Influence of the Alkali Treatment of HZSM-5 Zeolite on Catalytic Performance of PtSn-Based Catalyst for Propane Dehydrogenation
2013-07-25HuangLiZhouShijianZhouYumingZhangYiweiXuJunWangLi
Huang Li; Zhou Shijian; Zhou Yuming; Zhang Yiwei; Xu Jun; Wang Li
(1. School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189; 2. Nanjing Wolaide Energy Technology Co., LTD, Nanjing 210042)
Influence of the Alkali Treatment of HZSM-5 Zeolite on Catalytic Performance of PtSn-Based Catalyst for Propane Dehydrogenation
Huang Li1,2; Zhou Shijian1; Zhou Yuming1; Zhang Yiwei1; Xu Jun2; Wang Li2
(1. School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189; 2. Nanjing Wolaide Energy Technology Co., LTD, Nanjing 210042)
The porous material ATZ with micro-mesopore hierarchical porosity was prepared by alkali treatment of parent HZSM-5 zeolite and applied for propane dehydrogenation. The zeolite samples were characterized by XRD, N2-physisorption, and NH3-TPD analysis. The results showed that the alkali treatment can modify the physicochemical properties of HZSM-5 zeolite. In this case, the porous material ATZ showed larger external surface area with less acid sites as compared to the HZSM-5 zeolite. It was found out that the alkali treatment of HZSM-5 zeolite could promote the catalytic performance of PtSn/ATZ catalyst. The possible reason was ascribed to the low acidity of ATZ. Furthermore, the presence of mesopores could reduce the carbon deposits on the metallic surface, which was also favorable for the dehydrogenation reaction.
HZSM-5 zeolite; alkali-treatment; propane dehydrogenation; propene
1 Introduction
The catalytic dehydrogenation of propane is of great interest because of the growing demand for propene used as a basic petrochemical feedstock. Yet dehydrogenation of propane is a highly endothermic and equilibrium limited reaction that requires a relatively high temperature and low pressure to achieve high yield of propene[1-4]. Thus, the catalyst deactivation caused by the coke formation is inevitable because of the rigorous reaction conditions. It is the key to enhance the propene yield by developing new catalysts with high activity, high stability, and high selectivity[5-6].
Supported PtSn-based catalysts are considered to be a suitable kind of catalysts for propane dehydrogenation. In the last decade, supported PtSn-based catalysts have been extensively reviewed. In these bimetallic catalysts, the addition of alkali metals, alkaline earth metals, and rare-earth metals can result in increased catalytic activity, improved product selectivity and extended catalyst lifetime[7-11]. Besides, the adjustment of reaction conditions and the proper method for preparation of catalysts can also improve the catalytic activity of the bimetallic catalysts[12-16]. Recently, modification of physiochemical properties of the carrier is becoming a hotspot in the improvement of the PtSn-based catalysts. Duan Y., et al. upon preparation of Al2O3/SBA-15 catalyst through treating the SBA-15 zeolite with Al(OC3H7)3dissolved in anhydrous toluene have found out that PtSn/Al2O3/SBA-15 catalyst exhibited better catalytic performance in terms of activity and stability than that of PtSn/SBA-15 catalyst[17]. Wang Y., et al. reported a catalyst for propane dehydrogenation, which contained Pt and Sn supported on the mesoporous MgAl2O4modified by ZnO. The results revealed that the ZnO-modified catalysts could increase the Pt dispersion and enhance the interaction between the metal and the support[18]. However, the catalytic activity of PtSn-based catalysts supported on the mesoporous materials was still relatively low.
The ZSM-5 zeolite has been proposed as an efficient support for platinum catalyst used in the propane dehydrogenation reaction[19-20]. Thanks to its large surface area andparticular characteristics of channels, the catalysts using ZSM-5 zeolite as the support have better capacity to accommodate the coke deposition. Unfortunately, it should be noted that the pure ZSM-5 material possesses a large amount of acid sites, which are favorable to coke formation during the dehydrogenation reaction. Furthermore, it is well-known that the average diameter of Pt metal particles in the PtSn/HZSM-5 catalyst is about 16 nm-18 nm, which is too large to enter the main zeolite channels, so that the platinum particles are located mainly on the external surface of the zeolite[21]. As a consequence, the platinum particles are easily covered by coke deposits, leading to deactivation of the catalyst. Our previous work discussed the improvement of the catalytic performance of the PtSn/ZSM-5 catalyst through adding a promoter and using the synthesized heteroatom-containing ZSM-5 zeolite as the support[22-24]. Alternatively, there are heaps of work to do to further improve the catalytic property of the PtSn/ZSM-5 catalyst.
The alkali treatment is a novel and very simple postsynthesis strategy for modifying the ZSM-5 zeolite. Desilication by alkali treatment is a promising approach to achieve substantial mesoporous development in the ZSM-5 zeolite[25-26]. Nowadays investigations are concentrated on the application of the alkali-treated ZSM-5 zeolite in catalytic reaction. Li Y., et al. investigated Zn species on the alkali-treated ZSM-5 zeolite by the conventional liquid ion exchange and impregnation methods, and found out that the alkali-treated Zn/ZSM-5 catalysts displayed dramatically improved catalytic stability during aromatization of 1-hexene[27]. Hao K., et al. modified the ZSM-5 zeolite through alkali treatment and Fe-Tiloading. The results showed that the coexistence of mesomicroporosity and Fe-Ti-loading on ZSM-5 zeolite was beneficial to improvement of light olefins yield during catalytic cracking[28]. According to Su L., et al.[29], the Mo-modified catalysts derived from the alkali treatments showed a very high catalytic performance in the conversion of methane to aromatics (MDA) as compared with the conventional Mo/HZSM-5 catalyst. To the best of our knowledge, there is little literature information about the application of alkali-treated ZSM-5 zeolite in the propane dehydrogenation reaction.
In the present work, the as-synthesized ZSM-5 zeolite was treated in NaOH solution. Then the alkali-treated ZSM-5 zeolite was used as the support of the PtSn-based catalyst for propane dehydrogenation. The PtSn/HZSM-5 catalyst used as a reference was also tested. The goal of the present work was to investigate the effect of alkali treatment of ZSM-5 zeolite on the physicochemical characteristics and catalytic performance of the PtSn-based catalysts in the dehydrogenation of propane.
2 Experimental
2.1 Alkali treatment of HZSM-5 zeolite
The ZSM-5 zeolite was hydrothermally synthesized and the reagents used in the synthesis included silica sol, sodium meta-aluminate, NaOH, hexanediamine and distilled water. The molar composition of the gel was (60—100) SiO2: (0.15—0.3) Al2O3: 3.5 NaOH: 1400 H2O: 15 NH2(CH2)6NH2. Then, the mixture was transferred into a Teflon-lined stainless-steel autoclave and heated at 160 ℃ for 24 h. The as-synthesized zeolite was calcined at 550 ℃ in air for 8 h to decompose the organic template, followed by ion-exchanging with a 1 mol/L NH4Cl solution twice. The proton form of ZSM-5 zeolite (HZSM-5) was obtained by calcining the ammonium form of ZSM-5 zeolite at 580 ℃ for 6 h.
The alkali treatment procedure was carried out as follows: 10 g of HZSM-5 zeolite was dispersed in 50 mL of 0.8 mol/L NaOH solution. The mixture was stirred at 80 ℃for 2 h, and subsequently was cooled down immediately and filtered. Then, the alkali-treated zeolite was washed with deionized water to remove the remaining NaOH, followed by drying at 120 ℃ for 10 h. Finally, the sodiumtype zeolite was ion-exchanged two times with 1 mol/L NH4Cl solution. The proton form of zeolite was obtained by calcining the ammonium form of ZSM-5 zeolite at 580 ℃ for 6 h. The proton form of alkali-treated ZSM-5 zeolite was designated as ATZ.
2.2 Catalyst preparation
The PtSn/HZSM-5 and PtSn/ATZ catalysts were prepared by the sequential impregnation method in our laboratory according to a procedure referred to in our previous work[17,19]. The nominal composition of each sample included 0.5% of Pt, and 1.0% of Sn, respectively. Afterhaving been totally dried, the catalysts were fully agglomerated with 5% of alumina during the process of agglomeration. After drying, the catalysts were calcined at 500 ℃ for 5 h, and finally reduced under H2at 500 ℃for 8 h.
2.3 Catalyst characterization
The X-ray diffraction (XRD) patterns of different catalyst samples were obtained on a XD-3A X-ray powder diffractometer equipped with a copper anode tube. The Kα radiation was selected with a diffracted beam monochromator. An angular range 2θ from 5° to 50° was recorded using step scanning and long counting times to determine the positions of the ZSM-5 peaks.
The nitrogen adsorption-desorption isotherms were measured at -196 ℃ on a Micromeritics ASAP 2000 apparatus. Before measurements, the samples were degassed at a temperature of 300 ℃ and a vacuum of 1×10-3torr. The pore size distribution curve was derived from the analysis of the desorption branch of the isotherm. The specific surface area of the catalyst was obtained using the BET method. The micropore volume, mesopore volume, and external surface area were calculated by means of the tplot method. The pore size was reported as the average pore diameter of the sample.
The surface acidity of the catalyst was determined by NH3-TPD measurements using a Micromeritics ASAP 2750 apparatus. The sample (0.15 g) was preheated at 500 ℃ for 1 h, and then cooled to room temperature in a flowing He stream. At this temperature, sufficient pulses of NH3were injected until adsorption saturation. TPD analysis was carried out in a temperature range from 100 ℃ to 550 ℃ with a heating rate of 10 ℃ /min using helium (30 mL/min) as the carrier gas.
X-ray photoelectron spectra (XPS) measurements were carried out in a Thermo ESCALAB 250 instrument (USA) using non-monochromatic Al Kα 1486.6 radiation. All samples were reduced in situ under hydrogen at 500 ℃ for 1h. Then the spectra were recorded at room temperature under a reduced pressure of less than 5×10-9mbar. The binding energy values of the XPS signals were calibrated by using the Si 2p peak at 102.9 eV.
The amount of coke deposited on the catalyst was measured by means of the LCT thermogravimetric and differential thermoanalysis (TG-DTA). The sample, ca. 15 mg, was placed in a Pt cell and heated from room temperature to 800 ℃ at a heating rate of 10 ℃/min with a gas (air) feed rate of 50 mL/min.
Temperature-programmed oxidation (TPO) analysis was conducted with the same apparatus as that used for NH3-TPD analysis. About 0.05 g of sample was placed in a quartz reactor at room temperature, and then heated up to 700 ℃ at a heating rate of 10 ℃/min in a 5%O2/He mixture (at a flowrate of 30 mL/min).
2.4 Catalytic evaluation
Propane dehydrogenation was carried out in a conventional quartz tubular micro-reactor. The catalyst (with a mass of 1.5 g) was placed at the center of the reactor. The reaction was carried out at a temperature of 590 ℃, a pressure of 0.1 MPa, a H2/C3H8molar ratio of 0.25, and a weight hourly space velocity (WHSV) of 3.0 h-1. The reaction products were analyzed with an online gas chromatograph GC-14C equipped with an activated alumina packed column and a flame ionization detector (FID). The conversion of propane (X) is defined as the percentage of propane converted to all different products. The selectivity to propene (S) is defined as the amount of propene obtained divided by the amount of reactant converted to all products.
3 Results and Discussion
3.1 Zeolite characterization
Figure 1 shows the XRD patterns of different zeolite samples. The HZSM-5 zeolite showed a typical characteristic pattern of MFI structure. After alkali treatment, the structure of ATZ is similar to that of HZSM-5 zeolite, indicating that the characteristic structure of zeolite is not destroyed during the alkali treating process. However, the intensities of the corresponding peaks at angle 2θ=22°—24° decreased, denoting that the crystallinity of ATZ was degraded.
The nitrogen adsorption-desorption isotherms of different samples are shown in Figure 2. The parent HZSM-5 zeolite represented a type-I isotherm with a small N2uptake at higher relative pressure, which was typical of microporous materials without many mesopores. The isothermof ATZ was a typical Ⅳ isotherm exhibiting a hysteresis loop, which was usually associated with the filling and emptying of mesopores by capillary condensation[20]. The pore size distributions are also illustrated in Figure 2. The mesopores with a pore size of around 3.8 nm were formed after the alkali treatment of HZSM-5 zeolite.

Figure 1 XRD patterns of different zeolite samples
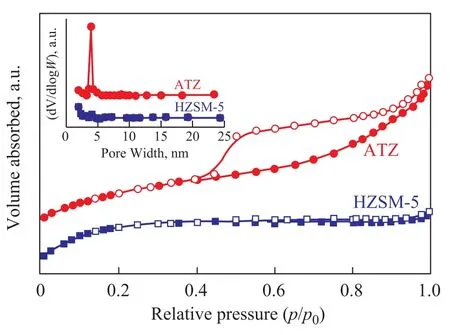
Figure 2 Nitrogen adsorption-desorption isotherms of different samples. The inlet is the pore size distribution of HZSM-5 before and after alkali treatment
Textural properties of samples calculated by the nitrogen desorption isotherms are listed in Table 1. It can be seen from Table 1 that N2sorption data show a reduction in the BET surface area (SBET), the surface area of micropores (Smicro), and the micropore volume (Vmicro) after the alkali treatment. In contrast, the external surface area (Sext) and the total pore volume (Vtotal) increased drastically, which could be attributed to the presence of surface cracks and defects resulted from the dissolution of framework silica and/or alumina atoms in zeolite surface. This may be explained by the fact that the treatment with alkali has dissolved a part of pore walls, resulting in the formation of mesopores[30].

Table 1 Characterization of data for different samples
The NH3-TPD profiles of different samples are displayed in Figure 3. The TPD curve for HZSM-5 zeolite exhibits two desorption peaks in the profile: the first peak is located at 250 ℃ and the other is at 480 ℃, attesting to the presence of weak acid sites and strong acid sites on the surface of zeolite, respectively[31]. As for ATZ, a distinct decrease of ammonia desorption at high temperature are observed, suggesting that the alkali treatment can lead to the decrease in the amount of strong acid sites in the HZSM-5 zeolite. Furthermore, the lower-temperature desorption peak areas decrease slightly. According to Ni Y., et al.[32], these phenomena are caused by removal of a few Al atoms that occurs concurrently with the preferential removal of Si atoms from the HZSM-5 framework. These results are in agreement with those reported by Hao K.[28]
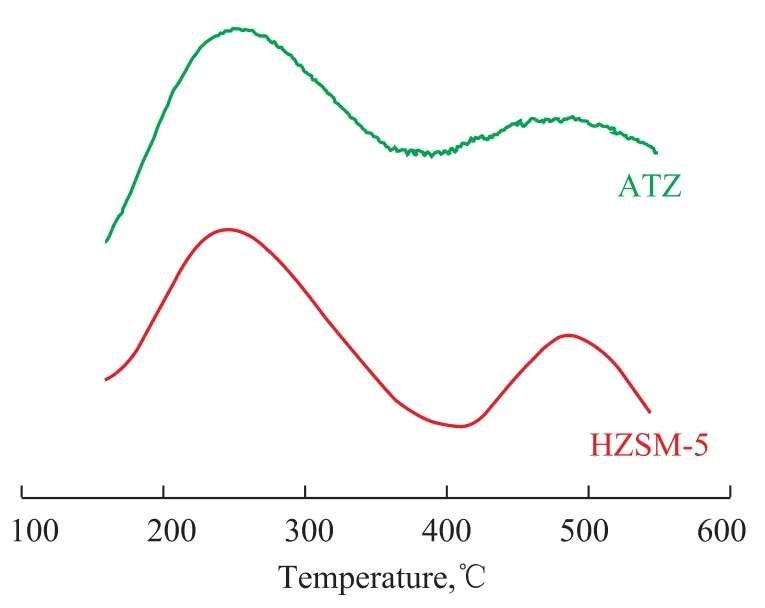
Figure 3 NH3-TPD profiles of different samples
On the basis of our previous study[2], the removal of framework aluminum atoms could weaken the interaction between Sn atoms and the support. Under these circumstances, the Sn species were more readily reduced and more Sn0species could be produced, which would lead to an irreversible deactivation of the catalyst. In order to obtain information about the oxidation state of the metal phase (Sn) on the catalyst surface, characterization of different catalysts by XPS was carried out. Figure 4 shows the Sn3d XPS region of different catalysts and the measured binding energies of the Si2p, Al2p, and Sn3d5/2levels are summarized in Table 2. It should first be notedthat in both spectra, the components at binding energies of 486.3 eV and 486.5 eV can be found, which are associated with a reduced tin phase, either in the metallic (Sn0) state or in the alloyed (SnPtx) state. On the other hand, two other signals can be observed at binding energies of 487.0 eV and 487.8 eV for the PtSn/HZSM-5 catalyst, which correspond to SnO and/or SnO2, respectively. Unfortunately, discrimination between SnO and SnO2is not possible by means of the XPS studies alone. Therefore, the only conclusion from these experiments is that most amount of tin exists exclusively in the oxidized form. With regard to the PtSn/ATZ catalyst, these two peaks are found at binding energies of 487.4 eV and 488.1 eV, respectively, which correspond to the oxidized tin species (SnO and/or SnO2). It can be seen from the data presented in Table 2 that the percentage of Sn0(26.7%) in the PtSn/ ATZ catalyst is slightly more than that in the PtSn/HZSM-5 catalyst (26.1%), denoting that the state of Sn species is almost free from the influence caused by the dealumination of HZSM-5 zeolite after the alkali treatment.

Figure 4 XPS profiles of different catalysts.
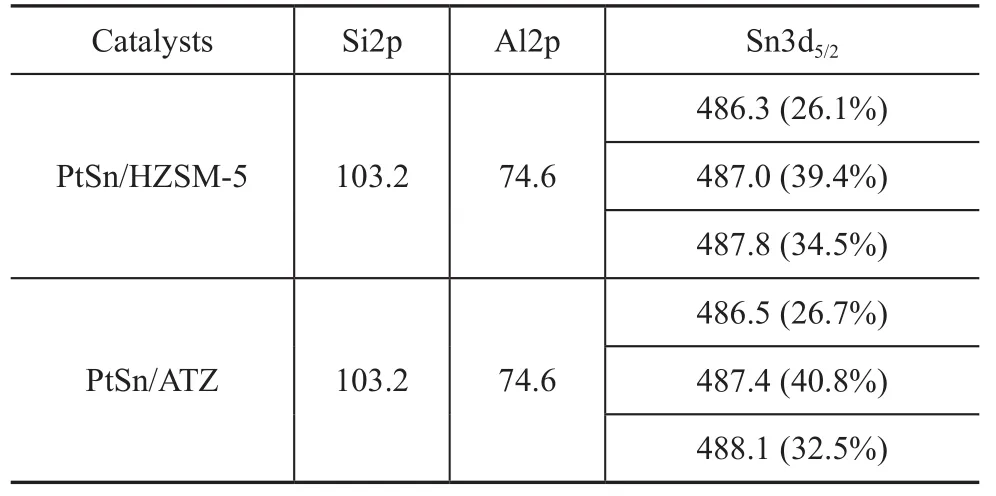
Table 2 XPS binding energies for the Si2p, Al2p and Sn3d5/2measured for different catalysts
3.2 Catalytic performance
Catalytic performance of different catalysts is shown in Figure 5. It can be seen from Figure 5 (a) that the propene yield of the PtSn/ATZ catalyst is relatively higher than that of the PtSn/HZSM-5 catalyst. After the dehydrogenation reaction had proceeded for 15 h, the propene yield of PtSn/HZSM-5 catalyst decreased from 14.85% to 9.54%. By contrast, the final propene yield of PtSn/ATZ catalyst after taking part in dehydrogenation reaction for 15 h was 11.55%. Beside this fact, it is interesting to note that the propene yield of both catalysts increased gradually, with the time-on-stream (TOS) increasing from 0 to 7 h. And then they decreased when the TOS increased from 7 h to 15 h. Figure 5 (b) shows the ethene yield of different catalysts. In all runs, the yield of ethene declined distinctly after the catalysts took part in the dehydrogenation reaction for 7 h. The tendency became slower with an increasing reaction time. The PtSn/HZSM-5 catalyst demonstrated a relatively high ethene yield.

Figure 5 Propene yield (a) and ethene yield (b) versus reaction time on different catalysts
To explain this finding, it should be emphasized that platinum in the bimetallic PtSn catalyst is the only active metal and propene is only formed on the metal through dehydrogenation; the main cracking product (ethene) ismainly formed via cracking reaction on the carrier and ethane is formed by hydrogenolysis of propane and by hydrogenation of ethene, with both reactions taking place on the metal[33]. Furthermore, the dehydrogenation of propane is assumed to proceed through carbonium-ion intermediates and the stronger acid sites generally promote the subsequent cracking reaction of the initially formedcarbenium ions[34]. The change of catalyst acidity may influence the catalytic performance effectively. In view of the NH3-TPD analysis, the HZSM-5 zeolite possesses more acidity amount than ATZ, especially in the strong acid sites. While the impregnation of Pt and Sn can hardly neutralize the acidity of HZSM-5 zeolite[8], the amount of acid sites on the PtSn/HZSM-5 catalyst is still more than that of the PtSn/ATZ catalyst. Consequently, the high acidity of PtSn/HZSM-5 catalyst is responsible for its higher yield of ethene.
At the beginning of the reaction, the rapid coking occurred in the metallic sites and the acid sites of the support that were active in the hydrogenolysis and cracking reactions, resulting in a gradual covering of the active sites and the acid sites of the support by carbon deposits. As a result, the side reactions are inhibited to some extent. According to this mechanism, the cracking reactions after 7 h on stream decrease apparently, leading to an increase in the propene yield and a decrease in the ethene yield during the reaction.
3.3 Coke analysis
Generally speaking, the carbon formed during the reaction can cover the active metal atoms, which is the main cause of catalyst deactivation. The coke deposition on the surface of different used catalysts was determined by TGDTA, and the corresponding data are depicted in Figure 6. The PtSn/HZSM-5 catalyst exhibited a 13.5% of coke deposits after having been subjected to dehydrogenation reaction for 15 h. In contrast to this, when the HZSM-5 zeolite was alkali treated, a less amount of coke (9%) was observed. Figure 7 shows the TPO profiles of different catalysts. There are two successive peaks representing two different carbon deposits in TPO pro file. The carbon deposits corresponding to the first peak at low temperature are the main one that covers the active metal, while the second peak at high temperature represents the coke located on the external surface of the support[8].

Figure 6 Coke amount deposited on different catalysts
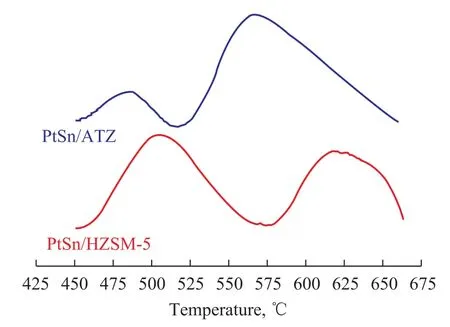
Figure 7 TPO profiles of different catalysts
It can be seen from Figure 7 that on the PtSn/HZSM-5 catalyst, both of the active metal atoms and the external surface of the support were covered by coke. Compared with the PtSn/HZSM-5 catalyst, the alkali treatment of the HZSM-5 zeolite had shifted the coke deposition peaks on both of the active metal atoms and the carrier surface towards the lower temperature region, suggesting that the deposited carbon had became more reactive. Depending on these findings and the TG-DTA analysis, it can be concluded that the alkali treatment of the HZSM-5 zeolite can decrease the intensity of coke deposition and reduce the accumulation of coke.
According to Afonso, et al.[35], olefins are primary precursors of the mechanism of coke formation. The intrinsic acidity of the support could promote the undesirable reactions such as cracking/isomerization to increase the carbon deposits. As it has been mentioned before, the alkali treatment could decrease the acidity of the HZSM-5 zeolite, which in consequence would reduce the amount and intensity of coke buildup. In addition, the relatively largeSextof ATZ may facilitate the coke precursors desorp-tion, and therefore, lead to a lower carbon deposition and weaker carbon formation intensity. Similar results have been obtained by Liu Z., et al.[28]
It can also be obviously seen from Figure 7 that on the PtSn/ATZ catalyst, most coke deposits are located on the external surface of the support. It is reasonable to deduce that a few Pt particles are located in the mesopores of ATZ, thus avoiding the rapid covering by carbon deposits. This phenomenon might be the reason why the PtSn/ ATZ catalyst exhibits higher propene yield than the PtSn/ HZSM-5 catalyst.
In summary, ATZ is a more attractive support material than the pure HZSM-5 zeolite for propane dehydrogenation. However, the propene yield of the PtSn/ATZ catalyst is still too low. According to our previous work[7-9], this result is attributed to the high acid amount and low “metallic function” of the PtSn/ATZ catalyst. The addition of promoter can overcome these drawbacks and ultimately improve the catalytic performance of the PtSn/ATZ catalyst. The follow-up work is being conducted.
4 Conclusions
ATZ with micro-mesopore hierarchical porosity was prepared by alkali treatment of the HZSM-5 zeolite and applied for propane dehydrogenation. When the HZSM-5 zeolite was treated by alkali, the total acidity decreased and the external surface area increased, thus reducing the accumulation of coke on the PtSn/ATZ catalyst. The XPS results have indicated that dealumination of HZSM-5 zeolite after the alkali treatment has little impact on the state of Sn species. The TPO analysis has shown that only a small percentage of carbon deposits is located on the metallic surface of PtSn/ATZ catalyst, implying that a part of Pt particles are located in the mesopores, thus avoiding the rapid covering by carbon deposits. In comparison with the traditional HZSM-5 zeolite, ATZ is a better porous material for propane dehydrogenation. In addition, more work should be done to further improve the catalytic performance of the PtSn/ATZ catalyst.
Acknowledgments:The authors are grateful to the Production and Research Prospective Joint Research Project (BY2009153) and Science and the National Nature Science Foundation of China (50873026,21106017) for financial support, and the Specialized Research Fund for the Doctoral Program of Higher Education of China (20100092120047).
[1] Zhang Y, Zhou Y, Qiu A, et al. Effect of alumina binder on catalytic performance of PtSnNa/ZSM-5 catalyst for propane dehydrogenation[J]. Ind Eng Chem Res, 2006, 45: 2213-2219
[2] Zhang Y, Zhou Y, Yang K, et al. Effect of hydrothermal treatment on catalytic properties of PtSnNa/ZSM-5 catalyst for propane dehydrogenation[J]. Microporous and Mesoporous Materials, 2006, 96: 245-254
[3] Cola P, Glaser R, Weitkamp J. Non-oxidative propane dehydrogenation over Pt-Zn-containing zeolites[J]. Appl Catal A: Gen, 2006, 306: 85-97
[4] Kogan S, Schramm H, Herskowitz M. Dehydrogenation of propane on modified Pt/θ-alumina performance in hydrogen and steam environment[J]. Appl Catal A: Gen, 2001, 208: 185-191
[5] Yu C, Xu H, Ge Q, et al. Properties of the metallic phase of zinc-doped platinum catalysts for propane dehydrogenation[J]. J Mol Catal A: Chemical, 2007, 266(1-2): 80-87
[6] Kumar. M, Chen. D, Walmsley. J, et al. Dehydrogenation of propane over Pt-SBA-15: Effect of Pt particle size[J]. Catal Commun, 2008, 9: 747-750
[7] Zhang S, Zhou Y, Zhang. Y, et al. Effect of K addition on catalytic performance of PtSn/ZSM-5 catalyst for propane dehydrogenation[J]. Catal Lett, 2010, 135: 76-82
[8] Zhang Y, Zhou Y, Qiu A, et al. Effect of Na addition on catalytic performance of PtSn/ZSM-5 catalyst for propane dehydrogenation[J]. Acta Phys-Chim Sin, 2006, 22(6): 672-678 (in Chinese)
[9] Huang L, Zhou S, Zhou Y, et al. Effect of strontium addition to platinum catalyst for propane dehydrogenation[J]. China Petroleum Processing and Petrochemical Technology, 2012, 14(3): 75-82
[10] Bai L, Zhou Y, Zhang Y, et al. Effect of magnesium addition to PtSnNa/ZSM-5 on the catalytic properties in the dehydrogenation of propane[J]. Ind Eng Chem Res, 2009, 48: 9885-9891
[11] Rui X, Ning B, Kam C, et al. The effect of lanthanum in dehydrogenation of propane on Pt-Sn bimetallic catalysts[J]. Catal Lett, 1998, 50: 219-223
[12] Fattahi M, Khorasheh F, Sahebdelfar S, et al. The effect of oxygenate additives on the performance of Pt-Sn/γ-Al2O3catalyst in the propane dehydrogenation process[J]. Scientia Iranica, 2011, 18(6): 1377-1383
[13] Nawaz Z, Tang X, Chu Y, et al. Influence of calcination temperature and reaction atmosphere on the catalytic properties of Pt-Sn/SAPO-34 for propane dehydrogenation[J]. Chinese Journal of Catalysis, 2010, 31(5): 552-556
[14] Late L, Rundereim J, Blekkan E. Selective combustion of hydrogen in the presence of hydrocarbons 1. Pt-based catalysts[J]. Appl Catal A: Gen, 2004, 262: 53-61
[15] Liu H, Zhang S, Zhou Y, et al. Effect of ultrasonic irradiation on the catalytic performance of PtSnNa/ZSM-5 catalyst for propane dehydrogenation[J]. Ultrasonic Sonochemistry, 2011, 18: 19-22
[16] Bai L, Zhou Y, Zhang Y, et al. Effect of calcination atmosphere on the catalytic properties of PtSnNaMg/ZSM-5 for propane dehydrogenation[J]. Catal Commun, 2009, 10(15): 2013-2017
[17] Duan Y, Zhou Y, Zhang Y, et al. Effect of aluminum modification on catalytic properties of PtSn-based catalysts supported on SBA-15 for propane dehydrogenation[J]. Journal of Natural Gas Chemistry, 2012, 21: 207-214
[18] Wang Y, Wang Y, Wang S, et al. Propane dehydrogenation over PtSn catalyst supported on ZnO-Modified MgAl2O4[J]. Catal Lett, 2009, 132(3/4): 472-479
[19] Kumar M, Holmen A, Chen D. The in fluence of pore geometry of Pt containing ZSM-5, beta and SBA-15 catalysts on dehydrogenation of propane[J]. Microporous Mesoporous Mater, 2009, 126(1): 152-158
[20] Lucas A, Valverde J, Sanchez P, et al. Hydroisomerization of n-octane over platinum catalysts with or without binder[J]. Appl Catal A: Gen, 2005, 282(1/2): 15-24
[21] Bai L, Zhou. Y, Zhang. Y, et al. In fluence of calcium addition on catalytic properties of PtSn/ZSM-5 catalyst for propane dehydrogenation[J]. Catal Lett, 2009, 129: 449-456
[22] Xue M, Zhou Y, Zhang Y, et al. Effects of Mg addition on catalytic performance of PtNa/Sn-ZSM-5 in propane dehydrogenation[J]. Acta Phys-Chim Sin, 2012, 28: 1-9
[23] Huang L, Zhou Y, Zhang Y. Propane dehydrogenation over PtSnNa catalyst supported on La-ZSM-5 zeolite[J]. China Petroleum Processing and Petrochemical Technology, 2010, 12(3): 18-24
[24] Zhang Y, Zhou Y, Huang L, et al. Sn-modified ZSM-5 as supported for platinum catalyst in propane dehydrogenation[J]. Industrial & Engineering Chemistry Research, 2011, 50: 7896-7902
[25] Ogura M, Shinomiya S, Tateno J, et al. Alkali-treatment technique-new method for modification of structural and acid-catalytic properties of ZSM-5 zeolites[J]. Appl Catal A: Gen, 2001, 219: 33-43
[26] Zhao L, Xu C, Gao S, et al. Effects of concentration on the alkali-treatment of ZSM-5 zeolite: a study on dividing points[J]. J Mater Sci, 2010, 45: 5406-5411
[27] Li Y, Liu S, Xie S, et al. Promoted metal utilization capacity of alkali-treated zeolite: Preparation of Zn/ZSM-5 and its application in 1-hexene aromatization[J]. Appl Catal A: Gen, 2009, 360: 8-16
[28] Hao K, Shen B, Wang Y, et al. Influence of combined alkaline treatment and Fe-Ti-loading modification on ZSM-5 zeolite and its catalytic performance in light olefin production[J]. Journal of Industrial and Engineering Chemistry, 2012, 18: 1736-1740
[29] Su L, Zhuang J, Wang H, et al. [J]. Catal Lett, 2003, 91(3-4): 155-167
[30] Choi D, Park J, Kim J, et al. Liquid-phase degradation of HDPE over alkali-treated MFI zeolites with mesopores[J]. Polym Degrad Stab, 2006, 91: 2860-2866
[31] Lobree L, Hwang In-Chul, Reimer J, et al. Investigations of the state of Fe in H-ZSM-5[J]. J Catal, 1999, 186: 242-253
[32] Ni Y, Sun A, Wu X, et al. Preparation of hierarchical mesoporous Zn/HZSM-5 catalyst and its application in MTG reaction[J], Journal of Natural Gas Chemistry, 2011, 20: 237-242
[33] Larsson M, Hulten M, Blekkan E, et al. The effect of reaction conditions and time on stream on the coke formed during propane dehydrogenation [J]. J Catal, 1996, 164(1): 44-53
[34] Katranas T K, Vlessidis A G, Tsiatouras V A, et al. Dehydrogenation of propane over natural clinoptilolite zeolites [J]. Micropo Mesopo Mater, 2003, 61(1/2/3): 189-198
[35] Afonso J C, Schmal M, Frety R. The chemistry of coke deposits formed on a Pt-Sn catalyst during dehydrogenation ofn-alkanes to mono-olefins[J]. Fuel Process Technol, 1994, 41(1): 13-25
[36] Liu Z, Fan W, Ma J, et al. Adsorption, diffusion and catalysis of mesostructured zeolite HZSM-5[J]. Adsorption, 2012, 18: 493-501
Recieved date: 2013-01-21; Accepted date: 2013-04-18.
Professor Zhou Yuming, Telephone: +86-25-52090617; E-mail: ymzhou@seu.edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Isolation and Characterization of a Thermophilic Oil-Degrading Bacterial Consortium
- Quantum Chemistry of PAHs Thermal Cracking with Different Hydrogenation Degree
- Experimental Study on Aqueous Phase Entrainment in a Mixer-settler with Double Stirring Mode
- Performance of Ni-based, Fe-based and Co-based Oxygen Carriers in Chemical-Looping Hydrogen Generation
- Effects of Temperature Gradient and Cooling Rate on the Formation of Methane Hydrates in Coarse Sand
- Development and Commercial Application of Third Generation Resid Hydrotreating Catalysts
