. A reason for this is the fact that a lot of inner silicon and aluminium active sites with positive charge might emerge on the surface after coal fly ash modification, as other authors have reported [41].
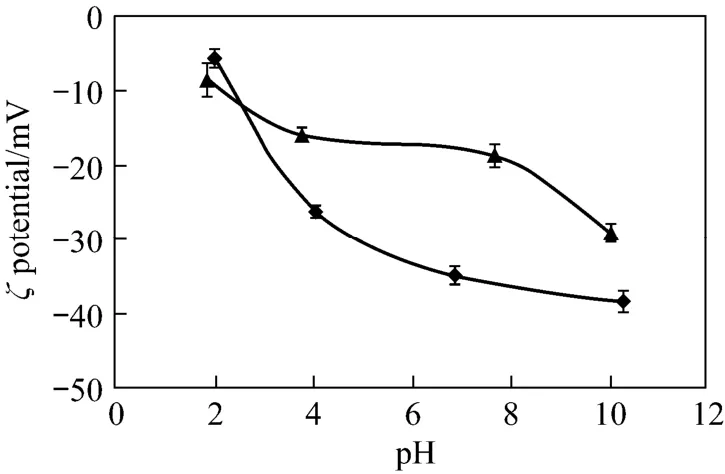
Figure 5Influence of pH on the zeta potential of CV-Z in distilled water solution and activated sludge (error bars: standard deviations)◆ CV-Z; ▲ activated sludge

Figure 6Zeta potential vs. CV-Z concentration (g·L−1) (error bars: standard deviations)
4CONCLUSIONS
In this study, CV-Z was tested as sorbent material for the sorption of nitrogen and as a ballasting agent to improve settleability of activated sludge from urban wastewaters. The ammonium absorption equilibrium data obtained with synthetic water are successfully modelled by Freundlich and Langmuir isotherms and show that CV-Z is a suitable agent for the removal of ammonium, with a maximum capacity of 13.7 mg·g−1.
In activated sludge plants presenting poor sludge settling performance, the application of inorganic ballasting agents may considerably improve sludge settling characteristics. The application of CV-Z, which is used for the first time to solve sludge settleability problems, has demonstrated its ballasting properties in the solid/liquid separation of activated sludge. The tests performed with the poorly settled sludge present a faster settling and a decrease of ζ even at the minimal CV-Z concentration used (1 g·L−1).
Besides its ballasting characteristics, the use of CV-Z has an added value, because this material can also remove nitrogen. At the minimal dose used to restore appropriate settleability (1 g·L−1), the total nitrogen content of the effluent water was reduced by 30%. The optimal dose to achieve a satisfactory economic and performance balance is probably not higher than 1 g of CV-Z per litre of sludge, which is an important consideration regarding sludge management and disposal. However, more tests should be conducted with a wider variety of sludge and additional experiments in continuous mode should be carried out to determine the optimum conditions more accurately.
Due to its high nitrogen absorption capability, the use of CV-Z may be appropriate even for well settled activated sludge which has a high concentration of ammonium in the effluent water. However, zeolite treatment of the water effluent in packed beds may be more appropriate for ammonium removal than adding CV-Z directly to the activated sludge.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Dr. Xavier Querol Group for the CV-Z zeolite synthesized in the Institute of Earth Sciences “Jaume Almera” (CSIC) Barcelona, Spain. Rodrigo Poblete thanks the Spanish Ministry of Science and Innovation for his Ph.D. research grant.
NOMENCLATURE
b Langmuir isotherm constant
Ce4NH+equilibrium concentration, mg·L−1
C04NH+inlet concentration, mg·L−1
K Freundlich isotherm constant, L·g−1
m mass of solid (CV-Z), g
n empirical constant in Freundlich isotherm
Q0Langmuir isotherm constant, mg·g−1
qeamount of4NH+adsorbed per unit mass of solid (4NH+/CV-Z), mg·g−1
R correlation coefficient
SVI sludge volume index, ml·g−1
V60settling sludge volume after 60 min, ml
X mass of ammonium adsorbed, mg
ζ zeta potential, mV
REFERENCES
1 Higgins, M.J., Novak, J.T., “The effect of cations on the settling and dewatering of activated sludges: Laboratory results”, Water Environ. Res.,69, 215-224 (1997a).
2 Higgins, M.J., Novak, J.T., “Dewatering and settling of activated sludges: The case for using cation analysis”, Water Environ. Res.,69, 225-232 (1997b).
3 Eikelboom, D.H., Grovenstein, J., “Control of bulking in a full-scale plant by addition of talc (PE 8418)”, Water S ci. Technol.,37, 297-301 (1998).
4 Piirtola, L., Hultman, B., Andersson, C., Lundeberg, Y., “Activated sludge ballasting in batch tests”, Water Res.,33, 1799-1804 (1999a).
5 Piirtola, L., Uusitalo, R., Vesilind, A., “Effect of mineral materials and cations on activated and alum sludge settling”, Water Res.,34, 191-195 (1999b).
6 Clauss, F., Balavoine, C., Helaine, D., Martin, G., “Controlling the settling of activated sludge in pulp and paper wastewater treatment plants”, Water Sci. Technol.,40, 223-229 (1999).
7 Chen, Y.C., Higgins, M., Murthy, S., Tesfaye, A., Bailey, W., Kharkar, S., Puterbaugh, S., “Comparison of lime and caustic addition for pH control and microbial communities on activated sludge settleability and plant performance. Implications for the field”, In: Proceedings of World Water and Environmental Resources Congress, Anchorage, Alaska (2005).
8 Kara, F., Gurakan, G.C., Sanin, F.D., “Monovalent cations and their influence on activated sludge floc chemistry, structure, and physical characteristics”, Biotechnol. Bioeng.,100, 231-239 (2008).
9 Park, C., Muller, C.D., Abu-Orf, M.M., Novak, M.T., “The effect of wastewater cations on activated sludge characteristics: Effects of aluminum and iron in floc”, Water Environ. Res.,78, 31-40 (2006).
10 Zhang, W., Rao, P., Zhang, H., Xu, J., “The role of diatomite particles in the activated sludge system for treating coal gasification wastewater”, Chin. J. Chem. Eng.,17, 167-170 (2009).
11 Du, Q., Liu, S.J., Cao, Z.H., Wang, Y.Q., “Ammonium removal from aqueous solution using natural Chinese clinoptilolite”, S ep. Purif. Technol.,44, 229-234 (2005).
12 Sarioglu, M., “Removal of ammonium from municipal wastewater using natural Turkish (Dogantepe) zeolite”, Sep. Purif. Technol.,41, 1-11 (2005).
13 Ghan, S.D., Bakshi, H.V., Bhoskar, B.T., “Removal of ammonium ions from wastewater using natural zeolites”, Asian J. Chem.,17, 634-636 (2005).
14 Wu, Z., An, Y., Wang, Z., Yang, S., Chen, H., Zhou, Z., Mai, S.,“Study on zeolite enhanced contact-adsorption regeneration-stabilization process for nitrogen removal”, J. Hazard. Mater.,156, 317-326 (2008).
15 Chang, H.L., Shih, W.H., “Synthesis of zeolites A and X from fly ashes and their ion-exchange behaviour with Cobalt ions”, Ind. Eng. Chem. R es.,39, 4185-4191 (2000).
16 Lee, M.G., Yi, G., Ahn, B.J., Roddick, F., “Conversion of coal fly ash into zeolite and heavy metal removal characteristics of the product”, Korean J. Chem. Eng.,17, 325-331 (2000).
17 Juan, R., Hernández, S., Querol, X., Andrés, J.M., Moreno, N.,“Zeolitic material synthesised from fly ash: use as cationic exchanger”, J. Chem. Technol. Biotechnol.,77, 299-304 (2002).
18 Juan, R., Hernández, S., Andrés, J.M., Ruiz, C., “Synthesis of granular ZM with high cation exchange capacity from agglomerated coal fly ash”, Fuel,86, 1811-1821 (2007).
19 Juan, R., Hernández, S., Andrés, J.M., Ruiz, C., “Ion exchange uptake of ammonium in wastewater from a Sewage Treatment Plant by zeolitic materials from fly ash”, J. Hazard. Mater.,161, 781-786(2009).
20 Querol, X., Moreno, N., Umaña, J., Juan, R., Hernández, S., Fernández-Pereira, C., Ayora, C., Janssen, M., García-Martínez, J., Linares-Solano, A., Cazorla-Amoros, D., “Application of ZM synthesised from fly ash to the decontamination of wastewater and flue gas”, J. Chem. Technol. Biotechnol.,77, 292-298 (2002).
21 Murayama, N., Yoshida, S., Takami, Y., Yamamoto, H., Shibata J.,“Simultaneous removal of NH+4and PO34−in aqueous solution and its mechanism by using zeolite synthesized from coal fly ash”, Sep. S ci. Tech nol.,38, 113-129 (2003).
22 Wu, D.Y., Zhang, B.H., Li, C.J., Zhang, Z.J., Kong, H.N., “Simultaneous removal of ammonium and phosphate by zeolite synthesized from fly ash as influenced by salt treatment”, J. Colloid Interface Sci.,304, 300-306 (2006).
23 Molina, A., Poole, C., “A comparative study using two methods to produce zeolites from fly ash”, Miner. En g.,17, 167-173 (2004).
24 Zhang, M., Zhang, H., Xu, D., Han, L., Niu, D., Tian, B., Zhang, J., Zhang, L., Wu, W., “Removal of ammonium from aqueous solutions using zeolite synthesized from fly ash by a fusion method”, Desalination,271, 111-121 (2011).
25 Moreno, N., Querol, X., Ayora, C., Fernández Pereira, C., Jansen-Jurkovicova, M., “Utilization of zeolites synthesized from coal fly ash for the purification of acid mine waters”, Environ. Sci. Thechnol.,35, 3526-3534 (2001a).
26 Moreno, N., Querol, X., Alastuey, A., García, A., López, A., Ayora, C., “Immobilization of heavy metals in polluted soils by the addition of zeolitic material synthesized from coal fly ash”, In: Proceedings of 2000 Fly Ash Utilization Symposium, University of Kentucky (2001).
27 Otal, E., Vilches, L.F., Moreno, N., Querol, X., Vale, J., Fernández Pereira, C., “Application of zeolitized coal fly ashes to the depuration of liquid wastes”, Fuel,84, 1440-1446 (2005).
28 Otal, E., Pereira C.F., Vilches, L.F., Querol, X., “Application of synthetic zeolites to the depuration of a waste landfill leachate”, In: Waste Management and the Environment, WIT Press, Southampton, Boston, 141-150 (2002).
29 Al-Degs, Y., Khraisheh, M.A.M., Allen, S.J., Ahmad, M.N., Walker, G.M., “Competitive adsorption of reactive dyes from solution: Equilibrium isotherm studies in single and multisolute systems”, Chem. Eng. J.,128, 163-167 (2007).
30 APHA, AWWA, WPCF Eds., Standard Methods for the Examination of Water and Wastewater, APHA Publishing, Washington DC., USA (2005).
31 Balci, S., “Nature of ammonium ion adsorption by sepiolite: analysis of equilibrium data with several isotherms”, Water Res.,38, 1129-1138 (2004).
32 Kadirvelu, K., Namasivayam, C., “Agricultural by-products as metal adsorbents: sorption of lead (II) from aqueous solutions onto coir pith carbon”, Environ. Technol.,21, 1091-1097 (2000).
33 Vadivelan, V., Kumar, K.V., “Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk”, J. Colloid Interface Sci.,286, 90-100 (2005).
34 Rao, M.M., Ramesh, A., Rao, G.P.C., Seshaiah, K., “Removal of copper and cadmium from the aqueous solutions by activated carbon from Ceiba pentandra hulls”, J. Hazard. Mater.,129, 123-129 (2006).
35 Balkaya, N., Cesur, H., “Adsorption of cadmium from aqueous solution by phosphogypsum”, Chem. Eng. J.,140, 247-254 (2008).
36 Chen, W., “Sedimentation and thickening”, In: Filtration Technology, Lu, W.M., Leu, W.F., Ed., Chap. 5, Gauli Book Co., Taipei (1994).
37 Mikkelsen, L.H., Keiding, K., “Physico-chemical characteristics of full scale sewage sludges with implications to dewatering”, Water Res.,36, 2451-2462 (2002).
38 Jin, B., Wilén, B.M., Lant, P., “A comprehensive insight into floc characteristics and their impact on compressibility and settleability of activated sludge”, Chem. Eng. J.,95, 221-234 (2003).
39 Liu, Y., Fang, H.H.P., “Influences of extracellular polymeric substances (EPS) on flocculation, settling, and dewatering of activated sludge”, Crit. Rev. Environ. Sci. Tech nol.,33, 237-273 (2003).
40 Saveyn, H., Pauwels, G., Timmerman, R., Meeren, P.V., “Effect of polyelectrolyte conditioning on the enhanced dewatering of activated sludge by application of an electric field during the expression phase”, Water Res.,39, 3012-3020 (2005).
41 Chen, C., Zhang, P., Zeng, G., Deng, J., Zhou, Y., Lu, H., “Sewage sludge conditioning with coal fly ash modified by sulfuric acid”, Chem. Eng. J.,158, 616-622 (2010).
10.1016/S1004-9541(13)60566-2
2012-09-07, accepted 2013-01-17.
* Supported by the Spanish Ministry of Science and Innovation, under the project FOXMORE (CTM2006-05114). ** To whom correspondence should be addressed. E-mail: eotal@us.es
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Halloysite Nanotube Composited Thermo-responsive Hydrogel System for Controlled-release*
- Experimental and Theoretical Studies of CO2Absorption Enhancement by Nano-Al2O3and Carbon Nanotube Particles
- A Novel γ-Alumina Supported Fe-Mo Bimetallic Catalyst for Reverse Water Gas Shift Reaction*
- Recent Advances in Separation of Bioactive Natural Products*
- Volumetric and Transport Properties of Aqueous NaB(OH)4Solutions*
- A New Tuning Method for Two-Degree-of-Freedom Internal Model Control under Parametric Uncertainty*
