Halloysite Nanotube Composited Thermo-responsive Hydrogel System for Controlled-release*
2013-07-31LINXi林茜JUXiaojie巨晓洁XIERui谢锐JIANGMingyue江明月WEIJie魏竭andCHULiangyin褚良银
LIN Xi (林茜), JU Xiaojie (巨晓洁)**, XIE Rui (谢锐), JIANG Mingyue (江明月), WEI Jie (魏竭) and CHU Liangyin (褚良银)**
School of Chemical Engineering, Sichuan University, Chengdu 610065, China
Halloysite Nanotube Composited Thermo-responsive Hydrogel System for Controlled-release*
LIN Xi (林茜), JU Xiaojie (巨晓洁)**, XIE Rui (谢锐), JIANG Mingyue (江明月), WEI Jie (魏竭) and CHU Liangyin (褚良银)**
School of Chemical Engineering, Sichuan University, Chengdu 610065, China
Halloysite nanotube-composited thermo-responsive hydrogel system has been successfully developed for controlled drug release by copolymerization of N-isopropylacrylamide (NIPAM) with silane-modified halloysite nanotubes (HNT) through thermally initiated free-radical polymerization. With methylene blue as a model drug, thermo-responsive drug release results demonstrate that the drug release from the nanotubes in the composited hydrogel can be well controlled by manipulating the environmental temperature. When the hydrogel network is swollen at temperature below the lower critical solution temperature (LCST), drug releases steadily from lumens of the embedded nanotubes, whereas the drug release stops when hydrogel shrinks at temperature above the LCST. The release of model drug from the HNT-composited hydrogel matches well with its thermo-responsive volume phase transition, and shows characteristics of well controlled release. The design strategy and release results of the proposed novel HNT-composited thermo-responsive hydrogel system provide valuable guidance for designing responsive nanocomposites for controlled-release of active agents.
halloysite nanotube, composite hydrogel, controlled release, thermo-responsive carrier, poly(N-isopropylacrylamide)
1INTRODUCTION
In recent years, nanotubes have gained significant attention as carriers for various active agents like drugs, genes and biocatalysts due to their excellent loading capacity and sustained-release property [1-5]. Among the existing nanotubes, halloysite nanotubes (HNT) have attracted increasing interests as excellent nano-containers for enzymes [6, 7] and drugs [8-12] due to their non-cytotoxicity [13-15] and availability [8, 16]. Nowadays, how to further enhance the controllability of the release process from HNT is still a challenging topic. Kelly et al. [9], Levis and Deasy [11] successfully delayed the drug release from HNT by coating them with a range of cationic polymers, and further did in vivo testing in dogs to test their sustained-release performance for the treatment of periodontitis. Veerabadran et al. [15] retarded the release of macromolecular dexamethasone by modified HNT with polyelectrolyte shells. However, these retardation releases are realized just by the static blocking with inert coating and therefore lack of controllability to certain extent.
For some diseases like chronic pain and hormone deficiencies, long-term treatment and multi-dose of medication are necessary. However, conventional drug delivery systems like implants and oral delivery systems usually fail to maintain the optimal drug concentration for a long time and need repeated drug administration [17, 18]. Novel drug delivery systems with excellent performance both in controllability and sustainability are desired. It is well known that introduction of stimuli-sensitive materials into delivery systems would improve their controllability and be helpful to realize the on-demand release [19-25]. Because temperature stimulus can be easily designed and controlled, it is a wise strategy to introduce thermo-responsive materials into drug delivery systems. Poly(N-isopropylacrylamide) (PNIPAM) hydrogel is a well-known thermo-responsive material with a dramatic volume phase transition at the lower critical solution temperature (LCST) around 32 °C. When environmental temperature is below the LCST, the PNIPAM hydrogel network is swollen, and above the LCST the hydrogel network would shrink dramatically [26-28]. This thermo-responsive phase transition behavior makes PNIPAM hydrogel a promising candidate for “smart” drug delivery system [21, 23, 29-32]. If the excellent controllability of PNIPAM can be incorporated with the excellent sustained-release property of HNT to achieve delivery systems with controlled and long-lasting drug release properties, it is feasible to realize pulsatile drug-release and maintain the desirable concentration of drug on demand, i.e., HNT-composited systems have the potential to be better drug carriers for the treatments of those diseases that require long-term treatment and multi-dose drug delivery. Unfortunately, such HNT-composited thermoresponsive controlled-release systems have not been reported yet up to now.
Here we report on a novel thermo-responsive controlled-release system, which is composed of HNT and PNIPAM hydrogel. The controlled-release mechanism of our proposed thermo-responsive hydrogel system is schematically illustrated in Fig. 1 (a). The nanotube-composited hydrogel system is constructedby crosslinking PNIPAM polymer chains with silane modified HNT, which could combine the advantages of the excellent controllability of PNIPAM hydrogel system and the sustained-release property of HNT. When temperature is below the LCST of the composited hydrogel, the hydrogel network is swollen and the molecular diffusion of the encapsulated drug would take place at the interface between the open ends of HNT and the PNIPAM hydrogel network. When temperature rises above the LCST, the hydrogel network which shrinks dramatically would block the open ends of nanotubes, and therefore obstruct the drug diffusion from the lumens of nanotubes. That is to say, by manipulating the environmental temperature, it could control the drug release from the lumen of the HNT and achieve the on-demand and long-lasting sustained-release.

Figure 1Schematic illustration of the thermo-responsive controlled-release mechanism (a) and preparation process (b) of the proposed HNT-composited PNIPAM hydrogel system
In the proposed novel controlled-release system, strong interfacial interaction between the inorganic and organic interfaces is ensured by the chemical bonds between the silane modified HNT and the PNIPAM hydrogel matrix. Furthermore, drug diffusion from the lumens of HNT could be controlled by manipulating open/close state of the ends of the HNT, which wouldbe open at temperature below LCST as hydrogel network swells and obstructed by the shrunken PNIPAM hydrogel network at temperature above LCST. The strategy and results presented in this study would provide valuable guidance for design and fabrication of stimuli-responsive nanocomposites for controlledrelease of active agents.
2EXPERIMENTAL
2.1Materials
Halloysite nanotubes (HNT) obtained from Sigma-Aldrich were used without further purification. N-isopropylacrylamide (NIPAM), kindly provided by Kohjin Co., Japan, was purified by recrystallization with a hexane/acetone mixture (50/50, by volume). Hydrogen peroxide (H2O2), purchased from Chengdu Kelong Chemical Reagent Co., China, was used to pretreat the halloysite nanotubes. Silane coupling agent 3-(methacryloyloxy)propyl trimethoxysilane (MPS), obtained from Chengdu Thinkbond Chemical Reagent Co., China, was used to modify the halloysite nanotubes. N,N-methylene-bis-acrylamide (MBA) used as crosslinker, ammonium persulfate (APS) used as initiator, tetramethylethylenediamine (TEMED) used as activator, sodium dodecyl sulfate (SDS) and polyvinyl alcohol (PVA, average polymerization degree is 1788±50) used as surfactants, were all purchased from Chengdu Kelong Chemical Reagent Co., China. Methylene blue (MB), provided by Tianjin Bodi Chemical Reagent Co., China, was used as the model drug to investigate the controlled-release behavior. Other reagents were of analytical grade and used as received. The deionized water from a Millipore Milli-Q water purification-system was used throughout the experiments.
2.2Modification of halloysite nanotubes
Chemical functionalization was utilized to enhance the interfacial interaction between the HNT and PNIPAM hydrogel network. The HNT were modificated with MPS to endow the outer surface of nanotubes with reactive CC bonds, which would react with the NIPAM monomer later during the radical polymerization [Fig. 1 (b)]. Firstly, the HNT were treated with 30% H2O2aqueous solution at 80 °C for 24 h, and then modified with 4% MPS in toluene at 110 °C for 12 h. The MPS-modified halloysite nanotubes (MPS-HNT) were purified by centrifugation with ethanol for more than five times and finally dried at 40 °C in vacuum oven for 12 h.
2.3Loading of methylene blue into halloysite nanotubes
Water-soluble methylene blue was chosen as model drug to investigate the controlled-release behavior of HNT-composited PNIPAM hydrogel. HNT tends to have a polyanionic surface at most pH conditions, and would readily bind cationic drugs from the solution. Moreover, as the inner diameter of HNT is around 10-15 nm, it is much easier for drugs with low molecular mass to be encapsulated. So, most reported drugs that encapsulated into the lumen of halloysite nanotubes are water soluble cationic drugs with low molecular mass ranging from 300-500. Methylene blue is a cationic compound with a molecular mass of 373. Therefore, methylene blue was used as a model drug to be encapsulated into the halloysite nanotubes. As shown in Fig. 2 (b), methylene blue was loaded into the narrow lumen of halloysite nanotubes by air pressure. Firstly, the air in the nanotubes was removed with a vacuum pump, and then degassed saturated aqueous solution of methylene blue was injected into the container with degassed halloysite nanotubes. After equilibration for 2 h, when the saturated methylene blue solution has adequately entered into the lumen of HNT, the methylene blue-loaded HNT were separated by filtration and dried via vacuum oven at 40 °C for 15 h. The whole process was repeated once to ensure the maximum loading amount of methylene blue.
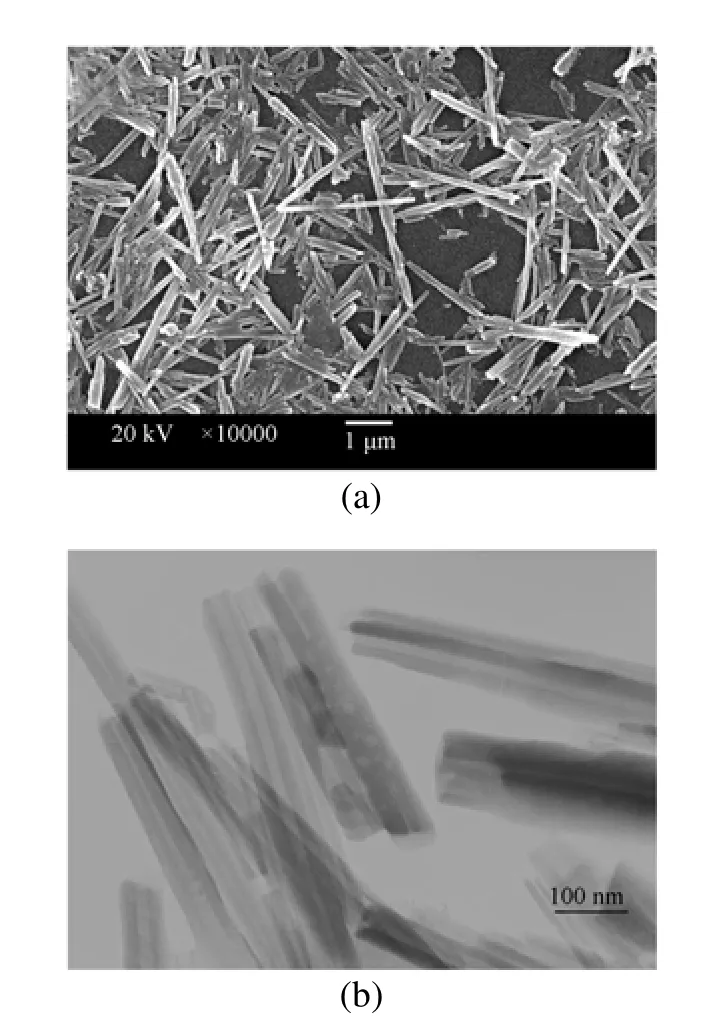
Figure 2SEM (a) and TEM (b) images of the halloysite nanotubes [the scale bar in (a) is 1 μm and that in (b) is 100 nm]
2.4Synthesis of halloysite nanotube-composited PNIPAM hydrogel
HNT-composited PNIPAM hydrogel with 10% content of HNT was synthesized by thermally initiated free-radical crosslinking polymerization. Briefly, 2 ml aqueous solution of monomer was composed of monomer NIPAM (1 mol·L−1), crosslinker MBA (0.02 mol·L−1), initiator APS (0.004 g), surfactants SDS (0.8 g·L−1) and PVA (20 g·L−1), and model drug methyleneblue (4.438 mg·ml−1). After methylene blue-loaded MPS-HNT [10% (by mass) to NIPAM monomer] were added into the monomer aqueous solution, the mixture solution was ultrasonically treated for 5 min to get good dispersion of nanotubes. Then, 50 μl TEMED used as reaction accelerator was added into the dispersed mixture. Then, the mixture was immediately transferred into a poly(tetrafluoroethene) (PTFE) tube with inner diameter of 6 mm, and the tube was sealed immediately. Finally, the PTFE tube was immersed in to a constant-temperature water bath at 25 °C for polymerization for 12 h. The resulted hydrogels were characterized by Fourier Transform Infrared Spectrometer (FT-IR, IR Prestige-21, Shimadzu, Japan).
For comparison, PNIPAM hydrogel was also prepared using the same protocol described above, except that HNT were not added. The prepared composite and pure PNIPAM hydrogels have the same columntype morphology of the same diameter at 25 °C.
2.5Componential and morphological analysis
The chemical components of HNT, MPS-HNT, HNT-composited hydrogel and MB-loaded HNT-composited hydrogel were analyzed by FT-IR spectrometer (IR Prestige-21, Shimadzu, Japan). Nanotube samples were dried at 30 °C via vacuum oven for 12 h and hydrogel samples were freeze-dried before use.
The microstructure of untreated HNT was observed using scanning electron microscopy (SEM, JSM-5900LV, JEOL, Japan) and transmission electron microscopy (TEM, JEM-100CX, JEOL, Japan). The microstructures of HNT-composited PNIPAM hydrogel and pure PNIPAM hydrogel were characterized with SEM. Samples were repeated washed with deionized water and freeze-dried before SEM observation.
2.6Thermo-responsive volume phase transition behaviors
The thermo-induced equilibrium volume phase transition behavior of HNT-composited hydrogel was investigated by measuring the diameter change of hydrogel discs in deionized water at various temperatures ranging from 21 to 49 °C. Before the experiment, HNT-composited hydrogel was cut into thin discs with the same thicknesses (2 mm) at 25 °C. The experiment was performed in a transparent glass container with a calibrated scale, and the container was placed in a temperature-controllable water bath with temperature fluctuation of ±0.1 °C. To ensure hydrogel discs reach their equilibrium states, the equilibrium process was maintained for at least 3 h at each scheduled temperature. The diameters of the hydrogel discs were recorded and measured by taking photos with a digital camera at a fixed vertical height, and then the photos of the hydrogel discs with the calibrated scale were analyzed via graph-processing software. To minimize the errors in measurements, the measurements were carried out at least three times for each equilibrium state.
Pure PNIPAM hydrogel was also tested as a reference.
2.7Thermo-responsive controlled-release from HNT-composited hydrogel
Methylene blue was used as the model drug to examine the controlled-release performance from the HNT-composited hydrogel. The thermo-induced release experiments were carried out at 23 °C and 44 °C, which were chosen below and above LCST respectively, by measuring the increased methylene blue concentration in the surrounding medium at the fixed time intervals during the release process. Briefly, in a typical release experiment at 23 °C, drug-loaded composited hydrogel with a length of 2 cm (25 °C) was cut into thin discs with the thickness of 2 mm, and put into a 25 ml beaker. Both the beaker containing hydrogel thin discs and another 150 ml beaker containing 100 ml deionized water were placed in the constant-temperature water bath at 23 °C. After temperature equilibrium for 0.5 h, the hydrogel discs were transferred into the breaker with deionized water at 23 °C, and timed immediately. To eliminate the effect of concentration polarization near the hydrogel surface, magnetic stirrer was used to gently stir the water around the hydrogel discs during the release process. Then, at fixed time intervals, the methylene blue concentration was determined using a UV-visible recording spectrophotometer at a wavelength of 626 nm. Each data was measured at least tree times to minimize the experimental error.
The released amount of methylene blue from the hydrogel at fixed time interval was calculated from the methylene blue concentration and the solution volume. To exclude the portion of drug exists on the hydrogel sample surface which was released during the equilibrium course, the amount of released methylene blue in the first 5 min was not used. The release rate was calculated by

where Mtis the amount of methylene blue released at time t, and M0is the total released amount of methylene blue which was measured 6 days later.
To further examine the dynamic thermo-induced controlled-release performance of the HNT-composited hydrogel, an experiment with changing temperature was carried out as follows. After the drug release from halloysite-nanotube-composited hydrogel at 23 °C for 1 h, the beaker was quickly transferred into a 43 °C water bath for 50 min and then swiftly transferred back into the 23 °C water bath again. 3 h later the procedure was repeated, that the beaker was transferred to the 43 °C again for 50 min and then transferred back into the 23 °C water bath. During the whole process, the temperature change inside the beaker and the released amount of methylene blue were both continuously recorded. Pure PNIPAM hydrogel was also tested as a reference.
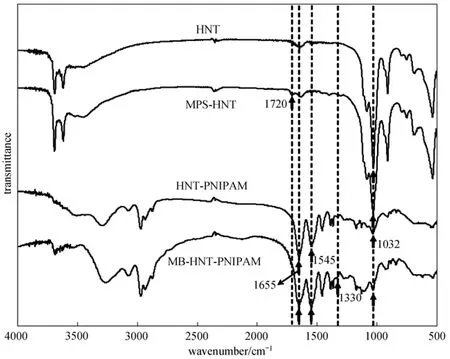
Figure 3FT-IR spectra of untreated HNT, MPS-HNT, HNT-composited PNIPAM hydrogel, and HNT-composited PNIPAM hydrogel loaded with MB
3RESULTS AND DISCUSSION
3.1Microstructure of halloysite nanotubes
The SEM image of the untreated HNT [Fig. 2 (a)] shows excellent tubular structure of the nanotubes. The length of HNT are about 1-4 µm. In the TEM micrograph [Fig. 2 (b)], the lumens of halloysite nanotubes are clearly visualized. The outer diameter of the nanotubes is about 100-200 nm and the inner diameter is around 10-15 nm.
3.2Composition and structure characterization of HNT-composited hydrogel
FT-IR spectrum of HNT-composited PNIPAM hydrogel confirms the successful synthesis of nanotube-composited hydrogel composed of both HNT and PNIPAM (Fig. 3). Comparing with the FT-IR result of untreated HNT, the peak at 1720 cm−1for stretching vibration of CO bond of MPS in the FT-IR spectrum of MPS-HNT confirms the successful modification of HNT with silane MPS. The appearance of the following characteristic peaks in the FT-IR spectrum of HNT-composited PNIPAM hydrogel (HNT-PNIPAM) suggests the successful synthesis of nanotube-composited hydrogel: peaks at 1545 cm−1for the bending vibration of NH and 1655 cm−1the stretching vibration of CO are the characteristic peaks of PNIPAM, and the peak at 1032 cm−1corresponds with the characteristic peak of HNT for the stretching vibration of Si O. Moreover, a more significant peak at 1330 cm−1in the FT-IR spectrum of MB-loaded HNT-composited hydrogel indicates the successful loading of methylene blue, which has a characteristic peaks at 1330 cm−1due to the stretching vibration of CN.
Compared with the pure PNIPAM hydrogel, the presence of HNT in the composited hydrogel network can be clearly seen from the SEM images of pure and HNT-composited PNIPAM hydrogels (Fig. 4). Both FT-IR spectra and SEM images verify the successful synthesis of HNT-composited hydrogels.
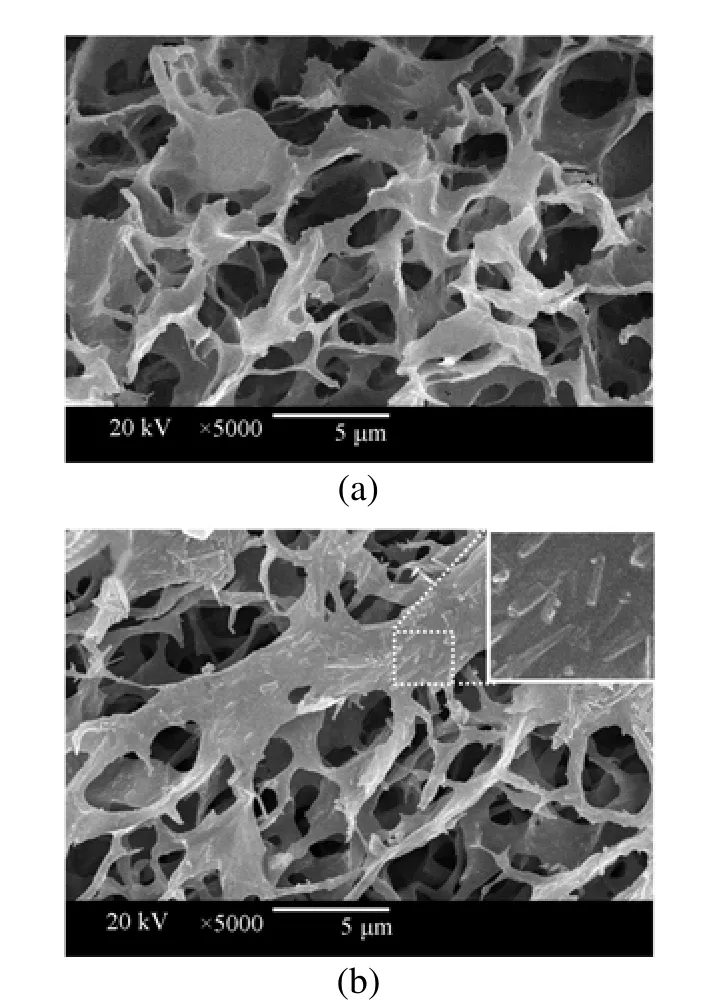
Figure 4SEM images of pure PNIPAM hydrogel (a) and HNT-composited PNIPAM hydrogel (b)
3.3Thermo-responsive volume phase transition and drug-release
The thermo-responsive characteristic of the HNT-composited PNIPAM hydrogel is studied by detecting the volume change of the hydrogel at various temperatures ranging from 21 to 49 °C. The thermoresponsive volume change of hydrogel is expressed using the volume change ratio of the equilibrium volume at a certain temperature (T/°C) to that at the lowest temperature during the measurement (21 °C). As expected form Fig. 5, the HNT-composited hydrogel undergoes a rapid volume change when environmental temperature is lowered across a certain temperature. Moreover, it can be seen clearly that the HNT-composited hydrogel has a slightly higher volume phase transition temperature than normal-type pure PNIPAM hydrogel. The reason could be that the incorporation of hydrophilic HNT into PNIPAM hydrogel increases the hydrophilicity of the hydrogel network and therefore lead to an increase in the LCST [33]. The excellent reversibility of the swelling/deswelling behavior of PNIPAM-based hydrogels have already been demonstrated in previous works [34], i.e., the thermoresponsive volume phase transition curves are the same no matter the temperature is increased or decreased across the LCST.

Figure 5Thermo-responsive volume phase transition curves of pure PNIPAM hydrogel and HNT-composited PNIPAM hydrogel△ PNIPAM; ▲ HNTs-PNIPAM
The thermo-responsive controlled-release behavior of HNT-composited hydrogel is studied at temperatures (23 °C) below and (44 °C) above the LCST, respectively. As shown in Fig. 6, methylene blue could easily diffuse from HNT-composited hydrogel as the hydrogel network is swollen at 23 °C, and the release profile resembles those of low molecular mass drugs from uncoated HNT that reported before [12, 15, 35], whereas at 44 °C the release of methylene blue decreases significantly due to the blocked ends of the nanotubes by the shrunken hydrogel. The drug releases from the HNT-composited hydrogel at different temperatures match well with it swelling/deswelling phase state. And it is clear that composited hydrogel has excellent thermo-responsibility and the drug release from the lumen of HNT can be well controlled just via the manipulation of environmental temperature. Therefore, the supposed on-demand release of drugs is achievable with temperature control upon the HNT-composited hydrogel.
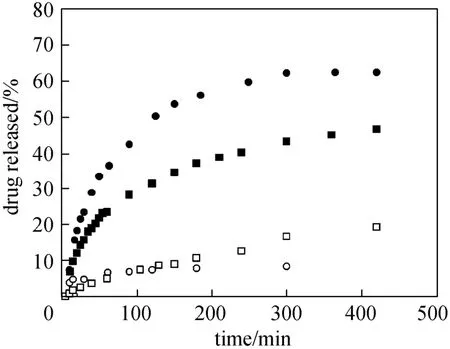
Figure 6 Temperature-dependent controlled-release behaviors of methylene blue molecules from pure PNIPAM hydrogel and HNT-composited PNIPAM hydrogel● PNIPAM-23 °C; ○ PNIPAM-44 °C; ■ HNTs-PNIPAM-23 °C;□ HNTs-PNIPAM-44 °C
Moreover, as shown in Fig. 6, the release rate of methylene blue from HNT-composited hydrogel is apparently much slower than that from pure PNIPAM hydrogel at 23 °C. There are mainly two reasons for this phenomenon. First, compared with the drug in the bulk hydrogel, the methylene blue in the lumen of the HNT must first travelling through the narrow lumen to reach the open ends of the halloysite. HNT has a high length-diameter ratio, so the diffusion of the drug molecules from the lumen into the hydrogel network would take some time. Second, methylene blue is a cationic compound and can bind to the polyanionic surface of the HNT, and this binding effect would delay the release of the drug molecules too. The results demonstrated that the composited hydrogel inherited the excellent sustained-release property of halloysite nanotubes.
3.4Dynamic drug-release of methylene blue from the HNT-composited hydrogel
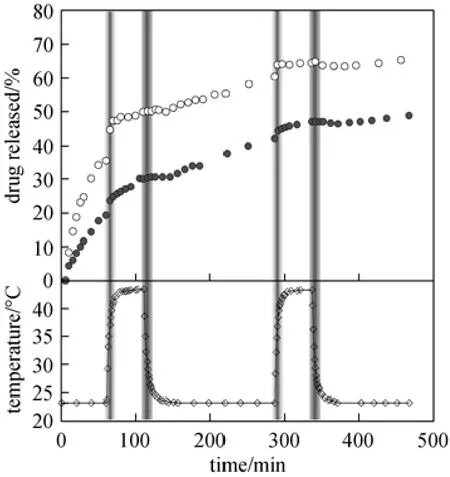
Figure 7Thermo-responsive dynamic release behaviors of methylene blue molecules from pure PNIPAM hydrogel and HNT-composited PNIPAM hydrogel (The environmental temperature switches between 23 and 43 °C periodically)○ PNIPAM; ● HNT-PNIPAM
To verify its long-lasting controlled-release performance, dynamic drug-release of methylene blue from the HNT-composited hydrogel with alternate changes of temperature across the LCST is investigated. As shown in Fig. 7, HNT-composited hydrogel exhibits excellent thermo-responsive and long-lasting sustained-release properties. During the two cycles of temperature change across the LCST, the thermoresponsively on/off switched release behaviors of methylene blue from the composited hydrogel match well with its reversible volume phase changes. When environmental temperature is below the LCST (at 23 °C), methylene blue is released from the hydrogel network stably as the hydrogel network is swollen. As the temperature is switched above the LCST (at 43 °C), a burst release of methylene blue is caused by the sudden shrinking of the hydrogel network. When the composited hydrogel reaches equilibrium state at 43 °C, the release of methylene blue dramatically slows down due to the shrunken hydrogel network that blocks the drug release from the lumen of the embedded HNT. As temperature is switched back to 23 °C, the release rate of methylene blue increases due to the deswelling of the hydrogel network. The pure PNIPAM hydrogel also responds to the temperature similarly. However, the release rate of methylene blue from pure PNIPAM hydrogel at the beginning is much faster than from HNT-composited hydrogel, and there is a sharp decrease of release rate when temperature was switched back to 23 °C at the second time and third time. After falling back to the environmental temperature at 23 °C at t=340 min, almost no release of methylene blue is observed from pure PNIPAM hydrogel. In comparison, the HNT-composited hydrogel, which still show certain release of methylene blue after being heated and cooled for twice, demonstrated better long-lasting sustained-release property.
A small amount of methylene blue still releases from the composited hydrogel network during the equilibrium state at 43 °C. This may be caused by the leakage of methylene blue from a few HNT that located at the surface of the hydrogel discs when the hydrogel network shrinks. To solve this problem, the HNT-hydrogel with another hydrogel layer can be employed to prevent the exposure of the open ends of the nanotubes.
4CONCLUSIONS
Novel thermo-responsive HNT-composited hydrogel with excellent controllability and sustained-release property is successfully fabricated by copolymerization of silane-modified biocompatible halloysite nanotubes and thermo-sensitive NIPAM monomer. The drug release from the nanotubes can be easily controlled by manipulating environmental temperature. When temperature is below the LCST of the hydrogel, drug release stably from lumens of the nanotubes as the hydrogel network is swollen. By heating the temperature above LCST, it can easily stop the drug release from the nanotubes because the shrunken hydrogel network would obstruct the ends of the nanotubes. Moreover, the dynamic drug-release result showed that this new delivery system could maintain a longer release performance compared with pure PNIPAM hydrogel as it still releases model drugs after two cycles of heating and cooling across through the LCST. As the controlled and long-lasting drug release properties of the proposed HNT-composited hydrogel enable it to maintain the desirable concentration of drug on demand, the HNT-composited hydrogel may be used as potential drug carrier for the treatments of diseases like chronic pain and hormone deficiencies, which require long-term treatment and pulsatile drug release.
REFERENCES
1 Martin, C.R., Kohil, P., “The emerging field of nanotube biotechnology”, Nat. Rev. Drug Discovery,2, 29-37 (2003).
2 Goldberg, M., Langer, R., Jia, X.Q., “Nanostructured materials for applications in drug delivery and tissue engineering”, J. Biomater. Sci. Polym. Ed.,18, 241-268 (2007).
3 Son, S.J., Bai, X., Nan, A., Ghandehari, H., Lee, S.B., “Template synthesis of multifunctional nanotubes for controlled release”, J. Controlled Release,114, 143-152 (2006).
4 Ji, S.R., Liu, C., Zhang, B., Yang, F., Xu, J., Long, J., Jin, C., Fu, D.L., Ni, Q.X., Yu, X.J., “Carbon nanotubes in cancer diagnosis and therapy”, BBA Rev. Cancer,1806, 29-35 (2010).
5 Gu, Z., Biswas, A., Zhao, M.X., Tang, Y., “Tailoring nanocarriers for intracellular protein delivery”, Chem. Rev.,40, 3638-3655 (2011).
6 Shchukin, D.G., Sukhorukov, G.B., Price, R.R., Lvov, Y.M., “Halloysite nanotubes as biomimetic nanoreactor”, Small,1, 510-513 (2005).
7 Zhai, R., Zhang, B., Liu, L., Xie, Y.D., Zhang, H.Q., Liu, J.D., “Immobilization of enzyme biocatalyst on natural halloysite nanotubes”, Catal. Commun.,12, 259-263 (2010).
8 Lvov, Y.M., Shchukin, D.G., Mohwald, H., Price, R.R., “Halloysite clay nanotubes for controlled release of protective agents”, ACS Nano.,2, 814-820 (2008).
9 Kelly, H.M., Deasy, P.B., Ziaka, E., Claffey, N., “Formulation and preliminary in vivo dog studies of a novel drug delivery system for the treatment of periodontitis”, Int. J. Ph arm.,274, 167-183 (2004).
10 Levis, S.R., Deasy, P.B., “Characterization of halloysite for use as a microtubular drug delivery system”, Int. J. Pharm.,243, 125-134 (2002).
11 Levis, S.R., Deasy, P.B., “Use of coated microtubular halloysite for the sustained release of diltiazem hydrochloride and propranolol hydrochloride”, Int. J. Pharm.,253, 145-157 (2003).
12 Price, R.R., Gaber, B.P., Lvov, Y.M., “In-vitro release characteristics of tetracycline HCl, khellin and nicotinamide adenine dineculeotide from halloysite; A cylindrical mineral”, J. Microencapsulation,18, 713-722 (2001).
13 Joussein, E., Petit, S., Churchman, J., Theng, B., Righi, D., Delvaux, B., “Halloysite clay minerals—A review”, Clay Minerals,40, 383-426 (2005).
14 Vergaro, V., Abdullayev, E., Leporatti, S., Lvov, Y.M., Zeitoun, A., Cingolani, R., Rinaldi, R., “Cytocompatibility and uptake of halloysiteclay nanotubes”, Biomacromolecules,11, 820-826 (2010).
15 Veerabadran, N.G., Mongayt, D., Torchilin, V., Price, R.R., Lvov, Y.M., “Organized shells on clay nanotubes for controlled release of macromolecules”, Macromol. Ra pid Commun.,30, 99-103 (2009).
16 Lin, Y., Ng, K.M., Chan, C.M., Sun, G.X., Wu, J.S., “High-impact polystyrene/halloysite nanocomposites prepared by emulsion polymerization using sodium dodecyl sulfate as surfactant”, J. Colloid Interface Sci.,358, 423-429 (2011).
17 Grayson, A.C.R., Choi, I.S., Tyler, B.M., Wang, P.P., Brem, H., Cima, M.J., Langer, R., “Multi-pulse drug delivery from a resorbable polymeric microchip device”, Nature Mater.,2, 767-772 (2003).
18 Carr, D.B., Sendil, D., “Current approaches to analgesic drug delivery for chronic pain”, Technol. Health Care,10, 227-235 (2002).
19 Qiu, Y., Park, K., “Environment-sensitive hydrogels for drug delivery”, Adv. Drug Delivery R ev.,53, 321-339 (2001).
20 Soppimath, K.S., Aminabhavi, T.M., Dave, A.M., Kumbar, S.G., Rudzinski, W.E., “Stimulus-responsive smart hydrogels as novel drug delivery systems”, Drug Dev. Ind. Pharm.,28, 957-974 (2002).
21 Chilkoti, A., Dreher, M.R., Meyer, D.E., Raucher, D., “Targeted drug delivery by thermally responsive polymers”, Adv. Drug Delivery Rev.,54, 613-630 (2002).
22 Thornton, P.D., Mart, R.J., Ulijn, R.V., “Enzyme-responsive polymer hydrogel particles for controlled release”, Adv. Mater.,19, 1252-1256 (2007).
23 Qu, T.H., Wang, A.R., Yuan, J.F., Gao, Q.Y., “Preparation of an amphiphilic triblock copolymer with pH- and thermo-responsiveness and self-assembled micelles applied to drug release”, J. Colloid Interface Sci.,336, 865-871 (2009).
24 Zhang, N.Y., Liu, M.Z., Shen, Y.G., Chen, J., Dai, L.L., Gao, C.M.,“Preparation, properties, and drug release of thermo- and pH-sensitive poly((2-dimethylamino)ethylmethacrylate)/poly(N,N-diethylacrylamide) semi-IPN hydrogels”, J. Mater. Sci.,46, 1523-1534 (2011).
25 Zhang, X.G., Teng, D.Y., Hou, J.L., Wang, X., Wang, Z., Li, C. X.,“Glucosamine-carrying temperature- and pH-sensitive microgels: Preparation, characterization, and in vitro drug release studies”, J. Colloid Interface Sci.,322, 333-341 (2008).
26 Freitas, R.F.S., Cussler, E.L., “Temperature sensitive gels as extraction solvents”, Chem. Eng. Sci.,42, 97-103 (1987).
27 Schild, H.G., “Poly(N-isopropylacrylamide): Experiment, theory and application”, Prog. Polym. Sci.,17, 163-249 (1992).
28 Suzuki, A., “Phase transition in gels of sub-millimeter size induced by interaction with stimuli”, Adv. Polym. Sci.,110, 199-240 (1993).
29 Gu, J.X., Xia, F., Wu, Y., Qu X.Z., Yang, Z.Z., Jiang, L., “Programmable delivery of hydrophilic drug using dually responsive hydrogel cages”, J. Controlled Release,117, 396-402 (2007).
30 Huang, G., Gao, G., Hu, Z.B., John, J.V.S., Ponder, B.C., Moro, D.,“Controlled drug release from hydrogel nanoparticle networks”, J. Controlled Release,94, 303-311 (2004).
31 Guo, J., Yang, W.L., Deng, Y.H., Wang, C.C., Fu, S.K., “Organicdye-coupled magnetic nanoparticles encaged inside thermoresponsive PNIPAM microcapsules”, Small,1, 737-743 (2005).
32 Yu, Y.L., Xie, R., Zhang, M.J., Li, P.F., Yang, L.H., Ju, X.J., Chu, L.Y., “Monodisperse microspheres with poly(N-isopropylacrylamide) core and poly(2-hydroxyethyl methacrylate) shell”, J. Colloid Interface S ci.,346, 361-369 (2010).
33 Feil, H., Bae, Y.H., Feijen, J., Kim, S.W., “Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers”, Macromolecules,26, 2496-2500 (1993).
34 Hu, L., Chu, L.Y., Yang, M., Yu, J., Wang, H.D., “A composite thermo-responsive membrane system for improved controlledrelease”, Chem. Eng. Technol.,30, 523-529 (2007).
35 Veerabadran, G., Price, R.R., Lvov, Y.M., “Clay nanotubes for encapsulation and sustained release of drugs”, ACS Nano,2, 115-120 (2007).
10.1016/S1004-9541(13)60572-8
2012-01-27, accepted 2012-07-11.
* Supported by the National Natural Science Foundation of China (20906064), the National Basic Research Program of China (2009CB623407), the Program for Changjiang Scholars and Innovative Research Team in University (IRT1163), and the Foundation for the Author of National Excellent Doctoral Dissertation of China (201163).
** To whom correspondence should be addressed. E-mail: chuly@scu.edu.cn; juxiaojie@scu.edu.cn
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Recent Advances in Separation of Bioactive Natural Products*
- Volumetric and Transport Properties of Aqueous NaB(OH)4Solutions*
- Experimental and Theoretical Studies of CO2Absorption Enhancement by Nano-Al2O3and Carbon Nanotube Particles
- A Novel γ-Alumina Supported Fe-Mo Bimetallic Catalyst for Reverse Water Gas Shift Reaction*
- Treatment of Sebacic Acid Industrial Wastewater by Extraction Process Using Castor Oil Acid as Extractant*
- A New Tuning Method for Two-Degree-of-Freedom Internal Model Control under Parametric Uncertainty*
