4种葡萄抗氧化活性比较
2013-03-03敬思群马泽鑫
普 燕,敬思群,马泽鑫
(新疆大学生命科学与技术学院,新疆 乌鲁木齐 830046)
4种葡萄抗氧化活性比较
普 燕,敬思群,马泽鑫
(新疆大学生命科学与技术学院,新疆 乌鲁木齐 830046)
通过测定总抗氧化能力、清除DPPH自由基能力、清除羟自由基(•OH)能力及清除超氧阴离子自由基(O2—·)的能力,评价4种葡萄3种提取物的体外抗氧化能力。通过动物实验评价玫瑰香葡萄水提物体内抗氧化活性,同时定性、定量分析玫瑰香葡萄水提物的活性成分。结果表明:4种葡萄的同一种提取物的体外抗氧化能力显著差异。综合分析玫瑰香葡萄水提物体外抗氧化能力最强。剂量800mg/(kg·d)玫瑰香葡萄水提物能显著提高小鼠血清及肝脏组织中超氧化物歧化酶(SOD)酶活力,降低肝脏组织中丙二醛(MDA)含量。玫瑰香葡萄水提物中含有多糖、有机酸、黄酮类、酚类等抗氧化活性物质,其中总酚含量为0.456mg/mL、总黄酮含量为4.37mg/mL。
抗氧化能力;葡萄;清除自由基;提取;体内
At present, more and more research on the grape’s antioxidant, grapes contain a large number of biologically active substances, such as grape polyphenols, flavonoids, proanthocyanidins and so on, this antioxidant components play a role in the prevention and improvement of many diseases[1]. Previous studies showed that grape seed extract have anti-inflammatory, anti-rheumatic, anti-allergic, antitumor, anti-aging, slow down the process of development of Parkinson’s disease, and the regulation of vascular cell function, inhibit LDL oxidation and reduce platelet. Protective effect of condensation[2-3]. Free radicals were the reason of evil, which can cause cancer, aging, cardiovascular and other degenerative diseases[4]. Many researches showed that the pathogenesis of many diseases may be relate to the imbalance on the produce and remove of the free radicals, and the performance show that the enhanced of the oxidation with the decreased antioxidant capacity[5]. Free radical generation and scavenging in vivo was imbalance in the case of steady-stage, the body produces many free radicals. These free radicals attacked normal cells, lead to normal cell dysfunction, such as atherosclerosis, heart disease, cerebral ischemia, Alzheimer’s disease, liver disease, diabetes, the aging, cancer and rheumatism, etc[6-8]. Today, the food industry was using synthetic antioxidants, such as BHA and BHT, the animal experiments show that they have a toxic and carcinogenic effects[9]. The disadvantages required the development of natural non-toxic anti-oxidants. The grapes were such natural plant resources, which posessed anon-toxic, efficient characteristics, and has antioxidant, in particular play a role in scavenging free radicals[10]. Bagchi et al.[11]research shows that in the 100 mg/L of the same concentration of procyanidins on superoxide anion and hydroxyl radical inhibition rate of 78%—81%, much higher than the vitamin C (12%—19%) and vitamin E (36%—41%) of the inhibition rate. Other research reports[12]showed that the ability of the catechin to capture the ABTS+· was higher than VC. Combinded with resveratrol, VC and VE can make synergistic effect, which can enhanced antioxidant activity[13]. Domestic and international research[14]showed that the free radical scavenging capacity of grape polyphenols was strongest in many plant polyphenols, which antioxidant activity was 50 times to VE and 20 times to VC. in vitro antioxidant evaluation, many researchers used to measure DPPH free radical scavenging capacity of samples. Many studies[15]have shown: DPPH method was rapid, simple, sensitive, direct and practical. In addition, there was a method that used the POV (peroxide value of oils and fats) to measure antioxidant capacity in vitro[16]. in vivo antioxidant evaluation was often through measure serum or liver homogenate MDA values to reflect the antioxidants ability to inhibit lipid peroxidation[17].
Because there were many difference to the same species of plants in different ecological environments, climatic factors, planting and management[18]. Grapes were no exception, the impact of its quality by region, and the special circumstances of Xinjiang’s geography and climate, grape resources also very rich, with broad prospects of research grape in Xinjiang. Therefore, The objectives of this work were to investigate four evaluation methods of antioxidants in vitro and by animal experiments of antioxidants in vivo to evaluate the antioxidant activities of distilled water, 80% ethanol and ethylacetate extracts of four kinds of grape grown in Xinjiang, such as the DPPH radical scavenging assay, superoxide radical scavenging assay, hydroxyl radical scavenging assay, prussian blue spectrophotometry measure, and methods of SOD kit and MDA kit. In addition, this study also chemical qualitative and quantitative analyse the active ingredient of the best antioxidant activity of grape extract, which providing the theoretical basis for development of grapes grown in Xinjiang.
1 Materials and Methods
1.1 Chemicals
DPPH (2,2-diphenyl-2-picrylhydrazyl radical), Nitrotetrazolium Blue chloride (NBT), L-methionine, lactoflavin, ethanol, ethylacetate, orthophenanthroline, hydrogen peroxide, trichloroacetic acid, sulfuric acid, bromine phenolic blue, alchlor, silicon tungsten acid, gallic acid, rutin, VC, SOD kit, MDA kit, Nanjing Built Biological Research Institute; Coomassie brilliant blue, bovine serum albumin, All of reagents were analytical gradeor highest grade available; Distilled water and ddH2O were used for all experiments.
1.2 Sample processing
Four grapes: Thompson seedless grape (Thompson seedless uvas), Munage grape (Munage uvas), America rubrum (Rubrum uvas) and Muscat hamburg grape (Muscat hamburg uvas) was obtained from Ürümqi market (Xinjiang Province, China). Grape juice preparation: the whole grape (include seeds, pulp and skin) pressed by philipsjuicer and the extracts were filtered, take the juice and avoide light at4 ℃ before use.
Aqueous extract sample preparation (AE): V(juice):V(water)=1:5, added distilled water and homogenate, after using ultrasonic cleaning machine (China) ultrasonic slurry auxiliary extraction 30 min and rest for2 h, filter, and the supernatant was collected by centrifugation (4000 r/min, 20 min), which was aqueous extract from grape juice.
Ethanol extract sample preparation (EE): V(juice): V(ethanol) =1:5, added ethanol (80%) and homogenate, after using ultrasonic cleaning machine (China) ultrasonic slurry auxiliary extraction 30 min and rest for2 h, filter, and the supernatant was collected by centrifugation (4000 r/min, 20 min), which was ethanol extract from grape juice.
Ethylacetate extract sample preparation (EAE): V(juice): V(ethylacetate) =1:5, added ethylacetate and homogenate, after using ultrasonic cleaning machine (China) ultrasonicslurry auxiliary extraction 30 min and rest for2 h, filter, and the supernatant was collected by centrifugation (4000 r/min, 20 min), which was ethyl acetate extract from grape juice.
1.3 Determination of antioxidant capacity
1.3.1 Determination of total antioxidant capacity
The total antioxidant capacity of the samples was measured according to the experiment procedure described by Sheng Wei[19]. Exactly sample (1 mL) was mixed with PBS (2.5 mL, pH 6.6) and K3Fe(CN)6solution (2.5 mL,1 g/100 mL), then the mixture were placed in water-bath pot (50 ℃, 20 min), added TCA solution (2.5 mL,10 g/100 mL) after. Then exactly 2.5 mL mixture mixed with 2.5 mL distilled water and FeCl3solution (2.5 mL, 0.1 g/100 mL), placed for10 min, theabsorbance of the sample solution (A) was measured at 700 nm using a UV/2000 UV-Vis spectrophotometer, to replace the sample with reagent as empty control, its absorbance was A0. Total antioxidant capacity can be expressed as Δ A (Δ A = A—A0). Experimental repeat three times.
1.3.2 Determination of scavenging DPPH free radical capacity
The free radical scavenging activity of the samples was measured according to the procedure described by Lian Xijun[20]. A sample solution (2 mL) was mixed with 2 mL DPPH ethanol solution(2 × 10-4mol/L), after shaking for 30 min, the absorbance was measured at 517 nm using a UV/2000 UV-Vis spectrophotometer and the clearance rate was calculated using the expression. Experimental repeat three times.
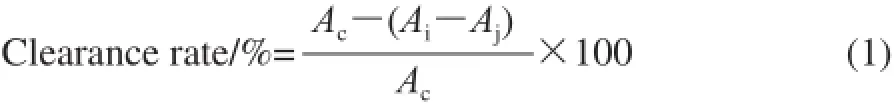
Here: Ai: absorbance of mixture of sample solution and the DPPH solution; Aj: absorbance of sample solution; Ac: absorbance of mixture of DPPH solution and extracted reagent.
1.3.3 Determination of scavenging hydroxyl radical (• OH) capacity
The scavenging •OH capacity of the samples was measured according to the method of phenanthroline-Fe2+oxidation by Zhao Yanhong et al[21]. Exactly PBS (4 mL pH 7.4) and mixed with phenanthroline solution (1.5 mL, 5 mmol/L). Then the mixture was mixed with FeSO4solution (1 mL, 7.5 mmol/L), sample (1 mL), the ddH2O (1.5 mL), and the H2O2solution (1 mL, 0.1%). Then the mixture was placed in water-bath pot (37 ℃, 60 min) finally. The absorbance was measured at 536 nm using a UV/2000 UV-Vis spectrophotometer, and the clearance rate was calculated using the expression. Experimental repeat three times.
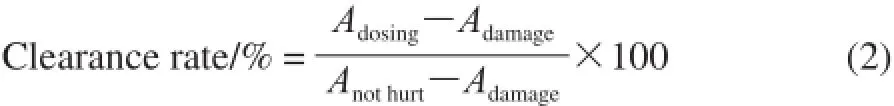
Here: Adosing: absorbance measured after adding the sample and the hydrogen peroxide; Adamage: absorbance measured after adding hydrogen peroxide instead of adding the sample; Anothurt: absorbance measured without adding the sample and the hydrogen peroxide.
1.3.4 Determination of scavenging superoxide anion radical (O2
—•) capacity The scavenging O2—• capacity was measured by the NBT reduction method[21]. Exactly PBS (1 mL, pH 7.8), and mixed with Riboflavin (0.5 mL, 3.3×10-5mol/L), Methionine (0.5 mL, 0.02 mol/L), NBT (0.5 mL, 5.1×10-4mol/L) and the sample (1 mL). Then placed them in the dark container (for fluorescent light 20 min). The absorbance was measured at 560 nm using a UV/2000 UV-Vis spectrophotometer, to replace the sample with reagent as empty control, its absorbance was A0, and the clearance rate was calculated using the expression. Experimental repeat three times.

1.3.5 Antioxidant activity evaluation test in vivo
AE from Muscat hamburg grape juice was concentrated for 1/10 (V/V) and keep in dark at 4 ℃ before use. Female mice, 5 weeks, KM, weighing 22—28 g, were supplied by 2003-0002/SCXK (Xin)Xinjiang Laboratory Animal Research center in China. The animals were housed in propylene cages at constant temperature of (20±2) ℃ and relative humidity of about 60%, with water and food. Five groups of 18—22 g KM mice (n=8) were used in this work, they were normal control group (NC), low dose group (LD, 400 mg/(kg • d)), middle dose group (MD, 600 mg/(kg • d)), high dose group (HD, 800 mg/(kg•d)) and positive control group (NC+, VC 800 mg/(kg •d), all calculated as body weight. Once a day, normal control group was given the same amount of saline water, persistent 30 d. Food and water intake by the animals was monitored daily and body weight was measured weekly. After the testing of 4 week, the mice was fasted overnight without limiting the water. Take blood from the eyes of the mice and the upper serum was collected after centrifugation (3500 r/min, 5 min). After the mice were sacrificed, organs were dissected out, strike out the the connective tissue and weighe it immediately. Physiological saline was added, to make 10% liver refining, the supernatant was collected by centrifugation (3500 r/min, 5 min). According to the test case methods and procedures determined. Measurement of serum superoxide dismutase (SOD) and liver homogenate SOD and MDA, total protein (Bradford method) were attached.
1.4 Qualitative analysis
The qualitative analysis assay about the AE from the Muscat hamburg grape juice was used according to the procedure described by Beijing University of Chinese Medicine[22].
1.5 Determination of phenolic and flavonoids contents
Total soluble phenolics content of the AE from the Muscat hamburg grape juice was determined with Folin-Ciocalteu reagent described by Li Jing et al[23]. The results were expressed as gallic acid equivalents (GAE) milligramsin per.gram of extract. Total flavonoids content of the AE from the Muscat hamburg grape juice was determined colorimetrically by a rutin standard curve[24]. The results were expressed as milligrams of rutin equivalents per gram of extract.
1.6 Statistical analysis
Using software SPSS 13.0 related data statistics processing, data representation±s. The single factor analysis of variance (one-way ANOVA) and multiple comparison (LSD) did significant differences between the comparison, significant level (P<0.05).
2 Results and Analysis
2.1 The total antioxidant capacity of different solvents extracts from grape juice

Table1 Total antioxidant capacity of grape juice extracted by water, ethanol and ethyl acetate(x±s)
Table1 shows the same four grape juice extract significant antioxidant activity, in which the AE from the Muscat grape juice, the highest total antioxidant capacity, Thompson seedless white grapejuice’s EE was the highest total antioxidant capacity, Thompson seedless’s EAE was the highest total antioxidant capacity. Comprehensive evaluation, the results of the method shown in the AE from the Muscat hamburg grape juice relatively high antioxidant activity.
2.2 The grape juice extracts on the DPPH free radical clearance rate

Table2 DPPH radical-scavenging rate of grape juice extracted by water, ethanol and ethyl acetate (x±s) %
Table2 shows the AE of four grapes juice and EE’s DPPH free radical clearance rates were obviously different, but the EAE of Muscat seedless and Muscat hamburg grape clearance rate were not obvious, in which aqueous extract of Muscat hamburg grape was the best, almost to 94.26% . The scavenging of DPPH free radical rate of the AE and the EE were obviously higher than the EAE. Meanwhile, the method was simple, the phenomenon was obvious, and it means that DPPH free radical method can effectively detect antioxidant activity of grape juice extracts.
2.3 The grape juice extracts on the •OH clearance rateTable3 shows clearance rates getting from this method were not very high, and negative rates could be found, and precipitation appeared in some samples affected determination. Which the AE from the Muscat hamburg grape juice scavenging • OH rate was the highest (18.74%). Table1 and Table2 shows the results differ greatly, which may be phenanthroline-Fe2+with some substances of grape juice extract, preventing accurate measurements. Also it meant that phenanthroline-Fe2+method was not effect to detect the antioxidant of grape juice extracts.

Table3 Hydroxyl radical-scavenging rate of grape juice extracted by water, ethanol and ethyl acetate (x±s) %
2.4 The grape juice extracts on the clearance rate of O2—•

Table4 Superoxide anion radical-scavenging rate of grape juice extracted by water, ethanol and ethyl acetate (x±s) %
Table4 shows light riboflavin-N blue tetrazolium (NBT) method for EE and EAE from grape juice by detection O2—•scavenging were effect, but the detection of EE in clearing O2—• were not obvious, and negative clearance were could been see. In contrast, this method results showed that the EE of Thompson seedless grape juice scavenging O2—• capacity was the best.
Four methods were used in vitro antioxidant evaluation of four kind of grape’s three extracts, comprehensive evaluation showed that aqueous extract of Muscat hamburg grape juice took up the best antioxidant activity. Therefore the aqueous extract of Muscat hamburg grape juice were usedfor animal experiments to evaluate its in vivo antioxidant effect. Results were as follows.
2.5 The determination results of antioxidant experiments in vivo

Table5 Antioxidant capacity in vivo of aqueous extract from Muscat ham burg grape (x±s)
The results from Table5 showed that the serum SOD activity of experimental mice done by gavage of aqueous extract of Muscat hamburg grape juice was significantly higher than the normal group, with the dose increase more the effect was better. HD group and the NC+group was significantly different, high dose illustrated more effective antioxidant than VC. In SOD activity of mice liver, only HD group and NC group and the NC+group were significantly different, indicated that only 800 mg/(kg•d) high dose of Muscat grape hamburg juice extract had effect on mice liver SOD activity. The results of liver MDA test showed that HD group and NC group were significantly different, but LD, MD group was not significant, indicated that the AE of Muscat hamburg grape juice had little effect on reducing the liver MDA values, dose group had little effect, only high dose group had effect, and the positive effect of VC control group was not significant.
2.6 Result of qualitative and quantitative analysis
Qualitative analysis show that the AE from the Muscat hamburg grape juice contains sugar, polysaccharides, organic acids, flavonoids, phenols and other active substances. Sugar, polysaccharide, phenolic and flavonoids were substances that have antioxidant activity which indicated that Muscat hamburg grape extract may have antioxidant activity.
The content of total phenolics in the AE from the Muscat hamburg grape juice, determined from regression equation of calibration curve (A=0.0105ρ—0.0048, R2=0.9991) and expressed in GAE, the content of flavonoids in AE from the Muscat hamburg grape juice, determined from regression equation of calibration curvethe (A=9.7321ρ—0.0019, R2=0.9996). The data showed that the AE from the Muscat hamburg grape juice, the total flavonoids content was up to 4.37 mg/mL and the total polyphenols was 0.456 mg/mL.
3 Discussion and Conclusion
The four in vitro evaluation and in vivo animal models experiment showed that there were difference exist in the antioxidant determination results among the four kind of grape, especially in phenanthroline-Fe2+method and light riboflavin-NBT method. The two methods determination results were not obvious, the test of negative clearance rate, indicated that the two methods were not suitable for evaluation of grape juice’s antioxidant activity. Measured the total antioxidant capacity and free radical scavenging DPPH method were simple and had obvious phenomenon can be used to indicate the antioxidation activity of four grape juice objectively.
Through animal experiments to test and verify the aqueous extract antioxidant of grape, the results confirmed the in vitro evaluation results. The fact that LD groupand NC group had no difference, which indicated that low doses of aqueous extract of grape juice had no effect on mice in vivo antioxidant aspect. But the MD and HD group were significantly different from NC group, and the MD and HD groups were significantly different from each other, these indicated that the medium dose and high dose group mice demonstrated obvious in vivo antioxidant effect. Muscat hamburg grape juice had obvious effect on mice antioxidant activity, it can effectively increasethe serum and liver SOD, it can reduce the MDA value of the liver. The water extract of Muscat hamburg grapes can improve the mice serum SOD activity and decrease MDA content in liver tissue.
By chemical qualitative experiment, the research obtained that aqueous extract of Muscat hamburg grape had active ingredient, the results showed that it contained phenols, flavonoids, polysaccharides and other substances with antioxidant activity, which total polyphenols was 0.456 mg/mL, total flavonoid 4.37 mg/mL was also good evidence of its high antioxidant activity. Inaddition the total flavonoids was the major antioxidant, while the polyphenols was due to the content of phenolic ompounds were low levels in grape juice, and mainly in the skin seeds of grape[25].
The results showed that: there were significantly difference among the same extracts of different kind of grape juice on antioxidant activity aspect in vitro. The in vitro antioxidant capacity of the AE from the Muscat hamburg grape juice was strongest. The AE (800 mg/(kg•d)) from the Muscat hamburg grape juice could increase serum and liver tissue SOD activity, and reduce the MDA content inliver tissue. The AE from the Muscat grape juice contains polysaccharides, organic acids, flavonoids, polyphenols and other antioxidant substances, which have total phenolics 0.456 mg/mL, total flavonoids 4.37 mg/mL.
[1] LÜ Yuze, SONG Yu, WU Guohong, et al. The antioxidant activity of grape polyphenols[J]. Food Science, 2006, 27(12): 213.
[2] BAGCHI D, BAGCHI M, STOHS S J, et al. Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention[J]. Toxicology, 2000, 148(2/3): 187-197.
[3] HUANG S S, TSAI M C, CHIH C L, et al. Resveratrol reduction of infarct size in longevans mice subjected to focal cerebral ischemia[J]. Life Science, 2001, 69(9): 1057-1065.
[4] MO Jian. Active oxygen and its biological function[J]. Progress in Biochemistry and Biophysics, 1981, 38(2): 23-29.
[5] VALKO M, LEIBFRITZ D, MONCOL J, et al. Free radical and antioxidant in normal physiological functions and human disease[J]. The International Journal of Biochemistry & Cell Biology, 2007, 39: 44-84.
[6] PERRY G, RAINE K A, NUNOMURA A, et al. How important is oxidative damage? Lesson from Alzheimer’s desease[J]. Free Radical Biol Med, 2000, 22: 831-834.
[7] KAMAT J P, DEVASAGAYAM T P A. Oxidative damage mitochondria in normal and cancer tissues, and its modulation[J]. Toxicology, 2000, 155: 73-82.
[8] MINUSSI R C, ROSSI M, BOLOGNA L, et al. Phenolic compounds and total antioxidant potential of commercial wines[J]. Food Chemistry, 2003, 82: 409-416.
[9] IQBAL S, HALEEM S, AKHTAR M, et al. Efficiency of pomegranate peel extracts in stabilization of sunflower oil under accelerated conditions[J]. Food Reasearch Internation, 2008, 41(2): 194-200.
[10] BAE S H, SUH H J. Antioxidant activities of five different mulberry cultivars in Koreal[J]. Food Science and Technology, 2007, 40(6): 955-962.
[11] BAGCHI D, GARG A, KROHN R L, et al. Oxygen freen radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro[J]. Res Commun Mol Pahol pharmacol, 1997, 95(2): 179-189.
[12] CASTILLO J, BENAVENTE G O, LORENTE J, et al. Antioxidant activity and radioprotective effects against chromosomal damage induced in vivo by X-rays of flavan-3-ols(procyanidins) from grape seeds(Vitis vinifera): comparative study versus other phenolic and organic compounds[J]. Journal of Agricultural and Food Chemistry, 200, 48(5): 1738-1745.
[13] CHANVITAYAPONGS S, DRACZYNKA-LUSIAK B, SUN A Y. Amelioration of oxidative stress by antioxidants and resveratrol in PCl2 cells[J]. Neuro Report, 1997, 8(6): 1499-1502.
[14] LÜ Lishuang, CAO Dong. Extract oligomeric procyanidins of defatted grape seed[J]. Journal of Wuxi University of Light Industry, 2001, 20(2): 208-210.
[15] PENG Changlian, CHEN Shaowei, LIN Zhifeng, et al. Detection of antioxidative capacity in plants by scavenging organic free radical DPPH[J]. Progress in Biochemistry and Biophysics, 2000, 27(6): 658-661.
[16] LI Yan, BAO Huiyan, LAI Xuxin, et al. Reseach on the qxidation and antioxidation of eddible oils and fats[J]. China Food Additives, 1997, 4: 4-9.
[17] CHENG Shoujiang, JIANG Song. Phenolic compounds and their antioxidant activity in fruits and vegertables[J]. Journal of Anhui Technical Teachers College, 2003, 17(2): 144-148.
[18] KELLEY M G. Interaction of nitrogen availability duaring bloom and light intensity during veraison[J]. American Journal of Enology and Viticulture, 1998, 49(3): 341-349.
[19] SHENG Wei, XUE Jianping, XIE Bijun. Study on antioxidant activity of extracts from bulbil of dioscorea opposita[J]. Food Science, 2009, 30(3): 92-93.
[20] LIAN Xijun. Study on antioxidant activity of resistant starch of differents weet potatoes by DPPH method[J]. Food Stuff and Fats, 2009, 6: 26-28.
[21] ZHAO Yanhong, LI Jianke, ZHAO Wei, et al. Evaluation on antioxidant activities of extracts from common edible and medicinal plants in vitro[J]. Food Science, 2009, 30(3): 104-106.
[22] Beijing University of Chinese Medicine. Chemistry of Chinese materia medica[M]. Shanghai: Shanghai People’s Publishing Office, 1976.
[23] LI Jing, NIE Jiyun, LI Haifeng, et al. Folin-Ciocalteus determination the total polyphenols of grape and wine[J]. Journal of Fruit Science, 2008, 25(1): 126-131.
[24] JING Siqum, ZHENG Li, SU Jun. Extraction and determination of total flavonoids and polysaccharides from persistent calyx of physalis alkekengil[J]. Food Research and Development, 2008, 29(6): 82-84.
[25] LI Jianhui, MA Huiqin, CHEN Shangwu. Stusies on antimicrobial effect of grape polyphenols[J]. Journal of Chinese Institute of Food Science and Technology, 2008, 8(4): 100-101.
TS218
A
1002-6630(2013)03-0109-06
2012-02-08
新疆大学青年教师科研启动基金项目(QN070119)
普燕(1978—),女,讲师,博士研究生,研究方向为生物工程。E-mail:yanpuxj@yahoo.com.cn
