A Probe into Process for Maximization of Low-carbon Olefins via Co-processing of Methanol and Heavy Oil
2013-07-25SongBaomei
Song Baomei
(Research Institute of Petroleum Processing, SINOPEC, Beijing 100083)
A Probe into Process for Maximization of Low-carbon Olefins via Co-processing of Methanol and Heavy Oil
Song Baomei
(Research Institute of Petroleum Processing, SINOPEC, Beijing 100083)
From the viewpoint of process specifics and thermodynamics, this article has put forward a route for maximization of low-carbon olefins via co-processing of methanol and heavy oil. Catalytic cracking experiments on co-processing of methanol and heavy oil at different ratios in a fixed fluidized bed reactor had been conducted. Test results have revealed that when 12.5% of methanol was blended to the heavy oil a good products distribution and relatively higher yield of lowcarbon olefins could be obtained. The overall yield of low-carbon olefins could reach 50.16%, with the yield of ethylene, propylene and butylene equating to 5.47 %, 28.93% and 15.76 %, respectively.
catalytic cracking; methanol; co-processing; low-carbon olefin
1 Introduction
Low-carbon olefins as basic organic chemical feedstocks have been playing a pivotal role in contemporary petrochemical industry. With the surging need for polypropylene (PP) and its derivatives, the demand for propylene is increasing with every year[1]. At present the production of methanol is increasing abruptly, because methanol can be manufactured from coal or natural gas, as well as from other routes. The process for indirect synthesis of lowcarbon olefins from methanol and dimethyl ether (DME) is becoming more matured. It can be learned from literature information[2-5]that the process for manufacture of olefins from methanol has its similarity with that of resid fluid catalytic cracking (RFCC). For example, both of these two processes use the reactor-regenerator system and adopt acid catalysts such as mesoporous catalysts with the reaction temperature ranging from 500—550 ℃coupled with atomized water. Furthermore, the conversion of methanol to olefins has to deliver a large amount of heat, while RFCC process must absorb a large amount of heat. If methanol is co-processed with heavy oil, this measure can stabilize the reaction temperature. Hence an idea relating to co-processing of methanol and heavy oil in FCC unit has been proposed in an attempt to increase the yield of low-carbon olefins in the reaction products, in particular the yield of propylene.
2 Experimental
2.1 Feedstocks
The heavy oil used in the experiments was a mixture of Daqing AGO blended with 30 percent of vacuum residue (VR) which was collected from the Yanshan Petrochemical Company, with its property presented in Table 1. Another feedstock was methanol, AR, which was manufactured by the Beijing Chemical Works.

Table 1 Feedstock property
2.2 Catalysts
Since the target reaction system contains not only large hydrocarbon molecules, but also small molecules of reactants and products, the selection of proper catalyst should consider these two factors. The macroporous Y zeolite was selected as the sites for reaction on large hydrocarbon molecules of heavy oil, and the mesoporous ZSM-5 zeolite was selected as the sites for reaction on small hydrocarbon molecules. The catalyst MMC-2 was composed of both the ZSM-5 zeolite and the Y zeolite, while the catalyst MMC-3-6 was mainly composed of the ZSM-5 zeolite. These two types of catalysts after having been respectively subjected to hydrothermal ageing under 100% steam at 790 ℃ for 14 h were mixed at a proper ratio to yield a catalyst MMY consisting of around 5% of the Y zeolite, with its basic property presented in Table 2.

Table 2 MMY Catalyst propert
2.3 Test method
The test equipment was a pilot-scale fixed fluidized bed (FFB). The catalyst was firstly put into the FBB reactor, into which methanol and atomized steam after having passed through the preheater were introduced to take part in the reaction on the catalyst at the hot fluidized state. The reaction products after being cooled down were separated into FCC gas and liquid. The FCC gas was analyzed by an Agilent 6890N gas chromatograph. The liquid mainly contained unconverted methanol, water and oil. The cooled water after reaction was collected and was weighed to measure the weight of methanol solution. The methanol content in the solution was determined with the internal standard method by a Varian CP-3800 gas chromatograph. The oil in the liquid product was analyzed to determine the content of gasoline, diesel and heavy oil fractions by simulated distillation method with an Agilent 6890N gas chromatograph, while the hydrocarbon group analysis of gasoline fraction was performed by an Agilent 6890 liquid chromatograph. The catalyst after reaction and steam stripping was regenerated through burning with oxygen, with the flue gas being analyzed by infrared spectroscopy to directly determine the coke yield.
2.4 Calculation method
The material balance is calculated based on the units of CH-CH2. For processing the material balance data the amount of methanol (mmethanol) involved in calculation is supposed to contain one CH2unit in a methanol molecule. If the methanol feed is supposed to bem, the methanol amount (mmethanol) involved in the calculation should be:

Likewise, the calculation of DME yield is based on the C2H4unit in DME. If the calculated DME yield is 10%, the actual equation for calculating the DME yield (YDME) should be:

3 Results and Discussion
3.1 Influence of different methanol blending ratios on material balance during coprocessing of methanol and heavy oil
The product distribution obtained during coprocessing of methanol and heavy oil in a laboratory FFB unit was investigated at a reaction temperature of 520 ℃, a catalyst/ oil mass ratio of 8, and an injected water ratio of 11% (based on the mass of feedstock), with the test results presented in Table 3. The results of catalytic cracking of pure heavy oil were obtained at a 0% of blended methanol in the feedstock, while the results of catalytic cracking of pure methanol were acquired at 100 % methanol in the feedstock. The influence of different methanol blendingratios on the catalytic cracking of heavy oil was studied based on the weight average data of methanol ratio in the feedstock which were calculated by changing the ratio between the results of catalytic cracking of 100% of heavy oil (with 0% of methanol) and 100% of methanol (with 0% of heavy oil), respectively.
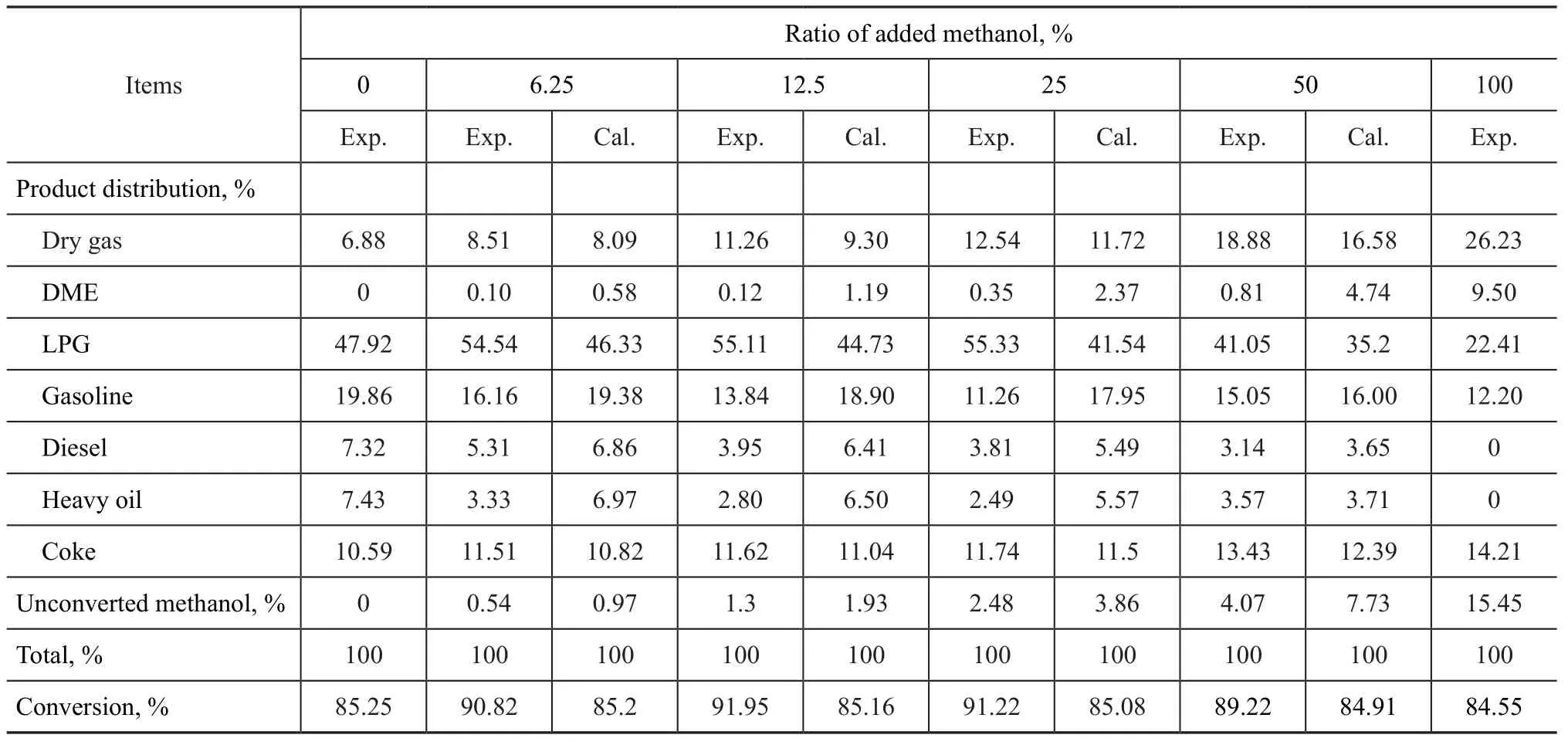
Table 3 Results of catalytic cracking of different methanol ratios in heavy oil in comparison with the calculated results
It can be seen from Table 3 that with an increasing ratio of blended methanol in the feedstock the amount of unconverted methanol and DME yield also increased, while the yield of diesel and heavy oil fractions decreased. This fact denoted that with the increase of methanol ratio in the feedstock the mass of unconverted methanol increased along with an increase in the mass of methanol converted to the product-DME, which was the characteristic product resulted from the methanol-involved reaction. In the meantime, the increasing ratio of methanol blended to the feedstock led to a decreasing amount of heavy oil and reduced diesel yield in the reaction products.
It can also be seen from Table 3 that compared to the calculated yield of reaction products, the actual yield of dry gas, coke and LPG and the feedstock conversion rate were higher, while the yield of DME, gasoline, diesel and heavy oil along with unconverted methanol was lower. Generally speaking, during co-processing of methanol and heavy oil because of the lower molecular weight of methanol than that of heavy oil at a same weight of reactants the molecules of methanol would occupy more active sites on the catalyst because its number of molecules is more that that of heavy oil, which would result in a decreased heavy oil conversion. However, the test result indicated an opposite trend, which might be attributed to the carbonium ions formed by methanol on the surface acid sites of the catalyst that could promote the initiation of chain cracking reaction in the heavy oil to increase the feedstock conversion rate.
The change in the content of dry gas components with a varying blending ratio of methanol in the feedstock is presented in Table 4. It can be seen from Table 4 that upon co-processing of methanol and heavy oil, the experimental yield of methane, ethane and ethylene was higher than the calculated data, while the experimental hydrogen yield was less than the calculated value. The hydrogen yield was higher when methanol was separately processed, but when methanol was processed in admixture with heavy oil the hydrogen yield was lower than the calculated value, which indicated that methanol molecules on the catalyst might engage in a chain transfer reaction with molecules of heavy oil to promote the catalytic cracking of heavy oil, resulting in an increased conversion of heavy oil. This phenomenon has revealed that the co-processing of methanol and heavy oil may involve interactions other than a combination of catalytic cracking reaction of methanol and heavy oil, respectively.

Table 4 Comparison between the experimental and calculated data on yield of dry gas components obtained from processing of feedstock with different methanol blending ratios w, %
3.2 Influence of different methanol blending ratios on low-carbon olefins yield
The experimental and calculated data on yield of low-carbon olefins obtained at different methanol blending ratios in the feedstock are presented in Figure 1. It can be seen from Figure 1 that blending a small amount of methanol to the heavy oil could increase the yield of propylene and butylenes. The experimental data on the yield of propylene and butylenes were higher than the calculated data, but the ethylene yield was quite close to the calculated value. Since ethylene is a product of thermal cracking reaction, and propylene and butylenes are the products of catalytic cracking reaction, it can be concluded that coprocessing of methanol with heavy oil can be conducive to catalytic cracking of heavy oil.
Judging from the experimental data, the overall yield of low-carbon olefins was the maximum (50.99%) at a 25% ratio of blended methanol in the feedstock, in which the yield of ethylene, propylene, and butylenes reaching 5.06%, 29.55% and 15.42%, respectively. But upon studying the products distribution in comparison with the case using a methanol blending ratio of 12.5% in the feedstock this case with 25% of blended methanol in the feedstock could increase the conversion rate by1.98%, while the yield of dry gas and coke was increased by 1.18% and 1.28%, respectively. According to a comprehensive assessment, at the said experimental conditions the optimal methanol blending ratio in the feedstock should be 12.5%.

Figure 1 Comparison between experimental and calculated data on yield of gaseous olefins obtained during processing of different blending ratios in feedstock
3.3 Influence of methanol blending ratio on gasoline composition obtained during coprocessing of methanol and heavy oil
The property of gasoline obtained during coprocessing of methanol and heavy oil at different ratios is presented in Table 5. It can be seen from the data listed in Table 5 that compared to the reaction for catalytic cracking of exclusive heavy oil the catalytic cracking of heavy oil blended with methanol had resulted in obviously reduced olefins content and increased aromatic content in gasoline fraction. In comparison with the calculated products yield, blending of methanol to heavy oil had led to a relatively low olefins content and higher aromatic content in gaso-line fraction, which might be ascribed to the enhanced hydrogen transfer reaction in the reaction system to transform olefins into aromatics after methanol blending into the feedstock.

Table 5 Influence of different methanol blending ratios in feedstock on gasoline composition w, %
4 Conclusions
(1) A route for obtaining low-carbon olefins was carved out via coprocessing of methanol with heavy oil. Coprocessing of methanol and heavy oil is an organic integration of methanol-to-olefin (MTO) technology with the catalytic cracking process, attesting to a breakthrough for manufacture of low-carbon olefins from renewable energy and heavy oil.
(2) The experimental results of coprocessing of methanol and heavy oil have revealed that methanol after having access to the catalyst bed has a synergistic effect on heavy oil to promote the catalytic cracking of heavy oil and increase the conversion of feedstock.
(3) The comprehensive assessment had shown that the optimal experimental regime was realized at a methanol ratio of 12.5% in the feedstock. At this test condition the yield of low-carbon olefins was as high as 49.68%, with the yield of ethylene, propylene and butylenes equating to 4.99%, 28.93%, and 15.76 %, respectively.
(4) In comparison with the outcome of catalytic cracking reaction of pure heavy oil, the blending of methanol to heavy oil under the specified experimental conditions could reduce the olefin content and increase the aromatic content in gasoline fraction.
[1] Qian Bozhang. Advances of technologies for increasing propylene production[J]. Petroleum Processing and Petrochemicals, 2001, 32(11): 19-23 (in Chinese)
[2] Cai Guangyu, Wang Qingxia, Yang Yonghe, et al. Conversion of methanol to lower olefins on high silica zeolites. I. Further improvement of catalyst performance [J]. Chinese Journal of Catalysis, 1988, 9(2): 145-151 (in Chinese)
[3] Rothaeme M, Holtmann H D. Methanol to propylene MTP—Lurgi’s way[J]. Erdöl Erdgas Kohle, 2002, 118(5): 234-237
[4] Stocker M. Methanol to hydrocarbons: Catalytic materials and behavior[J]. Microporous and Mesoporous Materials, 1999, 29(1/2): 3-48
[5] Chang D R, Jr Westrum E F, Sinke G C. The Chemical Thermodynamics of Organic Compounds[M]. New York: Wiley, 1969
Recieved date: 2013-03-14; Accepted date: 2013-04-18.
Ms. Song Beimei, Telephone: +86-10-82368471; E-mail: songbm.ripp@sinopec.com.
杂志排行
中国炼油与石油化工的其它文章
- Preparation and Catalytic Performance of Potassium Titanate Used as Soot Oxidation Catalyst
- Effects of Fatty Acids on Low-Sulfur Diesel Lubricity: Experimental Investigation, DFT Calculation and MD Simulation
- Influence of Carbon Content on S Zorb Sorbent Activity
- Propylene Polymerization Catalysts with Sulfonyl Amines as Internal Electron Donors
- Isolation and Characterization of a Thermophilic Oil-Degrading Bacterial Consortium
- Research on Catalytic Properties of Palladium Catalyst Prepared by Biological Reduction Method
