Synthesis of Hierarchical MgO-Containing Silicalite-1 Zeolites with High Hydrothermal Stability
2013-07-25CHENHongLiDINGJianWANGYiMeng
CHEN Hong-Li DING Jian WANG Yi-Meng
(Shanghai Key Laboratory of Green Chemistry and ChemicalProcesses,Department of Chemistry,East China Normal University,Shanghai 200062,P.R.China)
1 lntroduction
Zeolite catalysts are widely used in industry,1-5however,the catalysts slowly deactivate during catalytic reaction and regeneration due to the poor stability under the steam condition6,7and the presence of coke.8-13The zeolite catalyst with a high hydrothermal stability is therefore highly important for more regeneration cycles and extending the catalyst life.Many efforts have been taken to improve the hydrothermal stability of zeolites.It has been reported that the alkaline earth metal loaded catalysts had improved hydrothermal stability,which increased the useful life of catalyst during catalytic process and regeneration cycle.14Another key issue about catalyst life is to reduce the coke formation and/or enhance the coke tolerance.One of effective solutions is to introduce the mesopores into zeolite catalysts to provide more space for the coke deposition and reduce the diffusion limitation to improve the coke selectivity.15,16Furthermore,nano-sized zeolite is also promising to increase the diffusion capability,17,18while the introduction of metal oxide could interrupt the growing of zeolitic structure and thus inhibited the formation of bulky zeolite crystals.3
Moreover,alkaline metal oxides,such as CaO,SrO,BaO,and MgO,are also significant for some industrial applications including side chain alkylation,19-22cracking of tar components,5,23conversion of methanol to propylene,24the transformation of propane to propylene25and others.The incorporation of basic metal oxides benefits the formation of acid-base pairs,which may play an important role in the reactions.3For instances,the introduction of alkaline metal oxides into HZSM-5 zeolite could neutralize some acidity,alter the ratio of Brnsted acid to Lewis acid sites,and thus improve the selectivity of catalysts.24
In present work,magnesia was firstly introduced into porous silicaviasolid-state grinding and the subsequent calcinations,and then MgO-containing zeolite was obtainedviahydrothermal synthesis.The influence of MgO amount on the hydrothermal stability and the mesopore generation of the obtained MgO-containing zeolite was investigated.
2 Experimental
2.1 Preparation of hierarchical MgO-containing silicalite-1 zeolites
The precursor salt,Mg(NO3)2·6H2O(AR,SCRC),was manually ground with the porous silica,purchased from Qingdao Haiyang Chemical and Special Silica Gel Co.Ltd.(China),under ambient conditions.The resulting homogeneous powder was calcined at 550°C in air for 6 h with a heating ramp of 2 °C·min-1.The calcined samples were denoted asn%MgO/SiO2,wheren%represented the mass fraction of magnesia in the silica.In the hydrothermal process,the samples ofn%MgO/SiO2were mixed with aqueous TPAOH solution(1 mol·L-1,Aldrich)and calculated amount of water and stirred at room temperature for 2 h.The molar composition of reactant mixture was 1.0SiO2:0.1TPAOH:10H2O.The crystallization was accomplished at 175°C for 1 d.The solids were recovered by filtration,washed thoroughly several times with distilled water,and then dried overnight at 100°C.The occluded templating molecules of TPA+in as-made samples were removed by calcination at 550°C for 6 h in an air flow.The final obtained samples were labeled asn%MgO/silicalite-1.Part of MgO was removed by treating 0.2 g calcined samples with 30 mL 0.5 mol·L-1HCl at 70°C for 60 min.The products were obtained by centrifugation,and then dried at 100oC and denoted as H-n%MgO/silicalite-1.
Silicalite-1,MgO/silicalite-1,and H-MgO/silicalite-1 zeolites were hydrothermally treated at 800°C in 100%steam for 10 h.
2.2 Characterization
Powder X-ray diffraction(XRD)patterns were collected on a Rigaku-Ultima diffractometer(Japan)using CuKαradiation(λ=0.154184 nm)over a 2θrange from 5°to 35°,the accelerating voltage and the applied current were 35 kV and 25 mA,respectively.Transmission electron microscopy(TEM)experiments were conducted on TECNAI G2 F30(America)operating at 300 kV.Scanning electron microscopy(SEM)and EDX were performed on a scanning electron microscopy(type HITACHI S-4800,Japan)with an accelerating voltage of 3 kV.The amounts of Si and Mg in zeolites were quantified by inductively coupled plasma(ICP)on a Thermo IRIS Intrepid II XSP atomic emission spectrometer(America)after dissolving the samples in HF solution.The BET surface area(SBET)and pore parameters of the samples were determined by nitrogen adsorption-desorption measurements at 77 K on a nitrogen adsorption apparatus(BELSORP-max,Japan).Before the measurements,the samples were outgassed at 300°C in vacuum for 6 h.The pore size distributions were derived from the adsorption branches of the isotherms using the Barrett-Joyner-Halanda(BJH)method.The total pore volume(Vp)was estimated at a relative pressure of 0.99.
3 Results and discussion
As shown in Fig.1,no crystalline MgO phases are observed in the XRD patterns of all MgO/SiO2with different MgO contents,as the domain of MgO nanoparticles is too small to give XRD information,indicating that MgO can be highly dispersed on silica gel by the solid-state grinding method.
Fig.2 shows XRD patterns of MgO and samples synthesized at 175°C for 1 d with different amounts of MgO.All the zeolite samples have a typical MFI structure with characteristic diffraction peaks at 2θ=8.0°,8.9°,23.1°,23.3°,24.0°without any crystalline phases of MgO.The relative crystallinity of zeolite calculated from XRD data distinctly decreases when the amount of MgO increases from 0%to 30%,as listed in Table 1.The loss of relative crystallinity of MFI-structure can partly attribute to the occlusion of amorphous MgO phase or the fact that MgO may inhibit the growth of silicalite-1 phase.Moreover,the absence of the peaks corresponding to a magnesia phase in the XRD patterns of MgO/silicalite-1 proves that the crystallization process did not cause any further growth of highly dispersed MgO species on silica gel thus no large MgO particles presented in zeolite crystals.Very recently,Maoet al.24used wet impregnation method to introduce MgO into HZSM-5,where the crystalline phases of MgO were observed in XRD patterns with the loading amount of MgO larger than 4%.These results further prove that the solid-state grinding method favors high dispersion of MgO in matrix,making it possible to introduce more metal oxides with high dispersion in zeolite crystals.It is in agreement with results reported by Zhuet al.,26where manually grinding method was proved to be more effective to spontaneously disperse oxide species,like CuO,FeOx,and CrOx,onto SBA-15 than wetness impregnation.It might be explained by the absence of a solvent,solidstate grinding not only saves the time and energy needed but also inhibits the competitive adsorption of solvent molecules,which enhances the interaction between the guest species included and the silica matrix and thus facilitates the high dispersion of oxide species.

Fig.1 XRD patterns of samples(a)5%MgO/SiO2,(b)10%MgO/SiO2,and(c)30%MgO/SiO2

Fig.2 XRD patterns of samples(a)MgO,(b)pure silicalite-1,(c)5%MgO/silicalite-1,(d)10%MgO/silicalite-1,and(e)30%MgO/silicalite-1

Table 1 MgO amount of the calcined samples and relative crystallinity(R.C.)of the samples before and after the treatment at 800°C in 100%steam for 10 h
The uniformly distribution of MgO in 10%MgO/SiO2and zeolite crystals 10%MgO/silicalite-1 was further proved by TEM results.Very few MgO nano-domains in size of~15 nm are observed,as shown in inset of Fig.3b.Furthermore,some lattice fringes of MgO in parallel with black arrows(Fig.3b)are detected throughout the sample,indicating that the MgO species probably formed monolayer or submonolayer instead of large particles.There is no MgO particle formed in MgO-containing zeolites(Fig.3c),demonstrating that the crystallization process also did not cause any further growth of highly dispersed MgO species on silica gel.It is in agreement with the results suggested by XRD patterns.
The status of MgO was measured by EDX spectroscopy(Fig.4)and ICP data(Table 1).The MgO content of zeolites increases with an increase of the amount of MgO in the MgO/SiO2composites,as shown in Table 1.Moreover,for 5%MgO/silicalite-1 and 10%MgO/silicalite-1,the molar ratios of Mg/Si on the sample surface detected by EDX(nSi/nMg=191,92)are slightly higher than that of bulky sample determined by ICP(nSi/nMg=149,61),possibly attributed to MgO species which might be encapsulated in the zeolite crystals.
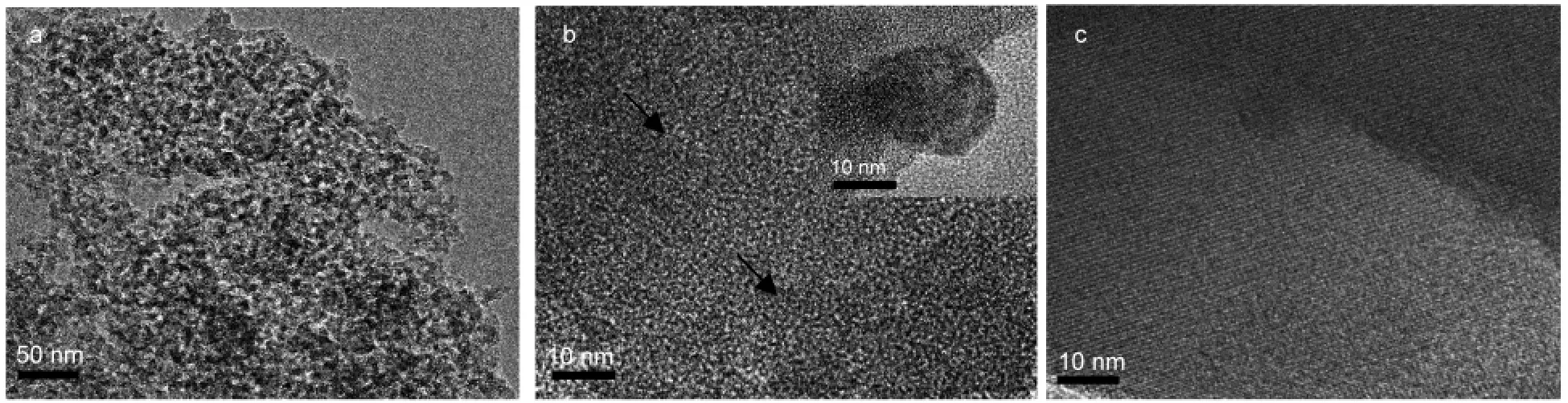
Fig.3 TEM images of samples(a,b)10%MgO/SiO2and(c)10%MgO/silicalite-1

Fig.4 SEM and EDX images(inset)of samples(a)5%MgO/silicalite-1 and(b)10%MgO/silicalite-1
As shown in the SEM images of Fig.5a,the calcination of magnesium nitrate itself at 550°C results in large particles of MgO,while no large MgO particles form in the MgO/SiO2samples,as shown in Fig.3.This indicates that manually grinding method is an effective method to spontaneously disperse metal oxide species on porous silica.The pure silicalite-1 contains relatively uniform twinned crystals in size of ~5 μm(Fig.5b),which can be observed over the whole specimen.When 5%MgO is introduced,the crystals are slightly irregular in morphology and the size obviously decreases around 2.5 μm×2.5 μm×1.3 μm.Interestingly,a small amount of non-uniform fragments in size of 80-240 nm are observed on crystals surface,consistent with low crystallinity in XRD measurement and very few twinned crystals observed in Fig.5c.With the amount of MgO further increasing to 10%,most crystals show un-defined and non-uniform morphology as shown in Fig.5d,which suggests that the presence of MgO strongly inhibits the zeolite growth.As there are no MgO crystalline phases in the XRD patterns of both 5%MgO/silicalite-1 and 10%MgO/silicalite-1 shown in Fig.2,these small particles randomly on the surface of zeolite crystals are possibly amorphous SiO2species rather than MgO particles.These results show that the crystal morphology and size distinctly vary with the loading amount of MgO species,indicating that the presence of MgO species does disturb the crystallization of silicalite-1 during hydrothermal synthesis and thus decrease the particle size of zeolite crystals.3
The hydrothermal stability of zeolites at 800°C in steaming is important because the steam easily destroys the zeolitic structure at high temperature.It has been reported that the alkaline earth metal loaded catalysts had enhanced hydrothermal stability which increased the catalyst life during catalytic processes.14One of the targets of this work is to synthesize the zeolites with improved hydrothermal stabilityviathe introduction of magnesium oxide.The pure silicalite-1 and MgO-loaded zeolites were subjected to hydrothermal treatment at 800°C in 100%steam with 4.4 g H2O every minute.The relative crystallinity of the samples before and after hydrothermal treatment is shown in Table 1.All samples lost some crystallinity due to the collapse of zeolitic structure under steam condition.With the introduction of MgO species(5%and 10%),the crystallinity retention of MgO/silicalite-1 after hydrothermal treatment obviously increased,demonstrating that the incorporation of magnesia in the crystals enhanced the hydrothermal stability of zeolites.However,30%MgO/silicalite-1 shows lower hydrothermal stability,as the poor crystallinity might result in low stability of the MFI crystals under the hydrothermal treatment.
To prove the positive impact of introduced magnesia in increasing hydrothermal stability of silicalite-1,MgO was removed by hydrochloric acid solution.The ICP data indicate that the content of MgO distinctly decreases after acid treatment.As shown in Table 1,the acid-washed samples have higher crystallinity compared with that of calcined 10%MgO/silicalite-1,indicating that part of MgO species has been removed during acid treatment.Furthermore,the hydrothermal stability of silicalite-1 with low magnesia content obviously decreases compared with that of samples without acid treatment,demonstrating that the incorporation of magnesia into silicalite-1 plays an important role in enhancing the hydrothermal stability of zeolites.

Fig.5 SEM images of(a)MgO,(b)pure silicalite-1,(c)5%MgO/silicalite-1,and(d)10%MgO/silicalite-1
Furthermore,the removal of MgO species may result in the Eadditional mesopores in zeolite samples.The presence of mes-opore created by acid treatment was further proved by nitrogen adsorption-desorption data(Fig.6 and Table 2).As shown in Table 2,porous silica possesses a very large pore volume of 1.02 cm3·g-1and high surface area of 406 m2·g-1.When 10%MgO is introduced,both pore volume and surface area decrease to 0.74 cm3·g-1and 253 m2·g-1,respectively.It might be explained by the collapse and occlusion of porous structure during the grinding and calcination.All MgO/silicalite-1 samples exhibit the combined characteristics of type I and type IV isotherms.For 5%MgO/silicalite-1 and 10%MgO/silicalite-1,there are hysteresis loops at high relative pressure ofp/p0>0.4 which is absent for pure silicalite-1,indicating the presence of some intracrystalline voids in zeolites which might be caused by the incorporation of MgO inhibiting the growth of silicalite-1 phase with long scale ordered structure.After the acid treatment,the hysteresis loops become more obvious,which is possibly attributed to the capillary condensation of nitrogen in the additional mesopores resulted by the removal of MgO domains during acid washing process.The detailed data are summarized in Table 2.All the samples have almost equivalent microporous volume.MgO/silicalite-1 composites show increased BET surface area(SBET),extra pore volume(Vextra),and external surface area(Sexter)compared with that of pure silicalite-1.Moreover,the extra pore volume increases with increasing the loading amount of MgO.When MgO is removed by hydrochloric acid,the extra pore volume distinctly increases,indicating the hierarchical porosity of acid treated samples is mostly contributed to the removal of MgO during acid treatment.It may improve the diffusion of reactants and products in catalytic process and thus possibly reduce the rate of the coke formation.

Fig.6 Nitrogen adsorption-desorption isotherms of(a)pure silicalite-1,(b)5%MgO/silicalite-1,(c)10%MgO/silicalite-1,(d)H-5%MgO/silicalite-1,and(e)H-10%MgO/silicalite-1

Table 2 Specific surface area and pore volume of the calcined samples
4 Conclusions
MgO species could be introduced into silica gel through solid-state grinding and the consequent calcination,which leads to highly dispersed MgO into the silica matrix.The MgO/silicalite-1 samples were synthesized under hydrothermal conditions using TPAOH as template.The calcined MgO/silicalite-1 samples show improved hydrothermal stability due to the introduction of MgO species.Furthermore,MgO species in MgO/silicalite-1 samples could be partially removed by acid treatment.The acid-washed MgO/silicalite-1 samples show increased crystallinity,surface area,total volume,and external surface area,which are benefit for the diffusion of reactants and products in catalytic process and reducing the rate of the coke formation.Both high hydrothermal stability and hierarchical porosity may increase the catalyst life during catalytic processes.In addition,this direct method can be extended to similar systems with the introduction of different alkaline metal oxide.
(1) Quann,R.J.;Green,L.A.;Tabak,S.A.;Krambeck,F.J.Industrial&Engineering Chemistry Research1988,27,565.doi:10.1021/ie00076a006
(2)Yamamura,M.;Chaki,K.;Wakatsuki,T.;Okado,H.;Fujimoto,K.Zeolites1994,14,643.doi:10.1016/0144-2449(94)90121-X
(3) Jiang,N.;Jin,H.;Jeong,E.Y.;Park,S.E.Journal of Nanoscience and Nanotechnology2010,10,227.doi:10.1166/jnn.2010.1513
(4)Asencios,Y.J.O.;Bellido,J.D.A.;Assaf,E.M.Applied Catalysis A:General2011,397,138.doi:10.1016/j.apcata.2011.02.023
(5) Li,X.L.;Yue,B.H.;Wang,X.G.;Yan,Y.F.;Kong,L.H.;Lu,X.G.;Ding,W.Z.Acta Physico-Chimica Sinica2009,25,762.[李雪玲,岳宝华,汪学广,鄢于飞,孔令华,鲁雄刚,丁伟中.物理化学学报,2009,25,762.]doi:10.3866/PKU.WHXB200904231
(6) Huber,G.W.;Guymon,C.G.;Conrad,T.L.;Stephenson,B.C.;Bartholomew,C.H.Stud.Surf.Sci.Catal.2001,139,423.doi:10.1016/S0167-2991(01)80226-3
(7) Chen,N.Y.;Mitchell,T.O.;Olson,D.H.;Pelrine,B.P.Ind.Eng.Chem.,Prod.Res.Dev.1977,16,247.
(8) Finnerty,C.M.;Coe,N.J.;Cunningham,R.H.;Ormerod,R.M.Catal.Today1998,46,137.doi:10.1016/S0920-5861(98)00335-6
(9) Beyne,A.O.E.;Froment,G.F.Chem.Eng.Sci.1993,48,503.doi:10.1016/0009-2509(93)80304-9
(10) Beyne,A.O.E.;Froment,G.F.Chem.Eng.Sci.1990,45,2089.doi:10.1016/0009-2509(90)80081-O
(11) Chen,D.;Rebo,H.P.;Moljord,K.;Holmen,A.Industrial&Engineering Chemistry Research1997,36,3473.doi:10.1021/ie9700223
(12) Triantafyllopoulos,N.C.;Neophytides,S.G.J.Catal.2003,217,324.
(13) Trimm,D.L.Catal.Today1999,49,3.doi:10.1016/S0920-5861(98)00401-5
(14) Voskoboynikov,T.V.;Pelekh,A.Y.;Senetar,J.J.OCP Catalyst with Improved Steam Tolerance.US Patent 12/639,577.X,2011-06-16.
(15) Choi,M.;Na,K.;Kim,J.;Sakamoto,Y.;Terasaki,O.;Ryoo,R.Nature2009,461,246.
(16) Verboekend,D.;Groen,J.C.;Pérez-Ramírez,J.Adv.Funct.Mater.2010,20,1441.doi:10.1002/adfm.200902205
(17)Chen,L.;Zhu,S.Y.;Wang,H.M.;Wang,Y.M.Solid State Sciences2011,13,2024.doi:10.1016/j.solidstatesciences.2011.08.033
(18)Chen,L.;Zhu,S.Y.;Wang,Y.M.;He,M.Y.New J.Chem.2010,34,2328.doi:10.1039/c0nj00316f
(19) Itoh,H.;Hattori,T.;Suzuki,K.;Murakami,Y.J.Catal.1983,79,21.doi:10.1016/0021-9517(83)90286-5
(20) Hathaway,P.E.;Davis,M.E.J.Catal.1989,119,497.doi:10.1016/0021-9517(89)90177-2
(21)Wieland,W.S.;Davis,R.J.;Garces,J.M.J.Catal.1998,173,490.doi:10.1006/jcat.1997.1952
(22)Hunger,M.;Schenk,U.;Weitkamp,J.J.Mol.Catal.A:Chem.1998,134,97.doi:10.1016/S1381-1169(98)00026-0
(23)Yue,B.H.;Kong,L.H.;Wang,X.G.;Lu,X.G.;Ding,W.Z.Chinese Jounal of Catalysis2010,31,218.[岳宝华,孔令华,汪学广,鲁雄刚,丁伟中.催化学报,2010,31,218.]
(24)Mao,D.S.;Guo,Q.S.;Meng,T.Acta Physico-Chimica Sinica2010,26,2242.[毛东森,郭强胜,孟 涛.物理化学学报,2010,26,2242.]doi:10.3866/PKU.WHXB20100814
(25)Zhang,F.;Miao,C.X.;Yue,Y.H.;Hua,W.M.;Gao,Z.Chin.J.Chem.2012,30,929.[张 帆,缪长喜,乐英红,华伟明,高 滋.中国化学,2012,30,929.]doi:10.1002/cjoc.201100379
(26)Wang,Y.M.;Wu,Z.Y.;Zhu,J.H.J.Solid State Chem.2004,177,3815.doi:10.1016/j.jssc.2004.07.013
