Propofol inhibits the adhesion of hepatocellular carcinoma cells by upregulating microRNA-199a and downregulating MMP-9 expression
2013-06-01
Hangzhou, China
Propofol inhibits the adhesion of hepatocellular carcinoma cells by upregulating microRNA-199a and downregulating MMP-9 expression
Jian Zhang, Dan Zhang, Guo-Qing Wu, Zhi-Ying Feng and Sheng-Mei Zhu
Hangzhou, China
BACKGROUND:Propofol is one of the extensively and commonly used intravenous anesthetics and has the ability to inf l uence the proliferation, motility, and invasiveness of many cancer cells. In this study, the effects of propofol on hepatocellular carcinoma cells invasion ability were examined.
METHODS:We assessed the invasion ability of HepG2 cellsin vitroby determining enzyme activity and protein expression of MMP-9 using gelatin zymography assay and Western blot. The real-time PCR was used to evaluate the effect of propofol on microRNA-199a (miR-199a) expression, and miR-199a-2 precursor to evaluate whether over-expression of miR-199a can affect MMP-9 expression. Finally, the effect of miR-199a on propofol-induced anti-tumor activity using anti-miR-199a was assessed.
RESULTS:Propofol signif i cantly elevated the expression of miR-199a and inhibited the invasiveness of HepG2 cells. Propofol also eff i ciently decreased enzyme activity and protein expression of MMP-9. Moreover, the over-expression of miR-199a decreased MMP-9 protein level. Interestingly, the neutralization of miR-199a by anti-miR-199a antibody reversed the effect of propofol on alleviation of tumor invasiveness and inhibition of MMP-9 activity in HepG2 cells.
CONCLUSION:Propofol decreases hepatocellular carcinoma cell invasiveness, which is partly due to the down-regulation of MMP-9 expression by miR-199a.
(Hepatobiliary Pancreat Dis Int 2013;12:305-309)
propofol; invasion; MMP-9; HepG2 cells; microRNA-199a; hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC), one of the most common malignancy tumors worldwide, is particularly high in much of east Asia and sub-Saharan Africa.[1]The vascular invasion propensity and high metastatic potential are the characteristics of HCC, which increase the diff i culties for the treatment.[2]It is well known that a lot of molecules are involved in tumor invasion and metastasis. Among these molecules, matrix metalloproteinases (MMPs) play an important role because they can degrade extracellular matrix (ECM) proteins.[3]It has been reported that the expression and activity of MMPs are increased in HCC and the increases are related to advanced tumor stage and poor survival.[4]
MicroRNAs (miRNAs) are a novel class of short, non-coding RNAs involved in post-transcriptional gene regulation by binding to the target site in the 3'-untranslated region (3'-UTR) of target mRNAs.[5]miRNAs involve in invasion and metastasis of a variety of tumors, such as liver, lung, colon and pancreatic cancers.[6]Microarray studies have determined a large number of miRNAs that are related to metastasis in HCC.[7]Shen et al[8]reported that microRNA-199a (miR-199a) was down-regulated in HCC. Decreased expression of miR-199a plays an important role in increasing cell invasion and metastasis in HCC.
Propofol (2, 6-diisopropylphenol) is one of the extensively and commonly used intravenous anesthetics. In recent years, more and more evidences indicatethat propofol has the ability to inf l uence the motility, proliferation, and invasiveness of cancer cellsin vitroandin vivo.[9-11]Therefore, propofol might be a better agent than other anesthetics for cancer surgery.[12]However, the anti-tumor function of propofol on HCC cells has not been reported. The present study was to determine the effect of propofol on the invasion of HCC cells and the molecular mechanisms related with this activity.
Methods
Chemicals and reagents
Dulbecco's modif i ed eagle medium (DMEM) was obtained from GIBCO (Invitrogen Company). Calf blood serum (CBS) was from Sigma Aldrich Chemical Co. (St. Louis, MO., USA). Propofol (2, 6-diisopropylphenol) was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Cytomatrix human vitronectin-coated strips were obtained from Chemicon International Inc. (California, USA). MMP-9 ELISA kit was from R&D (Abingdon, UK). Polyclonal antibody against β-actin and MMP-9 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA., USA). The Detergent Compatible (DC) Protein Assay kit was purchased from Bio-Rad Laboratories (Hercules, CA., USA).
Cell culture
Human hepatoma cell line HepG2 was purchased from the American Type Culture Collection (Manassas, VA). The cells were cultured in DMEM, containing 4 mmol L-glutamine, 10% CBS, 100 units/mL penicillin, 65 units/mL streptomycin, 1.5 g/L sodium bicarbonate, and 4.5 g/L glucose. Cultured cells were maintained in a humidif i ed incubator containing 5% CO2at 37 ℃ and were stimulated with propofol [dissolved in dimethyl sulfoxide (DMSO)] in complete DMEM medium. As a control, cultured cells were incubated in complete DMEM medium containing DMSO at a fi nal concentration of less than 0.1%.
Cell adhesion assay
Cytomatrix human vitronectin-coated strips were used to perform the activity of cell adhesion. Under serum-free conditions, the strips were incubated with 100 μL of HepG2 cells (105/mL) with control or different concentrations of propofol (1, 5, 10 μg/mL) at 37 ℃ for 45 minutes. Washed with phosphate buffer solution (PBS) for three times, the adhered cells were stained for 5 minutes with 0.2% crystal violet in 10% ethanol at 25 ℃. After PBS washing the excess stain for fi ve times, the stained cells were dissolved in 100 μL of solubilization buffer (1:1 mixture of 50% ethanol and 0.1 mol NaH2PO4, pH 4.5). The absorbance was read at 540 nm. The absorbance of dye in the control (without propofol treatment) cells was regarded as 100% adherence and the percentage adherence of propofol-treated cells was compared with that of the control cells.
Gelatin zymography assays
Gelatin zymography assays were used to detect the activity of MMP-9 in the medium according to the method described previously.[13]Brief l y, 20 μL of collected media were mixed with sodium dodecyl sulfate (SDS) sample buffer and subjected to SDS-PAGE electrophoresis without boiling or reduction. Washed with 2.5% Triton X-100, the gels were incubated in a reaction buffer (10 mmol/L CaCl2, 0.01% NaN3, and 40 mmol/L Tris-HCl, pH 8.0) at 37 ℃ for 12 hours. At last, Coomassie brilliant blue R-250 was used to stain the gels.
Quantitative real-time PCR (Q-PCR) analysis of miRNA expression
Approximately 5×106cells were treated without (Control) or with propofol for 24 hours. miRNAs were isolated and purif i ed using Trizol reagent (Invitrogen, USA), according to the manufacturer's protocol. The miR-199a level was measured by Q-PCR using TransStartTM SYBR Green qPCR Supermix (TransGen Biotech, Beijing, China). The U6 small nuclear RNA was used as an internal normalized reference. For miR-199a, the primers were as follows: forward, 5'-ACA CTC CAG CTG GGC CCA GTG TTC AGA CTA C-3' and reverse, 5'-CTC AAC TGG TGT CGT GGA GTC GGC AAT TCA GTT GAG GAA CAG GT-3'. For U6, the primers were as follows: forward, 5'-CTC GCT TCG GCA GCA CA-3' and reverse, 5'-AAC GCT TCA CGA ATT TGC GT-3'. The comparative threshold cycle (Ct) method was used to analyze fold change. The change of miRNA expression was calculated as fold-change, i.e. relative to control.
Western blot analysis
HepG2 cells were lysed with ice-cold lysis buffer containing: 150 mmol/L NaCl; 50 mmol/L Tris-HCl, pH 7.4; 1 mmol/L EDTA; 1% NP-40; and 1 mmol/L phenylmethylsulfonyl fl uoride. Protein concentration in the cell lysate was detected using the DC protein assay kit (Bio-Rad). After protein content was determined, Western blot analysis was performed.
Transfection procedures
miR-199a was overexpressed or knocked down bytransfection with 100 nmol/L of miR-199a-2 precursor or anti-miR-199a (Ambion) with lipo-fectamine 2000 (Invitrogen, USA) in antibiotic-free medium, at 30% to 50% conf l uency, in 60-mm dishes. Twenty-four hours after transfection, the cells were treated with or without propofol.
Statistical analysis
Statistical analysis was performed with SPSS software, version 13.0. Statistical analyses were performed using either an analysis of variance (ANOVA) or Student'sttest. Results are presented as means±SD. APvalue <0.05 was considered statistically signif i cant, and aPvalue <0.01 was highly signif i cant.
Results
The effects of propofol on HCC cells adhesion
HepG2 cell adhesion was signif i cantly inhibited by propofol at the concentration of 5 μg/mL after incubation for 24 hours. The inhibition was dosedependent (29.5% decrease at 5 μg/mL and 43.2% at 10 μg/mL;P<0.01 compared with the control (Fig. 1).
Inhibitory effects of propofol on MMP-9
We next examined the effect of propofol on MMP-9 activity in HepG2 cells. Gelatin zymography showed that MMP-9 activity was signif i cantly inhibited by propofol at the concentration of 5 μg/mL (P<0.01, Fig. 2A), and the inhibitory effect of propofol on the activity of MMP-9 was dose-dependent.
In order to determine the MMP-9 protein level in cells after treatment with propofol, Western blot analysis was made (Fig. 2B). Being consistent with the observed alteration of its activity, MMP-9 protein expression was signif i cantly reduced in HepG2 treated with propofol at 5 μg/mL (P<0.01 compared with control). The inhibitory effect of propofol on MMP-9 protein in HepG2 cells was dose-dependent, with a 46% inhibition at 5 μg/mL and a 68.4% decrease at 10 μg/mL (Fig. 2C).
Propofol stimulates miR-199a expression
Compared with the control, propofol increased miR-199a expression in HepG2 cells in a dose-dependent manner (Fig. 3). Propofol at 5 μg/mL increased the miR-199a expression in HepG2 cells to 2.23-fold. Propofol at 10 μg/mL elevated the miR-199a level to 3.53-fold.
Over-expression of miR-199a and MMP-9 expression
To determine whether miR-199a regulates MMP-9 expression in HepG2 cells, we determined the protein level of MMP-9 after transfection of miR-199a-2 precursor. The result of Q-PCR revealed that miR-199a-2 precursor signif i cantly increased the expression of miR-199a in HepG2 cells (Fig. 4A), suggesting that miR-199a-2 precursor is eff i ciently introduced into the cellsand induces miR-199a over-expression. Furthermore, transfection of miR-199a-2 precursor signif i cantly decreased MMP-9 expression (Fig. 4B).
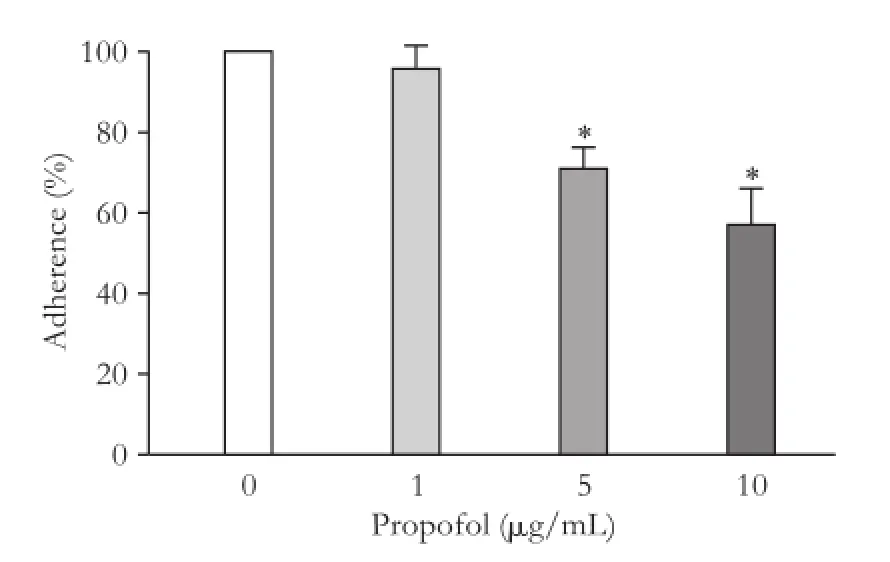
Fig. 1.Propofol induced impairment on adhesion ability of HepG2 cells. HepG2 cells were treated with various concentrations of propofol. Results are presented as the percentages of adhered cells in comparison with the control. *:P<0.01, compared with the control group.

Fig. 2.Propofol treatment induces down-regulation of MMP-9. Activity of MMP-9 was dose-dependently reduced in HepG2 cells after treated with propofol for 16 hours by Gelatin zymography assays (A). The protein levels in HepG2 cells were significantly decreased after propofol treatment (B). Fold changes of MMP-9 protein levels were determined (C). *:P<0.01, compared with the respective control groups.
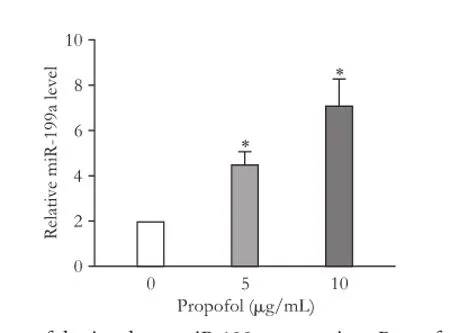
Fig. 3.Propofol stimulates miR-199a expression. Propofol treatment increased the expression of miR-199a in a dose-dependent manner. *:P<0.01, compared with the control group.
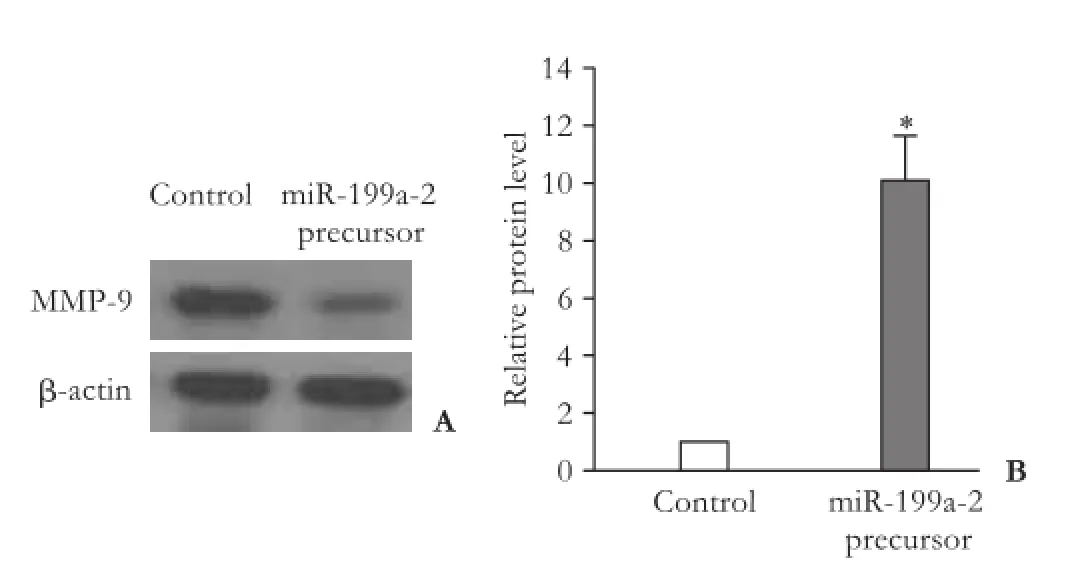
Fig. 4.Over-expression of miR-199a can inhibit MMP-9 expression. Western blot showed that transfection of miR-199a-2 precursor decreased MMP-9 protein expression (A). miR-199a-2 precursor can significantly increase the expression of miR-199a (B). *:P<0.01, compared with the control group.
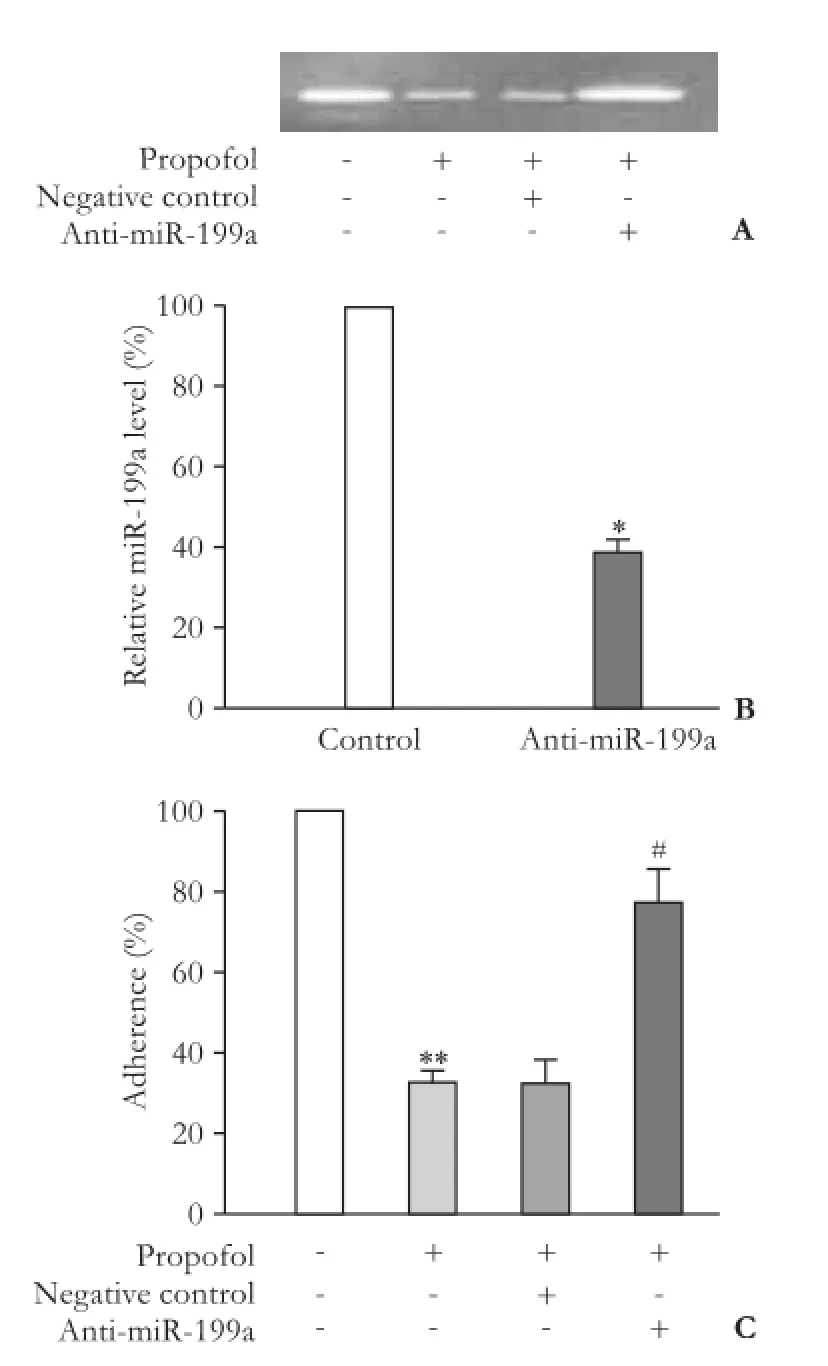
Fig. 5.Anti-miR-199a can reverse the effect of propofol. Gelatin zymography revealed that anti-miR-199a significantly increased MMP-9 activity in cells after treatment with propofol (A). AntimiR-199a significantly reduced the expression of miR-199a in HepG2 cells. *:P<0.01, vs negative control group (B). Anti-miR-199a signif i cantly increased invasion ability of HepG2 cells after propofol treatment (5 μg/mL). **:P<0.01 compared with the control group (without propofol treatment). #:P<0.01, compared with propofol-treated group transfected with negative control (C).
MiR-199a elimination and cell adhesion
We used anti-miR-199a antibody to evaluate the role of miR-199a in the effect of propofol on the inhibition of HepG2 cells adhesion anti-miR-199a antibody signif i cantly reduced the expression of miR-199a, suggesting that anti-miR-199a antibody eff i ciently penetrated into HepG2 cells (Fig. 5A). Furthermore, anti-miR-199a antibody reversed the inhibitory effect of propofol on cell adhesion (Fig. 5B) and neutralized the inhibitory effect of propofol on MMP-9 activity in HepG2 cells (Fig. 5C).
Discussion
HCC has become one of the most common malignancies worldwide, and has high metastatic potential.[2,14]In this study we investigated the effect of propofol on invasion ability of hepatoma cell line HepG2. It is well known that cell adhesion is essential in tumor metastasis and the changing adhesive preferences of cancer cells to fi broblasts play an important role in the metastatic process.[15]We evaluated the effect of propofol on HepG2 cells invasion ability using cell adhesion assay and found that propofol mitigated the cell invasiveness (Fig. 1). Our results were consistent to the study of propofol on breast cancer cells invasion.[16]
Increasing evidence showed that propofol perfectly fi ts anesthesia in tumor surgery.[16,17]In clinical anesthesia, the target plasma propofol concentrations for general anesthesia are 3-8 μg/mL[18]and these concentrations of propofol effectively inhibited invasion of HCC. We found that 5 μg/mL propofol signif i cantly inhibited invasion ability of HepG2 cells. To further explain the molecular mechanism involved in the suppression of invasion of these cells, we investigated the effect of propofol on the expression and activity of MMP-9. MMP-2 and MMP-9 which degrade ECM components and play an important role in HCC invasion.[3,4]Miao et al[11]reported that propofol treatment signif i cantly decreased the expression of MMPs and subsequently inhibited the invasive activity of colon carcinoma cells. We also found that propofol signif i cantly inhibited the activity of MMP-9 in HepG2 cells but had no effect on MMP-2 expression (data not shown).
It has been reported that the expression of miR-199a was often down-regulated in HCC.[19,20]When the expression of miR-199a was up-regulated, it could reduce the invasion ability of HepG2 cells.[8]Thus, miR-199a may be useful as a new effectively therapeutic target for HCC. In this study, the over-expression of miR-199a inhibited MMP-9 protein expression,indicating that miR-199a played a role in cancer invasion through down-regulation of MMP-9. Besides, propofol stimulated the expression of miR-199a in HepG2 cells. More importantly, neutralizing miR-199a with antimiR-199a antibody reversed the effect of propofol on the alleviation of the invasiveness of HepG2 cells and on the inhibition of MMP-9 expression. These results suggested that the propofol suppression of HCC cell invasion was partly due to the miR-199a down-regulation of MMP-9. However, the mechanism of miR-199a regulation of MMP-9 expression should be clarif i ed in future.
In conclusion, the concentrations of propofol in clinical application inhibited the invasion of HCC cells effectively and miR-199a possibly contributes to this antitumor action of propofol. Further studies are needed to validate its clinical relevance.
Contributors:ZSM proposed the study. ZJ and ZSM performed the research and wrote the fi rst draft. ZD, WGQ and FZY collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. ZSM is the guarantor.
Funding:This study was supported by a grant from the Medical Scientif i c Research Fundation of Zhejiang Province (2013KYA060).
Ethical approval:Not needed.
Competing interest:No benef i ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist 2010;15:5-13.
2 Ribatti D, Vacca A, Nico B, Sansonno D, Dammacco F. Angiogenesis and anti-angiogenesis in hepatocellular carcinoma. Cancer Treat Rev 2006;32:437-444.
3 Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002;2:161-174.
4 Ogasawara S, Yano H, Momosaki S, Nishida N, Takemoto Y, Kojiro S, et al. Expression of matrix metalloproteinases (MMPs) in cultured hepatocellular carcinoma (HCC) cells and surgically resected HCC tissues. Oncol Rep 2005;13:1043-1048.
5 Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281-297.
6 Nicoloso MS, Spizzo R, Shimizu M, Rossi S, Calin GA. MicroRNAs--the micro steering wheel of tumour metastases. Nat Rev Cancer 2009;9:293-302.
7 Budhu A, Jia HL, Forgues M, Liu CG, Goldstein D, Lam A, et al. Identif i cation of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology 2008;47:897-907.
8 Shen Q, Cicinnati VR, Zhang X, Iacob S, Weber F, Sotiropoulos GC, et al. Role of microRNA-199a-5p and discoidin domain receptor 1 in human hepatocellular carcinoma invasion. Mol Cancer 2010;9:227.
9 Altenburg JD, Harvey KA, McCray S, Xu Z, Siddiqui RA. A novel 2,6-diisopropylphenyl-docosahexaenoamide conjugate induces apoptosis in T cell acute lymphoblastic leukemia cell lines. Biochem Biophys Res Commun 2011;411:427-432.
10 Mammoto T, Mukai M, Mammoto A, Yamanaka Y, Hayashi Y, Mashimo T, et al. Intravenous anesthetic, propofol inhibits invasion of cancer cells. Cancer Lett 2002;184:165-170.
11 Miao Y, Zhang Y, Wan H, Chen L, Wang F. GABA-receptor agonist, propofol inhibits invasion of colon carcinoma cells. Biomed Pharmacother 2010;64:583-588.
12 Inada T, Kubo K, Shingu K. Possible link between cyclooxygenase-inhibiting and antitumor properties of propofol. J Anesth 2011;25:569-575.
13 Yang SF, Chu SC, Chiang IC, Kuo WF, Chiou HL, Chou FP, et al. Excessive matrix metalloproteinase-9 in the plasma of community-acquired pneumonia. Clin Chim Acta 2005;352: 209-215.
14 Pang RW, Poon RT. From molecular biology to targeted therapies for hepatocellular carcinoma: the future is now. Oncology 2007;72:30-44.
15 Bogenrieder T, Herlyn M. Axis of evil: molecular mechanisms of cancer metastasis. Oncogene 2003;22:6524-6536.
16 Siddiqui RA, Zerouga M, Wu M, Castillo A, Harvey K, Zaloga GP, et al. Anticancer properties of propofoldocosahexaenoate and propofol-eicosapentaenoate on breast cancer cells. Breast Cancer Res 2005;7:R645-654.
17 Wu RS, Liu KC, Tang NY, Chung HK, Ip SW, Yang JS, et al. cDNA microarray analysis of the gene expression of murine leukemia RAW 264.7 cells after exposure to propofol. Environ Toxicol 2011 Jul 22.
18 Coetzee JF, Glen JB, Wium CA, Boshoff L. Pharmacokinetic model selection for target controlled infusions of propofol. Assessment of three parameter sets. Anesthesiology 1995;82: 1328-1345.
19 Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong Q, et al. Identif i cation of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell 2011;19:232-243.
20 Jiang J, Gusev Y, Aderca I, Mettler TA, Nagorney DM, Brackett DJ, et al. Association of MicroRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin Cancer Res 2008;14:419-427.
Received October 15, 2012
Accepted after revision April 8, 2013
AuthorAff i liations:Department of Anesthesiology, First Aff i liated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Zhang J, Feng ZY and Zhu SM); Department of Gynaecology and Obstetrics, Hangzhou Cancer Hospital, Hangzhou 310002, China (Zhang D); and Department of Oncology, Zhejiang Provincial People's Hospital, Hangzhou 310014, China (Wu GQ)
Sheng-Mei Zhu, PhD, Department of Anesthesiology, First Aff i liated Hospital, Zhejiang University School of Medicine, 79 Qingchun Rd., Hangzhou 310003, China (Tel: 86-571-87236169; Email: smzhu20088@yahoo.com.cn)
© 2013, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(13)60048-X
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Surgical treatment of hepatocellular carcinoma with inferior vena cava tumor thrombus:a new classification for surgical guidance
- Pancreatic duct disruption and nonoperative management: the SEALANTS approach
- Pancreatic Castleman disease treated with laparoscopic distal pancreatectomy
- Inferior vena cava obstruction and collateral circulation as unusual manifestations of hepatobiliary cystadenocarcinoma
- Clinical features and outcomes of patients with severe acute pancreatitis complicated with gangrenous cholecystitis
- Double-blind randomized sham controlled trial of intraperitoneal bupivacaine during emergency laparoscopic cholecystectomy
