Determining the optimal time for bortezomib-based induction chemotherapy followed by autologous hematopoietic stem cell transplant in the treatment of multiple myeloma
2013-06-12
Department of Hematology, The First Affiliated Hospital, SunYat-sen University, Guangzhou 510080, China
Determining the optimal time for bortezomib-based induction chemotherapy followed by autologous hematopoietic stem cell transplant in the treatment of multiple myeloma
Junru Liu*, Juan Li*, Beihui Huang, Dong Zheng, Mei Chen, Zhenhai Zhou, Duorong Xu, Waiyi Zou
Department of Hematology, The First Affiliated Hospital, SunYat-sen University, Guangzhou 510080, China
Corresponding to:Juan Li, MD, PhD. Department of Hematology, The First Affiliated Hospital, SunYat-sen University, Guangzhou 510080, China. Email: lijuanzy@yeah.net.
In our study, we determined the efficacy of bortezomib-based induction therapy followed by autologous stem cell transplant (ASCT) in newly diagnosed and relapsed/refractory (R/R) multiple myeloma (MM) patients and compared the advantages of early versus late transplant. We used a retrospective analysis to examine 62 patients, including 46 cases of newly diagnosed MM (early transplant group) and 16 cases of relapsed/refractory MM (late transplant group). All of these patients
Multiple myeloma; autologous stem cell transplant; bortezomib; International Staging System stage
Scan to your mobile device or view this article at:http://www.thecjcr.org/article/view/1509/2477
Introduction
The treatment of multiple myeloma (MM) has improved enormously over the last ten years, due to the development and availability of new drugs, such as bortezomib, lenalidomide, and thalidomide (1,2). Before the emergence of these new drugs, Vincristine-Adriamycin-Dexamethasone (VAD) combination chemotherapy followed by an autologous hematopoietic stem cell transplant (ASCT) was the standard therapeutic approach for MM patients qualified for a transplant (3). Compared with traditional therapeutic methods, ASCT can improve the quality of life in MM patients (4).
Studies have demonstrated that ASCT, either as a first-line therapy (early transplant) or as a salvage therapy (late transplant), can improve patients’ survival (5). A prospective study showed that the overall survivals (OS) of early and late transplant were equivalent; however, patients with an early transplant had a longer event-free survival (EFS), a higher quality of life, and required a shorter course of chemotherapy (6). Therefore, early transplant was recommended. However, in the era of new therapeutic drugs, the efficacy of early versus late transplants in MM patients needs to be determined (7,8). A study conducted at the Mayo Clinic investigating the clinical efficacy of early versus late transplant after an immunomodulatory agent-based induction therapy, demonstrated that the time to progression (TTP) of early transplant patients was longer than that of those receiving late transplants (7). The phase III Intergroup Francophone Myeloma/Dana-Farber Cancer Institute trial (IFM/DFCI 2009), which focuses on comparing the clinical benefit of early versus late transplant following Revlimid-Velcade-dexamethasone (RVD)-based induction therapy, has not yet concluded (8).
The emergence of new drugs has had a major impact on MM induction therapy (9). Compared with traditional VAD chemotherapy, bortezomib-based induction therapy can significantly enhance remission quality for patients, and a high quality remission response can further lead to a long progression-free survival (PFS) and overall survival (OS) (9,10). Should patients with a suitable indication for transplant be treated with bortezomib-based induction therapy followed by ASCT immediately after diagnosis? Alternatively, should the patients first be treated with traditional chemotherapy and subsequently treated with bortezomib-based induction therapy followed by ASCT when relapse occurs? Using retrospective analysis, we examined the efficacy and side effects of bortezomib-based induction therapy followed by ASCT on newly diagnosed and relapsed/refractory MM patients who were registered into our clinic between June of 2006 and September of 2011. Our study was designed to provide a reference for clinicians to choose between an early or late transplant for MM patients.
Patients and methods
Patients
A retrospective analysis was performed on 62 MM patients who received a bortezomib-based induction regimen followed by autologous hematopoietic stem cell transplant in our clinic between June 2006 and September 2011, including 46 newly diagnosed patients and 16 relapsed/refractory patients. The previous treatments received by the relapsed/ refractory patients included the VAD regimen, the vincristine + adriamycin + dexamethasone + melphalan (VADM) regimen, the liposomal doxorubicin + vincristine + dexamethasone (DVD) regimen, the fludarabine + mitoxantrone + dexamethasone (FMD) regimen, and thalidomide.
Treatment regimen
Induction therapy
Bortezomib + Dexamethasone (BD) regimen: Bortezomib (Millennium, Inc.) was given at 1.3 mg/m2on days 1, 4, 8, and 11 of each treatment cycle, and Dexamethasone at 20 mg/m2was administered intravenously (IV) on days 1 to 4. Each treatment cycle lasted for 3 weeks. PAD regimen: liposomal adriamycin at 40 mg/m2(Kai Lao, Schering-Plough Healthcare Products, Inc.) was administered with the BD regimen on the fourth day of each treatment cycle. One treatment cycle was 28 days. VDDT regimen: bortezomib at 1.3 mg/m2was given on days 1, 8, 15, and 22 of each treatment cycle. Liposomal doxorubicin at 20 mg/m2was given on days 4 and 18. Dexamethasone was administered intravenously at 20 mg/d on days 1 to 4. Thalidomide was administered at 200 mg QN on days 1 to 28. Each treatment cycle lasted for 28 days.
Autologous hematopoietic stem cell transplant
For patients with normal renal function, peripheral blood stem cell transplant (PBSCT) was selected. For patients with renal insufficiency (serum creatinine >2 mg/dL), autologous bone marrow transplant (ABMT) was selected. A total of 46 patients received PBSCT, and 16 patients received ABMT. Prior to PBSCT, patients were treated with a mobilizing regimen consisting of a combination of cyclophosphamide (CTX) and granulocyte colony-stimulating factor (G-CSF). CTX 3.0-5.0 g/m2was administered on day 1, and G-CSF at 300 µg/d was given daily beginning on day 2 until the stem cell collection procedure was completed. Peripheral blood stem cell collection was performed on days 9 to 12. The conditioning regimen consisted of melphalan at 200 mg/m2. If a patients’ serum creatinine level was higher than 2 mg/dL, the dose of melphalan was reduced to 100-140 mg/m2.
Maintenance therapy
All patients were given the maintenance regimen immediately after the transplant. Maintenance therapyregimen: Thalidomide 200 mg QN was administered. Appropriate dose adjustments were made for patients who were unable to tolerate the dosage.
Efficacy assessments
Patients had a detailed physical examination before and after each treatment regimen, before and after the stem cell mobilization procedure, and before and after transplant. The physical examination included a chest X-ray, electrocardiogram (ECG), bone marrow examination, serum immunofixation electrophoresis, quantitative serum immunoglobulin testing, urinary Bence Jones protein electrophoresis, and quantitative 24 hours urine proteins and light chains examination. Wholebody X-rays were performed every 6 months or when new bone lesions occurred. During chemotherapy and transplant, patients’ liver and kidney function and complete blood count were examined weekly, and adverse reactions were carefully analyzed and recorded. The efficacy was assessed according to the European Blood and Marrow Transplant Group (EBMT) Standard and was classified as complete remission (CR), near complete remission (nCR), partial remission (PR), minimal response (MR), no change (NC), or progressed disease (PD). The side effects and drug toxicity were graded according to the United States National Cancer Institute Common Toxicity Criteria (NCI-CTC, version 3).
Follow-up
All patients had follow-up visits until September 30, 2011. The median follow-up period was 26.5 months (7-61 months).
Statistical analysis
The data were analyzed using the SPSS 13.0 statistical software. Count data were compared using the Chi-Square (χ2) test, continuous data were analyzed using a t-test, and continuous data that were not normally distributed were analyzed using a Wilcoxon rank-sum test. OS was defined as the time from the commencement of bortezomibbased regimen to death for any reason. PFS referred to the length of time after receiving the bortezomib-based regimen to disease progression or death. Survival analysis was performed using the Kaplan-Meier method, and the differences between the survival curves were analyzed using the log-rank test. Logistic regression analysis was used for multivariate stepwise regression analysis. P-values less than 0.05 were considered to be statistically significant.
Results
Patients’ characteristics
The gender, age, disease stage, types of M protein, and the method of receiving transplant were not statistically significant different between the two patient groups (P>0.05,Table 1). The median time from diagnosis to receiving transplant therapy for the newly diagnosed patient group was 5 months (3-10 months) and 20.5 months for the relapsed/refractory patient group (5-67 months).
The efficacy of bortezomib-based induction therapy followed by ASCT on newly diagnosed and relapsed/ refractory MM patients
When the efficacy assessment for the induction therapy was greater than nCR, the patients were given an ASCT. If the efficacy assessment failed to reach a status greater than nCR, the patients were treated with ASCT after 4 courses of induction therapy. The newly diagnosed patient group had 10, 11, and 25 cases of patients receiving 2, 3, or 4 courses of the induction therapy, respectively. The relapsed/ refractory patient group had 7, 5, and 4 cases of patients receiving 2, 3, or 4 courses of the induction therapy, respectively. The median course for the BD regimen was 4 and 3 courses for the newly diagnosed and relapsed/ refractory groups, respectively, which was not a statistically significant difference. The overall response rate (ORR = CR + nCR + PR) of bortezomib-based induction therapy for the newly diagnosed MM patient group was 91.3%, and the ORR was 81.2% for the relapsed/refractory MM patient group. The difference between the two groups was not statistically significant (P=0.361). We further compared the response level greater than nCR between the two groups. The newly diagnosed group had 32 cases of patients whose response to induction therapy was greater than nCR (69.5%); while the relapsed/refractory patient group had 9 cases (56.2%). The difference between the two groups was not statistically significant (P=0.369).
Comparison of the efficacy of ASCT on newly diagnosed and relapsed/refractory MM patient groups
The post-ASCT ORR (CR + nCR + PR) of the newly diagnosed and relapsed/refractory groups was 97.8% and 93.8%, respectively. The newly diagnosed patient group had 39 cases with a post-ASCT remission level higher than nCR (84.8%), while the relapsed/refractory grouphad 13 cases (81.3%) in this category. The differences in ORR and remission level between the two groups were not statistically significant (P=0.453 and 0.709, respectively, seeTable 2). The rate of remission greater than nCR in both groups of patients increased after ASCT. The responses greater than nCR in the newly diagnosed group increased from 69.5% to 84.8%, while the relapsed/refractory group increased from 56.2% to 81.3%. The differences in post-ASCT ORR or CR/nCR between the two groups were not statistically significant (P=0.453 and 0.709, respectively).
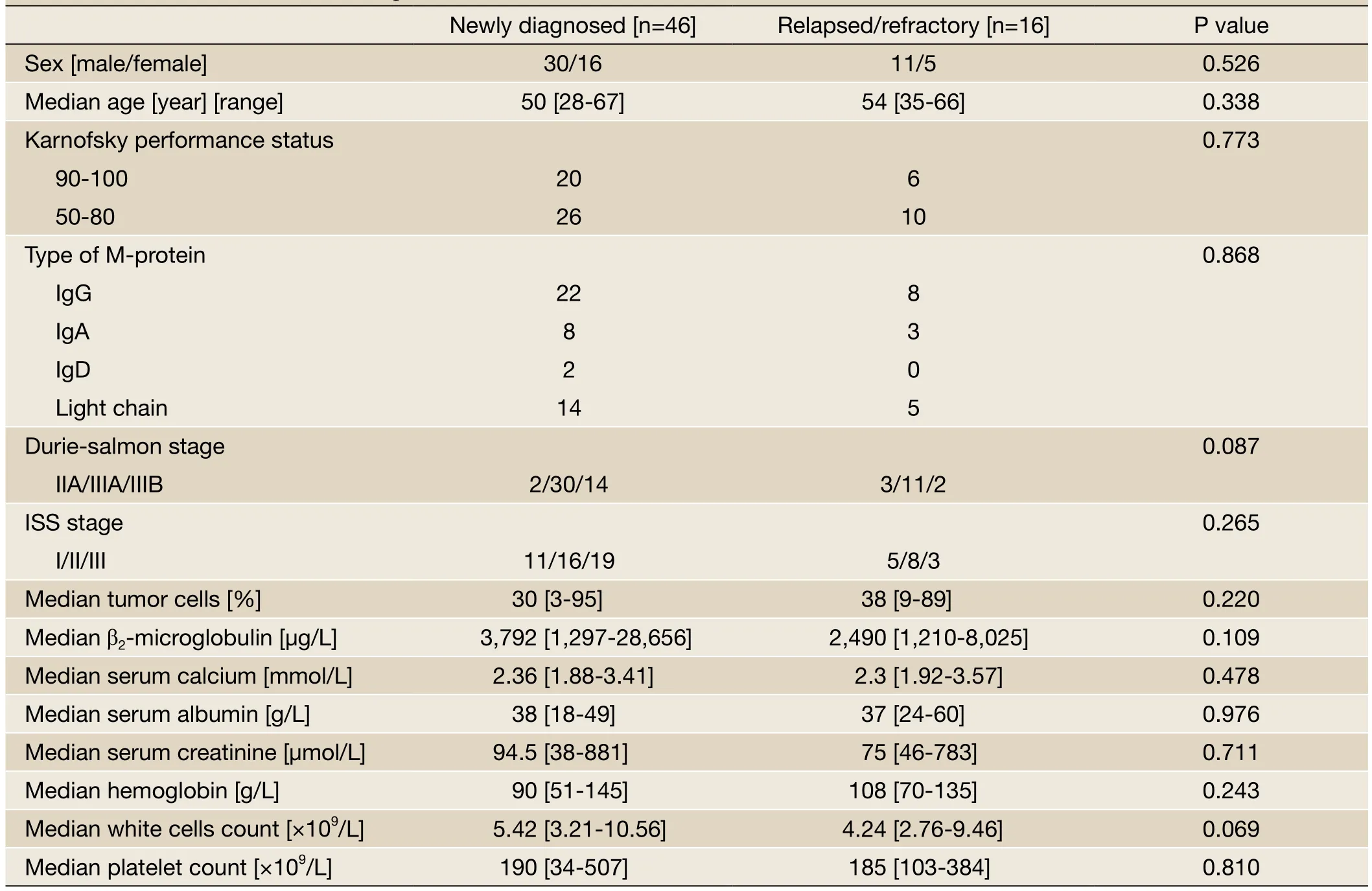
Table 1 Baseline characteristics of all patients
Comparison of the effect of the stem cell collection method on newly diagnosed and relapsed/refractory MM patient groups
The newly diagnosed group had 37 cases of patients receiving a peripheral stem cell transplant and 9 cases receiving a bone marrow transplant. The relapsed/ refractory group had 9 cases of patients receiving a peripheral stem cell transplant and 7 cases receiving a bone marrow transplant. Differences in the responses observed between the two groups receiving the different transplant methods were not statistically different. The number of mononuclear cells (MNCs) collected from the newly diagnosed group was (4.20±1.79)×108/kg, and the number of the CD34+cells was (4.01±1.42)×106/kg. We further analyzed the patient cases in the relapsed/ refractory group who had previously received alkylating agent therapy. There were a total of 10 patients who had previously undergone melphalan chemotherapy, and the median dose of melphalan was 384 mg (48 to 768 mg). The number of MNCs collected from these patients was (2.77±1.29)×108/kg, and the number of the CD34+cells was (2.39±0.32)×106/kg, both of which were statistically significantly different from the newly diagnosed group (P=0.021 and 0.041, respectively). However, there was no statistically significant difference in these values between the newly diagnosed patient group and the relapsed/refractory patients who had not previously undergone alkylating agent therapy (P=0.288 and 0.957, respectively,Figure 1). Thevalues from the relapsed/refractory patients with previous alkylating agent therapy were statistically significantly different compared with that of the relapsed/refractory patients without previous alkylating agent therapy (P=0.016 and 0.047, respectively).
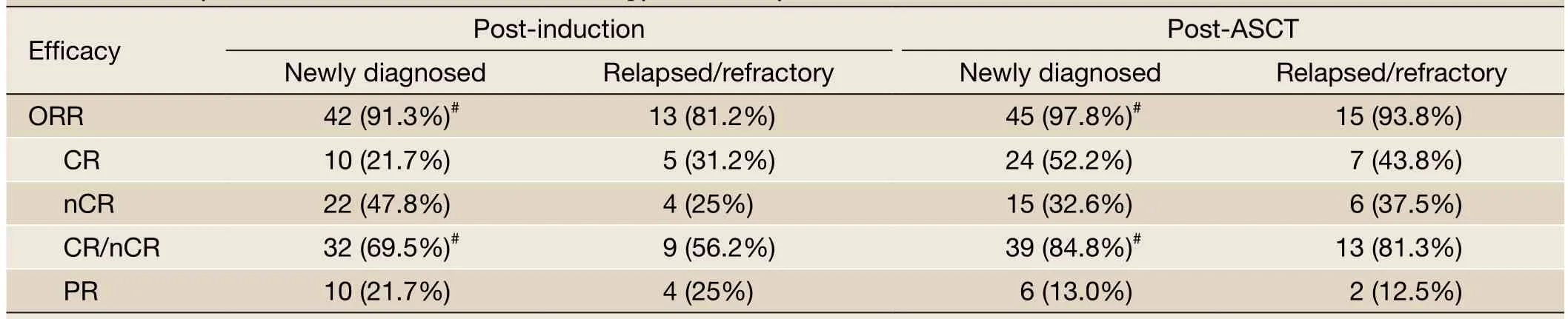
Table 2 Efficacy of bortezomib-based induction therapy followed by ASCT

Figure 1 MNC and CD34+ cells collected from newly diagnosed and R/R MM patients with and without previous exposure to an alkylating agent.#P<0.05vs.newly diagnosed and R/R without exposure to alkylating agent;*P<0.05vs.newly diagnosed and R/R without exposure to alkylating agent
The median time for collecting stem cells from the newly diagnosed group (n=37) and from the relapsed/refractory group (n=9) was 11 days (9 to 13 days) and 10.5 days (9 to 12 days), respectively. There was no statistically significant difference between the two groups (P=0.762). The average number of times stem cells were collected for the newly diagnosed group was 1.32±0.47 and 1.45±0.52 for relapsed/ refractory group. There was no statistically significant difference between the two groups (P=0.780).
Comparison of hematopoietic reconstitution after transplant of the newly diagnosed MM group and the relapsed/refractory MM group
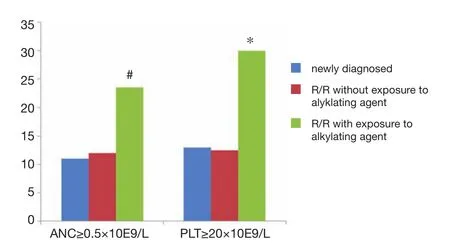
Figure 2 Engraftment of neutrophils and platelets in newly diagnosed and R/R MM patients with and without previous exposure to an alkylating agent.#P<0.05vs.newly diagnosed and R/R without exposure to alkylating agent; *P<0.05vs.newly diagnosed and R/R without exposure to alkylating agent
The median time required for neutrophils to recover to more than 0.5×109/L after transplant in the newly diagnosed group (n=46) was 11 days ( 9 to 23 days) and 14.5 days (11 to 43 days) in the relapsed/refractory group (n=16). The difference between these two values was statistically significant (P=0.003). The median time required for blood platelets (PLT) to recover to more than 20×109/L in the newly diagnosed group was 13 days (0 to 120 days) and 21.5 days (10 to 88 days) in the relapsed/refractory group. There was a statistically significant difference between these two values (P=0.031). The timing of G-CSF application after transplant was 8.5 days (3 to 22 days) and 13 days (8 to 35 days) for the newly diagnosed group and the relapsed/ refractory group, respectively. The difference between the two values was statistically significant (P=0.004, seeFigure 2). We further analyzed the hematopoietic reconstitution in the relapsed/refractory patients who had previously received alkylating agent therapy. The neutrophil recovery time was 23.5 days (11 to 43 days). PLT recovery time was 30 days (11 to 88 days). The timing of G-CSF application was 20 (8 to 35 days). All of these times weresignificantly longer than those in newly diagnosed group and the relapsed/refractory patients that had not previously received alkylating agent therapy (P=0.05).
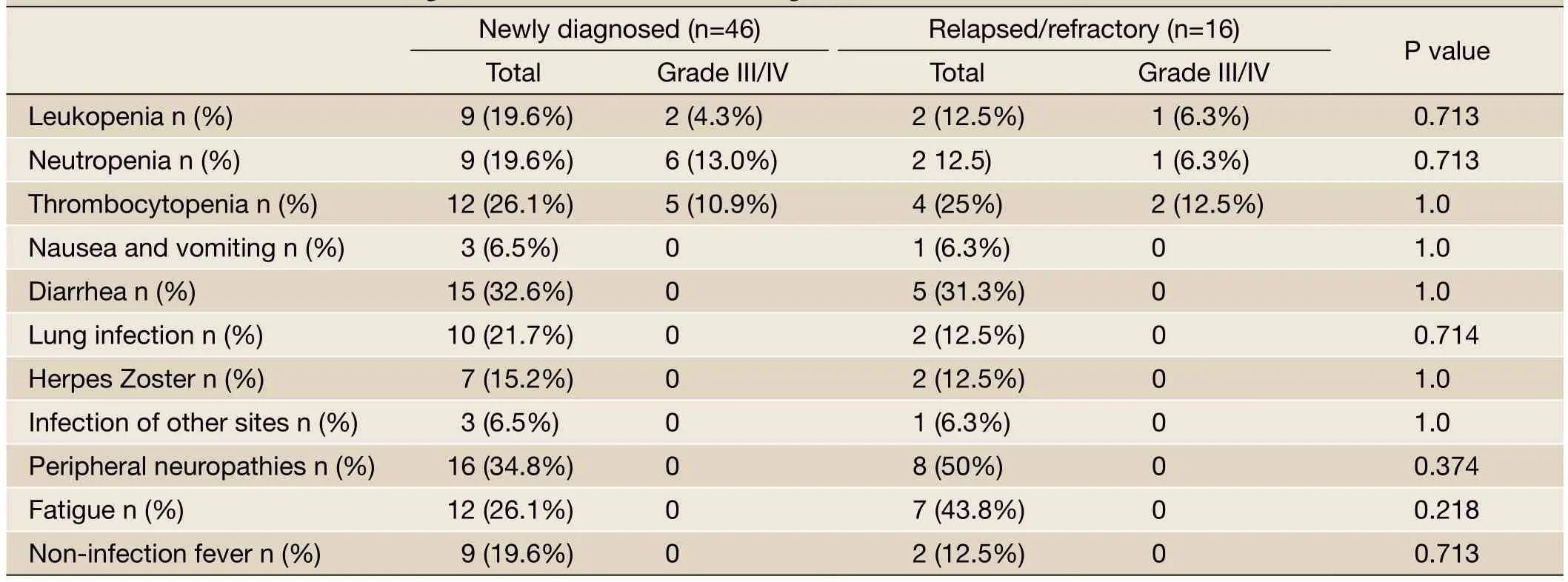
Table 3 Common side effects during bortezomib-based induction regimen
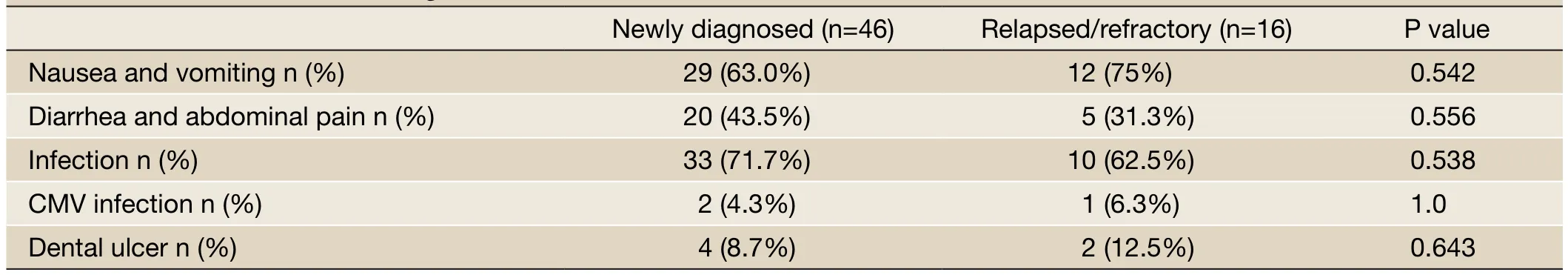
Table 4 Common side effects during ASCT
Comparison of the side effects of bortezomib-based induction therapy and ASCT on the newly diagnosed and relapsed/refractory groups
The common side effects observed during bortezomibbased induction therapy included diarrhea, fatigue, peripheral neuritis, infection, platelet reduction, and white blood cell reduction. Most of these side effects were grade I to II. The dose of Velcade was reduced to 1.0 mg/m2for two patients from the newly diagnosed group due to the pain associated with grade I peripheral neuritis. Other side effects disappeared by themselves after symptomatic treatments were applied and the induction therapy ended, in which case the side effects did not affect the next course of treatment. The difference in the incident rates of side effects in the two groups were not statistically significant (Table 3). The common side effects observed during transplant included nausea, vomiting, anorexia, diarrhea, fatigue, and infection. There was no statistically significant difference in the side effects between the two groups (Table 4).
Overall survival (OS)
The median follow-up period was 26.5 months (7 to 61 months). The OS rate in the newly diagnosed group for 1, 2, and 3 years was 95.7%, 89.9%, and 79.9%, respectively. The median survival time of the newly diagnosed group had not reached that of all patients. The OS rate in the relapsed/refractory group for 1, 2, and 3 years was 93.8%, 79.8%, and 57.1%, respectively. The median survival time in the relapsed/refractory group was 42 months (the 95% confidence interval was 34 to 50 months). The difference in the OS rate between the two groups was not statistically significant (P=0.058, seeFigure 3A). We further examined the OS during the period from diagnosis to the end of follow-up. The median survival time in newly diagnosed group had not yet reached that of all patients during the period. The median survival time in relapsed/refractory group was 62 months (the 95% confidence interval was 35 to 89 months). The OS of two groups did not show astatistically significant difference for this period (P=0.522, seeFigure 3B).

Figure 3 Overall survival after treatment with bortezomib-based regimen followed by ASCT. A. Comparison of OS after induction therapy between newly diagnosed and R/R MM patients; B. Comparison of OS after diagnosis between newly diagnosed and R/R MM patients

Figure 4 Progression-free-survival after treatment with a bortezomib-based regimen followed by ASCT
Six patients from the newly diagnosed group died, including five patients whose disease progressed after transplant, and one patient who died from liver failure due to a Hepatitis B outbreak after transplant. Eight patients from the relapsed/refractory group died, including 5 patients with progressed disease or relapse, one patient who died from liver failure due to a Hepatitis B outbreak, and one patient who died from an unknown reason. For the two patients who died from Hepatitis B outbreak, both of them voluntarily stopped taking an anti-Hepatitis B virus drug and died 3 to 4 months after transplant due to the Hepatitis B outbreak. Their MM remained at CR state when they died.
Progression-free survival (PFS)
The PFS in the newly diagnosed group for 1, 2, and 3 years was 95.1%, 72.4%, and 65.8%, respectively. The median PFS in the newly diagnosed group was 48 months (the 95% confidence interval was 37 to 59 months). The PFS in the relapsed/refractory group for 1, 2, and 3 years was 93.8%, 51.1%, and 14.6%, respectively. The median PFS in the relapsed/refractory group was 25 months (the 95% confidence interval was 19 to 31 months). The difference in the PFS between the two groups was statistically significant (P=0.005,Figure 4). A multivariate analysis of including additional factors that may affect PFS, such as age, gender, ISS stage, different types of M protein, Durie-Salmon (DS) classification, and the time to receive transplant, demonstrated that the time to receive transplant, different types of M protein, and ISS stage were all independent factors influencing PFS (Table 5).
Discussion
Based on the overall efficacy analysis, both the newly diagnosed MM patients and the relapsed/refractory MM patients for whom conventional therapies failed showeda high remission rate in response to bortezomib-based induction therapy. The overall response rate (CR + nCR + PR) was greater than 80%. In particular, the proportion of patients with a high remission level (≥ nCR) was significantly increase compared with that of conventional chemotherapies and was greater than 50% for both groups. This result further suggests that bortezomib and conventional chemotherapy drugs do not exhibit crossresistance and that previous conventional therapeutic regimens did not affect bortezomib efficacy. The phase III Assessment of Proteasome Inhibition for Extending Remissions (APEX) clinical trial also confirmed that other drug treatment regimens, including thalidomide and VAD, administered before bortezomib do not affect the efficacy of bortezomib (9). After both patient groups received ASCT, the proportion of patients with high remission responses in both groups increased significantly, although the overall response rate did not increase further. Several studies have suggested that high efficacy of a drug treatment regimen prior to transplant is correlated with the prognosis and long-term survival of a patient (10). Therefore, both newly diagnosed and relapsed/refractory patients can benefit from bortezomib-based induction therapy followed by ASCT.
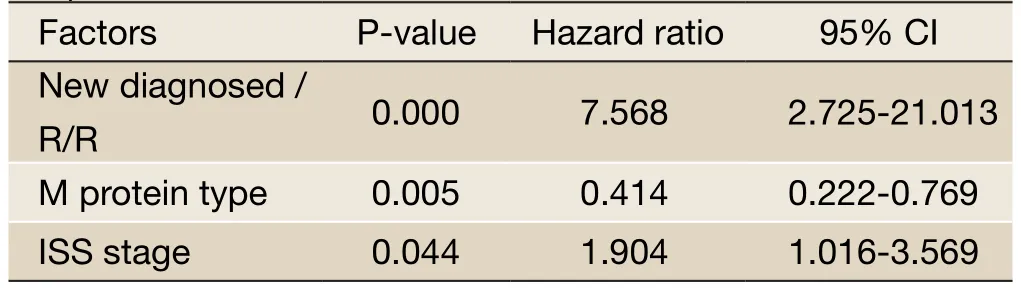
Table 5 Multivariate analysis of PFS in patients with multiple myeloma
Multiple studies have demonstrated that the bortezomibbased induction regimen rapidly reduces tumor cell burden without affecting stem cell collection (11,12). This study also revealed that stem cell collection from all of the patients after the induction therapy was successful and that all of the patients had a successful hematopoietic reconstitution after transplant. This result further suggests that bortezomib does not have toxic effect on bone marrow stem cells and therefore could be used as a safe and effective induction therapeutic agent prior to transplant. However, we found differences in stem cell collection and hematopoietic reconstitution between the newly diagnosed and relapsed/ refractory groups. During previous chemotherapy, the patients from the relapsed/refractory group had mostly received an alkylating agent therapy. One of the patients received the highest dose at 768 mg of Melphalan. We also found that the numbers of MNC and CD34 positive cells collected from the relapsed/refractory patients who had previously received an alkylating agent regimen were lower than those from the newly diagnosed group. Further observation on post-transplant hematopoietic reconstitution showed that differences in neutrophil recovery, platelet recovery, and the timing of granulocyte colony stimulating factor (G-CSF) application were all statistically significant between the two groups. The neutrophil recovery and platelet recovery were slower, and the timing of G-CSF application was longer in the relapsed/refractory patients who had received a previous alkylating agent therapy than was observed in the newly diagnosed patients and relapsed/ refractory patients that had not previously received an alkylating agent therapy.
ASCT is currently considered an important therapeutic approach for MM. In the era of new drugs, the optimal time point for transplant remains inconclusive. Kumaret al.treated newly diagnosed MM patients with immunomodulatory agent-based induction chemotherapy and studied the effect of early transplant and late transplant in these patients (8), early transplant was defined as receiving a transplant within one year after diagnosis, and late transplant was defined as receiving a transplant one year after diagnosis. Their results demonstrated that the 4-year survival was 73% for patients of both early and late transplant. The TTP was 20 months for early transplant and 16 months for late transplant if counted from post-transplant and was 25.3 months for early transplant and 15.9 months for late transplant if counted from diagnosis (P=0.09). Although the difference was not statistically significant, TTP of early transplant patients was slightly longer than that of late transplant patients. The 4-year overall survival (OS) counted from transplant of early transplant patients was better than that of late transplant patients (65.7%vs.56.3%, P=0.03). Our study demonstrated that the OS of the newly diagnosed group was not significantly different from that of the relapsed/ refractory group, as counted either starting from diagnosis or starting from entry into the study. The PFS in the newly diagnosed group was significantly longer than that of the relapsed/refractory group (P=0.005). Multivariate analysis showed that the time of receiving transplant was an independent prognostic factor influencing PFS. Although the relapsed/refractory patients survived, most of them already had a progressed stage of the disease, and the quality of their life was much worse than that of the newly diagnosed patients. Moreover, the progression of the disease directly affects the patient’s survival time. Therefore, it ispossible that the OS of the two groups might eventually become different as the follow-up time extends because the majority of the relapsed/refractory patients already had progressed disease, although the OS was not significantly different in our current study. This hypothesis remains to be tested in further follow-up observations.
We used retrospective analysis to discover that the bortezomib-based induction regimen followed by ASCT was effective when used either as a first-line therapy (early transplant) or as a salvage treatment (late transplant). The overall response rate and remission quality were not different between the two groups. Patients from both groups benefited from the therapy. The side effects during induction and transplant were not different between the two groups. However, the number of stem cells collected and hematopoietic reconstitution in patients from the relapsed/ refractory group who had previously received alkylating agent therapy were less than those in the newly diagnosed group. Early transplant can prolong patients’ PFS but not OS. To have a long PFS and high quality of life, early ASCT should be recommended immediately after diagnosis for patients in a suitable condition for transplant.
Acknowledgements
Disclosure:The authors declare no conflict of interest.
1. Cavo M, Baccarani M. The changing landscape of myeloma therapy. N Engl J Med 2006;354:1076-8.
2. Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008;111:2516-20.
3. Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med 1996;335:91-7.
4. Koreth J, Cutler CS, Djulbegovic B, et al. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: A systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transplant 2007;13:183-96.
5. Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood 1998;92:3131-6.
6. Anderson KC, Alsina M, Bensinger W, et al. NCCN clinical practice guidelines in oncology: multiple myeloma. J Natl Compr Canc Netw 2009;7:908-42.
7. Dunavin NC, Wei L, Elder P, et al. Early versus delayed autologous stem cell transplant in patients receiving novel therapies for multiple myeloma. Leuk Lymphoma 2012. [Epub ahead of print].
8. Kumar SK, Lacy MQ, Dispenzieri A, et al. Early versus delayed autologous transplantation after immunomodulatory agents-based induction therapy in patients with newly diagnosed multiple myeloma. Cancer 2012;118:1585-92.
9. Eom HS, Min CK, Cho BS, et al. Retrospective comparison of bortezomib-containing regimens with vincristine-doxorubicin-dexamethasone (VAD) as induction treatment prior to autologous stem cell transplantation for multiple myeloma. Jpn J Clin Oncol 2009;39:449-55.
10. Harousseau JL, Attal M, Avet-Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol 2010;28:4621-9.
11. Badros A, Goloubeva O, Fenton R, et al. Phase I trial of first-line bortezomib/thalidomide plus chemotherapy for induction and stem cell mobilization in patients with multiple myeloma. Clin Lymphoma Myeloma 2006;7:210-6.
12. Corso A, Barbarano L, Mangiacavalli S, et al. Bortezomib plus dexamethasone can improve stem cell collection and overcome the need for additional chemotherapy before autologous transplant in patients with myeloma. Leuk Lymphoma 2010;51:236-42.
Cite this article as:Liu J, Li J, Huang B, Zheng D, Chen M, Zhou Z, Xu D, Zou W. Determining the optimal time for bortezomib-based induction chemotherapy followed by autologous hematopoietic stem cell transplant in the treatment of multiple myeloma. Chin J Cancer Res 2013;25(2):166-174. doi: 10.3978/j.issn.1000-9604.2013.02.02

10.3978/j.issn.1000-9604.2013.02.02
bortezomib-based induction therapy followed by ASCT. The efficacy and side effects of the treatment regimen were analyzed. Patients’overall survival (OS) and progression-free survival (PFS) times were determined. The ratio of complete remission to near-complete remission (CR/nCR) was 69.5% versus 56.2% (P=0.361), respectively, for the early transplant group versus the late transplant group, respectively, after receiving bortezomib-based induction therapy; the overall response rates of the two group were 91.3% and 81.2%, respectively (P=0.369). After receiving ASCT, the CR/nCR of the two groups increased to 84.8% and 81.3%, respectively. The median time required for neutrophil engraftment of the early transplant group and the late transplant group was 11 and 14.5 days, respectively (P=0.003); the median time required for platelet engraftment was 13 and 21.5 days (P=0.031), respectively. There were no significant differences in the toxic side effects observed during induction therapy and ASCT between the two groups. The OS of the two groups was not statistically different (P=0.058). The PFS of the early transplant group and the late transplant group was 41.6 and 26.5 months, respectively (P=0.008). Multivariate analysis demonstrated that the time of receiving ASCT, the types of M protein, and the International Staging System (ISS) stage were all independent factors that influenced PFS. In conclusion, patients in a suitable condition for ASCT should be recommended to have an early ASCT immediately after diagnosis.
Submitted Jan 14, 2013. Accepted for publication Feb 19, 2013.
*These authors contributed equally in this study.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Safety and efficacy of trimodality therapy in patients undergoing extrapleural pneumonectomy
- Treatment of mesothelioma: still a long way to go!
- Role of contrast-enhanced ultrasonography in percutaneous radiofrequency ablation of liver metastases and efficacy evaluation
- Bevacizumab rescue therapy extends the survival in patients with recurrent malignant glioma
- Bortezomib, dexamethasone plus thalidomide for treatment of newly diagnosed multiple myeloma patients with or without renal impairment
- Giant neurogenic tumors of mediastinum: report of two cases and literature review
