Research on Catalytic Properties of Palladium Catalyst Prepared by Biological Reduction Method
2013-07-25ZhangFengFuJiquan
Zhang Feng; Fu Jiquan
(School of Materials Science and Engineering, Beijing Institute of Fashion Technology, Beijing 100029)
Research on Catalytic Properties of Palladium Catalyst Prepared by Biological Reduction Method
Zhang Feng; Fu Jiquan
(School of Materials Science and Engineering, Beijing Institute of Fashion Technology, Beijing 100029)
This paper relates to highly dispersed supported Pd/MWCNTs and Pd/α-Al2O3catalysts prepared by biological reduction method. The physico-chemical properties and the difference in catalytic activity of Pd catalysts prepared by biological reduction method and chemical method, respectively, were investigated using XRD, TEM and specific surface characterization methods. The catalytic properties of catalysts were studied through activity evaluation means. The test results showed that the catalysts prepared by biological method were characteristic of small Pd nanoparticle size, good dispersion and low agglomeration, while possessing a high activity and stability in styrene hydrogenation reaction in comparison with catalysts prepared via the chemical method.
Pd/MWCNTs; Pd/α- Al2O3; biological reduction; ginkgo leaf; Pd nanoparticles
1 Introduction
Catalyst with precision metal nanoparticles deposited on a support plays a dominant role in the field of oil refining, petrochemical industry and environmental protection thanks to its superior catalytic activity and selectivity. The traditional methods for preparing precious metal nanoparticles include physical method and chemical method. The catalyst preparation process using the physical method is simple, but the requirement on equipment is high and the production cost is expensive; the chemical method is flexible and diversified, but it should be realized with chemical reagents, which will bring about some environmental pollution problems. The biological reduction method for preparing precious metal nanoparticles is becoming a research focus in the field of nanometer scale technology along with the increasingly active research on greenization technology for preparation of materials[1]. Upon using biological method to prepare precious metal nanoparticles, there is no need to add additional chemical reagents, and pollution is reduced along with a full utilization of rich biological resources and biomass as the reducing agent[2-4]. Huang, et al.[5]prepared gold and silver nanoparticles through reduction using camphor leaf extract, and the addition of chemical reagent was not needed during the preparation process. Carbon nanotubes (CNTs) having good chemical stability and high specific surface area are the excellent support for active catalytic component[6-7]. The researchers have developed CNTs as the catalyst support that has been used in hydrogenation, dehydrogenation, and oxidation reactions[8-9]. The research on catalyst prepared by biological reduction method using carbon nanotubes as the support has not been reported previously.
This study prepared a series of catalysts using ginkgo leaf extract as a reducing agent, with MWCNTs (multi-walled carbon nanotubes) and α-Al2O3serving as the support. The catalysts were characterized through XRD, TEM and specific surface area measurements, and the performance of catalysts was investigated through styrene hydrogenation to produce ethylbenzene for activity evaluation purposes.
2 Experimental
2.1 Preparation of extract
Five gram of ginkgo leaf powder was added to a conical flask filled with 250 mL of deionized water. The conical flask was oscillated for 12 h at 60 ℃. Insoluble biomasswas removed by centrifugation after being cooled down. The obtained supernatant liquid was the ginkgo leaf extract at a concentration of 20 g/L, which was then stored in refrigerator at 4 ℃ prior to use.
2.2 Preparation of catalyst
A series of palladium catalysts supported on MWCNTs (including MWCNTs-0, Pd/MWCNTs-C, and Pd/ MWCNTs-B) and on α-Al2O3(including α-Al2O3-0, Pd/ α-Al2O3-C, Pd/α-Al2O3-B) were prepared respectively. Herein, 0 denotes the pure support (the pure support was prepared in order to study the influence of the said support on catalytic activity), C denotes the chemical method, and B denotes the biological method. A required concentration of palladium nitrate solution was prepared. An excess of palladium solution was used to impregnate multi-walled carbon nanotubes and α-Al2O3was used as the support for 6 h, followed by drying for 12 h at 60 ℃. Pd/MWCNTs-C catalyst and Pd/α-Al2O3-C catalyst were prepared by the chemical method. A certain amount of palladium nitrate solution was mixed with the same amount of ginkgo leaf extract. The excessive amount of the solution was used to impregnate multi-walled carbon nanotubes and α-Al2O3for 12 h. Thus, the Pd/MWCNTs-B catalyst and Pd/ α-Al2O3-B catalyst were prepared by the biological method after being dried for 24 h at 60 ℃. A series of catalysts using α-Al2O3as the support were calcined respectively for 2 h at 500 ℃, and were then reduced in the hydrogen flow.
2.3 Characterization of catalysts
The crystalline structure of catalysts was analyzed using a D8 Advance high-power X-ray diffractometer with a rotating target (made by the Bruker company), operating at a tube voltage of 40 kV, a tube current of 250 mA, a scan step size of 0.02°/step, and a scan range of 10°—90°. The morphology of catalysts was observed using a JOEL’s JEM2100 transmission electron microscope. The highresolution TEM images were obtained by a high magnif ication transmission electron microscope (JEM2100) at an accelerating voltage of 200 kV after dipping the ultra-thin carbon film in reaction solution followed by drying. Specific surface area and pore volume of each catalyst were measured by utilizing a pore distribution and specific surface measuring instrument made by the Beijing Jingweigaobo Science and Technology Development Center, with aP/P0in the range of 0.05—0.95.
2.4 Evaluation of catalyst activity
The reaction of styrene hydrogenation to form ethylbenzene was used as the probe reaction for evaluating the catalyst activity. The main reaction proceeds according to Formula (1), and this reaction only generates ethylbenzene under this condition, with the catalytic selectivity reaching 100%. 0.1 g of catalyst (with a particle size of 60-100 mesh) was weighed and added to the continuous micro-reactor device. H2flow was controlled at a rate of 80 ml/min, and the space velocity on catalyst was 20 h-1, with anhydrous ethanol used as solvent at a volume ratio of 1:1. The reaction temperature was 120 ℃, and sampling was performed at regular intervals. The composition of reaction products was analyzed by a GC-7890Ⅱ gas chromatography system (made by the Beijing Tianmei Instrument Company), equipped with a HJ-1 capillary column measuring Ф 0.25 mm×25 m and a FID detector, with the temperature in column equating to 100 ℃, in the sample room—200 ℃, and in the detection room—200 ℃. The content of each component was calculated by means of the area normalization method.
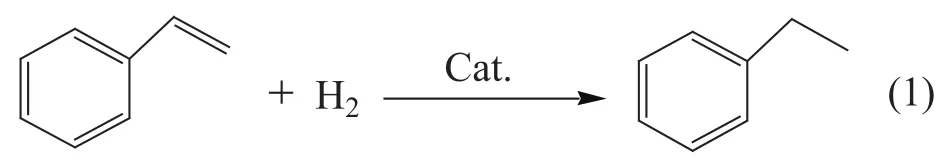
3 Results and Discussion
3.1 Characterization of catalysts
3.1.1 XRD study
Six prepared catalysts were characterized by XRD method in order to investigate whether the palladium loading could affect the structure of catalyst support and the crystalline structure of palladium on the catalyst.
Figure 1 depicts XRD patterns of a series of catalysts supported on MWCNTs, and Figure 2 shows XRD patterns of a series of catalysts supported on α-Al2O3. It can be seen from the spectral line of MWCNTs-0 shown in Figure 1 that the diffraction peaks of crystal planes (002) and (101) in carbon nanotubes appear at 2θ=25.87° and 42.7°, respectively, while other peaks are not obvious. The dif-fraction peak of crystal plane (002) is wide, and its diffraction peak is high, suggesting that the degree of longrange order of nanostructure is poor. This may be caused by superposition of diffraction peaks of impurities such as amorphous carbon and graphite particles. It is known that the carbon nanotubes-supported Pd still retains the characteristics diffraction peaks of crystal planes (002) and (101) in carbon nanotubes and their diffraction peak intensity is relatively weak upon comparing the spectral lines of MWCNTs-0, Pd/MWCNTs-C and Pd/MWCNTs-B presented in Figure 1. These results indicated that the structure of carbon nanotubes was not destroyed, and could serve as excellent support of palladium. It can be learned from Figure 1 that Pd/MWCNTs-B has characteristics diffraction peaks of crystal planes (111), (200), (220) and (311) of Pd at 2θ of 40.06°, 46.59°, 68.13° and 82.11°, respectively upon comparing XRD patterns of Pd/ MWCNTs-C and Pd/MWCNTs-B. This has revealed that ginkgo leaf extract could reduce Pd2+ions to Pd0species, which were then deposited on the MWCNTs support. So ginkgo leaf extract is a good reducing agent for Pd2+ions. It can also be learned from XRD patterns that there was a diffraction peak of Pd0phase at 33.04°, indicating that a part of Pd0species was also deposited on the surface of MWCNTs. The reason was that the grain size of Pd0supported on the surface of MWCNTs was small, and it could be oxidized easily by the oxygen in air. So it could be reduced first in hydrogen stream before the commencement of catalytic reaction in the presence of Pd/MWCNTs catalyst.

Figure 1 XRD patterns of MWCNT and Pd/MWCNTs catalyst samples

Figure 2 XRD patterns of α-Al2O3and Pd/α- Al2O3catalyst samples
It can be seen from the XRD patterns of Pd/α–Al2O3-C and Pd/α–Al2O3-B presented in Figure 2 that the supported Pd samples show characteristics diffraction peaks of crystal planes (111), (200) and (220) of Pd species at 2θ of 39.67°, 46.0° and 67.02°, respectively. This suggests that palladium species also existed in the form of single element on the surface of α–Al2O3support.
3.1.2 TEM study
XRD characterization confirmed that the palladium loading did not affect the structure of support, and palladium existed in the form of single element. TEM characterization was carried out in order to further study the surface morphology of the catalyst as well as the concentration and particle size of palladium particles.
Figure 3 shows HRTEM images of Pd/MWCNTs catalysts. It can be seen from Figure 3 that the MWCNTs supported Pd species retain a good tubular morphology.

Figure 3 HRTEM images of Pd/MWCNTs catalysts
We can see from the HRTEM images by comparing Pd/ MWCNTs-C and Pd/MWCNTs-B that Pd nanoparticles are supported on the inner wall of MWCNTs in the Pd/ MWCNTs-C catalyst. However, the distribution of Pd nanoparticles is more uniform, and the Pd nanoparticles are supported on the outer and inner walls in the Pd/ MWCNTs-B catalyst prepared by the biological reduction method. It can be concluded that Pd nanoparticles had obvious lattice fringes as demonstrated by the highresolution lattice image of the Pd/MWCNTs-B catalyst. Thus, the formation of crystalline Pd was verified, which was consistent with the XRD characterization results. The particle size distribution of Pd nanoparticles existing in the form of single element in Pd/MWCNTs-B and Pd/ MWCNTs-C catalysts was calculated, with the results shown in Figure 5. We can see that when the particle size is small, the range of particle size distribution is narrow, and the average size of Pd particles is about 4 nm in the Pd/MWCNTs-B catalyst. We also can see that when the particle size is big, the range of particle size distribution is broader, and the average size of Pd particles is about 14.8 nm in the Pd/MWCNTs-C catalyst.
Figure 4 shows HRTEM images of Pd/α-Al2O3catalyst. It can be seen from Figure 4 that α-Al2O3-supported Pd retains a good morphology. By comparing TEM images of Pd/α-Al2O3-C and Pd/α-Al2O3-B we can know that palladium nanoparticles supported on the surface of α-Al2O3can agglomerate to certain extent to form larger particles than that prepared by the biological method. However, palladium particle distribution in Pd/α-Al2O3-B catalyst prepared by the biological reduction method is more uniform and the particle is finer. This phenomenon may be attributed to the existence of biomass which makes the distribution of active components of catalyst more uniform during its preparation by the biological method, and Pd species exist in the form of single element in a reduced state. Thus, the agglomeration of active components was effectively avoided during the process of calcination. It should be noted that Pd supported on α-Al2O3at first existed in an ionic form, then turned to be single elemental Pd after calcination and reduction that took place in chemical immersion method. However, Pd was reduced directly on the support upon being treated by the biological reduction method. The two preparation methods were different in nature. It can be concluded that the supported Pd nanoparticles had obvious lattice fringes upon analyz-ing the high-resolution lattice images of Pd nanoparticles obtained by the biological reduction method. This outcome confirmed the existence of crystalline Pd, which was consistent with the XRD characterization results.

Figure 4 HRTEM images of Pd/α- Al2O3catalysts

Figure 5 Particle size distribution for supported Pd nanoparticles Frequency
3.1.3 Specific surface area
The specific surface area and pore volume of two series of catalysts were characterized, and the results are listed in Table 1.
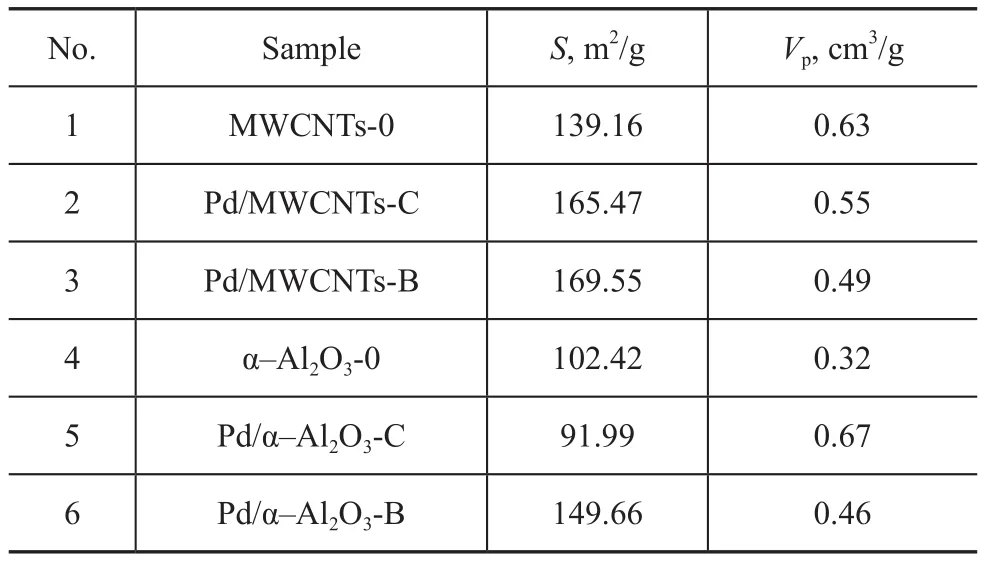
Table 1 BET surface area measurements
It can be seen from Table 1 that the specific surface of catalyst samples varies to different extent ranging from 139.16 m2/g for support itself to 165.47 m2/g for catalyst prepared by the chemical method and 169.55 m2/g for catalyst prepared by the biological method due to the use of different methods for supporting palladium with MWCNTs. The reason is that the carbon nanotube is characteristic of a tubular structure. The overall specific surface of catalyst increases due to large specific surface area of the palladium particles that are deposited on inner and outer walls of the nanotubes. The specific surface area of catalyst prepared by the biological method was larger than that of catalyst prepared by the chemical method (as evidenced by comparison between Pd/MWCNTs-C and Pd/MWCNTs-B). The reason may be that Pd particles prepared by the biological method were larger in dimension, and were deposited on the inner and outer walls in a reduced state, leading to larger specific surface area. The pore volume of palladium catalyst with MWCNTs functioning as the support was smaller. This is because Pd particles supported on MWCNTs were embedded inside the carbon nanotubes, resulting in the blocking of a part of carbon nanotubes. Some carbon nanotubes were hollow, leading to the reduction of effective pore volume. This finding was consistent with the results shown by TEM measurements.
For catalysts supported on α-Al2O3, the specific surface area of Pd/α-Al2O3-C prepared by the chemical method was 91.99 m2/g, which was lower than 102.42 m2/g for the α-Al2O3-0 support. However, the specific surface area of Pd/α-Al2O3-B prepared by the biological method was 149.66 m2/g, which was greater than the α-alumina support. This is probably because palladium particles prepared by the chemical method agglomerated on the surface of α-Al2O3during the process of calcination, and the particle size was greater, which blocked a part of the carbon nanotubes. The palladium nanoparticles supported on the surface were mainly in the form of single element on the Pd/α-Al2O3-B catalyst, and its particle size was small, which would not cause the blocking of nanotubes. The protective effect of biomass could be better utilized, so agglomeration would not easily take place during calcination. Due to large specific surface area of small particles, the overall specific surface area of the catalyst increased. The increase of pore volume was probably due to the acid etching effect of palladium nitrate solution (with a pH value of 1.0) on the surface of support during the process of impregnation, leading to an increased pore volume[10]. The pore volume of Pd/α-Al2O3-B was smaller compared with Pd/α-Al2O3-C due to the buffering effect of biomass, which could weaken the acid etching effect during the process of catalyst preparation by the biological method.
3.2 Evaluation of catalyst activity
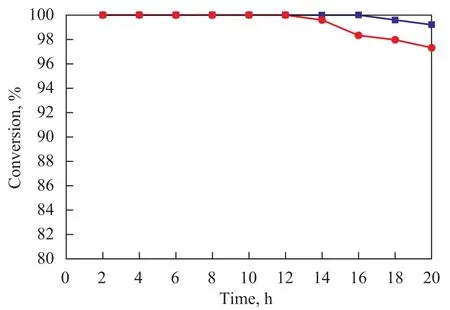
Figure 6 Effect of reaction time on conversion of styrene
The conversion of styrene during catalytic hydrogenation reaction on Pd/α-Al2O3-C and Pd/α-Al2O3-B catalysts is shown in Figure 6.It can be seen from Figure 6 that two catalysts have a high activity and stability in catalytic hydrogenation reaction of styrene. The activity of each catalyst is still high even after continuous reaction for 20 h. The catalytic performance of catalyst prepared by the biological method is better. The space velocity on styrene hydrogenation catalyst was increased from 20 h-1to 200 h-1in order to evaluate the catalytic activity of various catalysts. The activities of 6 catalyst samples were evaluated, and the results are shown in Figure 7. It can be seen from Figure 7 that the catalytic activity of catalysts varies significantly. The catalytic activity of MWCNTs-0 and Pd/α-Al2O3-0 comprising pure support is close to zero, indicating that pure support materials MWCNTs and α-Al2O3have no catalytic activity.

Figure 7 Effect of different catalysts on conversion of styrene
Upon comparing Pd/MWCNTs-C, Pd/MWCNTs-B and Pd/α-Al2O3-C and Pd/α-Al2O3-B catalysts, it can be learned that the catalytic activity of catalyst using MWCNTs as the support was higher than that of catalyst using α-Al2O3as the support, which might be probably attributed to the nanometer effect of carbon nanotubes. The structure of carbon nanotube itself provides a larger specific surface area, which significantly increases the support area and contributes to the uniform distribution of active component of catalyst. This view has already been demonstrated from previous characterizations. Therefore, the catalysts using MWCNTs as the support have a higher activity based on the same amount of catalyst support.
The results showed that Pd/MWCNTs-C and Pd/ MWCNTs-B catalysts all had a higher activity with carbon nanotubes serving as the support. The high activity of these two catalysts identified at the beginning of reaction was resulted from the hydrogen reduction of catalysts. Pd/ MWCNTs-B catalyst began to show a much higher activity than that of Pd/MWCNTs-C catalyst in 2.5 h after the start of run. The reason is that the dispersion of palladium particles prepared by the biological reduction method was better, resulting in a uniform distribution of palladium on the inner and outer walls of carbon nanotubes with high stability. However, the stability of Pd/MWCNTs-C catalyst prepared by the chemical method was lower, which was consistent with the results obtained from TEM characterization. The activity of two catalysts all decreased due to the loss of active component with the extension of reaction time. Although the activity of both of them decreased, the activity of Pd/MWCNTs-B was slightly higher than that of Pd/MWCNTs-C after 2.5 h of reaction. In general, the activity of catalysts prepared by the biological method was higher than that of catalysts prepared by the chemical method.
The activity of Pd/α-Al2O3-B catalyst prepared by the biological method was even higher than that of Pd/α- Al2O3-C catalyst prepared by the chemical method as indicated by the two curves of catalysts using α-Al2O3as the support.
4 Conclusions
1) MWCNTs containing no functional groups can be used as a good support for palladium catalyst. The MWCNT-supported catalyst had a higher activity compared with traditional Al2O3-supported catalyst, and had revealed considerable development potential.
2) The biological reduction method using ginkgo leaf extract can directly reduce Pd2+ions to Pd0species. The biological method has the advantages of good dispersion, small particle size of active component and low agglomeration of Pd particles.
3) Palladium catalysts prepared by the biological reduction method had higher activity and better stability than that of catalysts prepared by the chemical method under the reaction system adopted by this study.
[1] Klefenz H. Nanobiotechnology: From molecules to systems[J]. Engineering in Life Science, 2004, 4(3): 211-218
[2] Zheng Bingyun, Huang Jiale, Sun Daohua, et al. Research progress on biosynthetic technology of noble metal nanomaterials[J]. Journal of Xiamen University (Natural Science), 2011, 50(2): 378-386 (in Chinese)
[3] Fu Jinkun, Liu Yueying, Fu Jinyin, et al. Preparation of supported palladium catalyst by biochemical method [J]. Journal of Xiamen University (Natural Science), 2000, 39(1): 67-71 (in Chinese)
[4] Huang Jiale. Plant-mediated synthesis of silver and gold nanomaterials by biomass-based reduction and their potential applications[D]. Xiamen: Xiamen University, 2009 (in Chinese)
[5] Huang J Y, Li Q B, Sun D, et al. Biosynthesis of silver and gold nanoparticles by novel sundried cinnamomum camphora leaf [J]. Nanotechnology, 2007, 18(10): 105104
[6] Ye X R, Lin Y H, Wai C M. Decorating catalytic palladium nanoparticles on carbon nanotubes in supercritical carbon dioxide[J]. Chem Commun, 2003(5): 642-643
[7] Yu R, Chen L, Liu Q, et al. Platinum deposition on carbon nanotubes via chemical modification[J]. Chem Mater, 1998, 10(3): 718-722
[8] Li Xueting, Zang Pengyuan, Ye Qiuming, et al. Palladium on multi-walled carbon nanotubes (Pd/MWCNTs): Preparation and application in Heck reaction[J]. Chinese Journal of Inorganic Chemistry, 2011, 27(8): 1550-1554 (in Chinese)
[9] Cao Youming, Wang Zhiyong. Preparation and catalytic properties of SWNTs-supported Pd catalyst[J]. Acta Phys-Chim Sin, 2009, 25(5): 825-828 (in Chinese)
[10] Liu Huiping. Synthesis and characterization of novel mesoporous Al2O3and catalytic hydrogenation performance of Pt supported on mesoporous Al2O3[D]. Shanghai: East China University of Science and Technology, 2009 (in Chinese)
Recieved date: 2013-01-31; Accepted date: 2013-03-30.
Professor Fu Jiquan, Telephone: +86-10-64288291; E-mail: fujq010@sina.com.
杂志排行
中国炼油与石油化工的其它文章
- Preparation and Catalytic Performance of Potassium Titanate Used as Soot Oxidation Catalyst
- Effects of Fatty Acids on Low-Sulfur Diesel Lubricity: Experimental Investigation, DFT Calculation and MD Simulation
- Influence of Carbon Content on S Zorb Sorbent Activity
- Propylene Polymerization Catalysts with Sulfonyl Amines as Internal Electron Donors
- Isolation and Characterization of a Thermophilic Oil-Degrading Bacterial Consortium
- A Probe into Process for Maximization of Low-carbon Olefins via Co-processing of Methanol and Heavy Oil
