基于HPLC-DAD技术对大鼠灌胃八珍汤后尿液样品处理方法的研究
2012-12-22李文兰代岐昌季宇彬
李文兰,杨 洋,白 晶,2,代岐昌,季宇彬,2
1哈尔滨商业大学生命科学与环境科学研究中心;2国家教育部抗肿瘤天然药物工程研究中心,哈尔滨150076
Introduction
Bazhen decoction is composed of Radix Ginseng,Radix Rehmanniae,Rhizoma Atractylodis Macrocephalae,Radix Paeoniae Alba,Radix Angelicae Sinensis,Rhizoma Chuanxiong,Poria and Radix Glycyrrhizae.It treats the syndrome of deficiency of both qi and blood by enriching the blood and replenishing qi.The modern pharmacology investigations indicated that Bazhen decoction can transform the hemorheology,enhance the accommodation of immune system and balance the endocrine function[1-3].After making the pharmacological effects,the chemical components of Chinese medicine will be eliminated from the body through various metabolic pathways.Most of components absorbed into blood can be detected in urine,so it is very important to identify the chemical compositions of urine.
High performance liquid chromatography(HPLC)has played a dominant role in the analysis of pharmaceuticals[4].In this study,urine samples after oral administration of Bazhen decoction were treated by n-butanol (saturated with water),ethyl acetate,SPE column and so on.The optimal gathering time for urine samples was determined by HPLC-DAD[5-9].
Materials and Methods
Materials
Instruments:Agilent 1100 LC system(including quaternary gradient pump,vacuum degasser,autosampler,column oven and DAD detector);HPLC-3D ChemStation;TDL80-2B Feigecentrifuge;XW-80A Vortex Shaker;H66MC ultrasonator.
Reagents:homemadeultra-purewater,formicacid (HPLC grade,lot number 20050912,Tianjin Kermel Chemical Reagent Co.,Ltd.),acetonitrile(HPLC grade,lot number 2008082801,Shangdong Yuwang Industrial Co.,Ltd.)and methanol(HPLC grade,lot number 20080919142,Shangdong Yuwang Industrial Co.,Ltd.).Chinese herb medicine:Radix Ginseng,Radix Rehmanniae,Rhizoma Atractylodis Macrocephalae,Raidix Paeoniae Alba,Radix Angelicae Sinensis,Rhizoma Chuanxiong,Poria,and Radix Glycyrrhizae were purchased from Baofeng Medicine commercial Limited company and identified by Professor Zhang Delian.
Animal:body weight 200 ±20 g,male Wistar rats,Changchun National Biological Industry Base Laboratory Animal Center,Lot:SCXK-(Kyrgyzstan)2003-2004.
MethodsHPLC-DAD analysis conditions
Column:Hydrosphere C18(5 μm,4.6 mm×250 mm); mobile phase:acetonitrile-0.15%formic acid;column temperature:30℃;flow rate:0.5 mL/min;Injection volume:10 μL.Mobile phase gradient program as follows:

Table 1 Gradient elution program of mobile phase
Preparation of the solution taken orally
Weigh Bazhen decoction all parties 75 g,extracted with methanol for 2 times(in the condition of 70℃),first 8 times amount,refluxed 120 min,then 6 times amount,refluxed 90 min.Combined with the filtrate and vacuum recovery to dry,and further diluted with distilled water to get final concentration of 3 g/mL of Bazhen decoction for intragastric administration.
Collection of urine samples
Wistar male rats were fasted(free water)12 h,weight,treatment group were given at a dose of 1.5 mL/100 g body weight,the blank group were given distilled water,then were placed in metabolic cages and continue to fasting(free water).Urine samples were collected at 0-4,4-8,8-16,16-24 h,then were stored in a freezer at -20℃ and set aside.
Results and Discussion
Results
Comparison of three different sample preparation n-Butanol extraction
Frozen urine samples were thawed at room temperature and centrifuged for 10 min at 3000 r/min.2 mL of the supernatant was extracted 3 times with 2 times volumes of n-butanol(saturated with water).The n-butanol layer were combined and concentrated to dry in vacuo.Then,the extract was dissolved with 1 mL acetonitrile by ultrasound and filtrated through 0.22 μm membrane.The filtrate was analyzed by HPLC(Fig.1).HPLC analysis revealed that more polar components were extracted with n-butanol than non-polar components,and some components were lost.So,it didn’t meet experiment requirement.
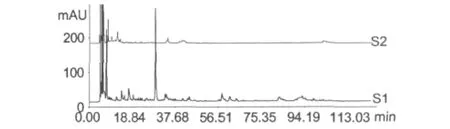
Fig.1 The chromatograms of drug-containing urine(S1) and blank urine(S2)extracted by n-butanol
Ethyl acetate extraction
Frozen urine samples were thawed at room temperature and centrifuged for 10 min at 3000 r/min.2 mL of the supernatant was extracted 3 times with 2 times volumes of ethyl acetate.The ethyl acetate layer were combined and concentrated to dry in vacuo.Then,the extract was dissolved with 1 mL acetonitrile by ultrasound and filtrated through 0.22 μm membrane.The filtrate was analyzed by HPLC(Fig.2).Ethyl acetate could only extract the least minor component in the urine samples after oral Bazhen decoction.It does not meet the test requirements.
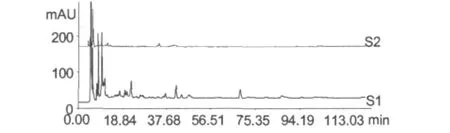
Fig.2 The chromatograms of drug-containing urine (S1)and blank urine(S2)extracted by ethyl acetate
Solid phase exatraction
Frozen urine samples were thawed at room temperature and centrifuged for 10 min at 3000 r/min.1 mL of the supernatant was loaded on a solid-phase extraction cartridge.The cartridge was wash with 1 mL of distilled water firstly,and then eluted with 2 mL of methanol and 1 mL of acetonitrile successively.The eluates were combined and concentrated to dry in vacuo,and the residue was dissolved with 1 mL acetonitrile for analysis by HPLC(Fig.3).It was observed that the urine samples purified by SPE gave more chromatography peaks than other samples.SPE was suitable for extracting the analytes of interest from the urine sample and provided a purified extract for the determination.
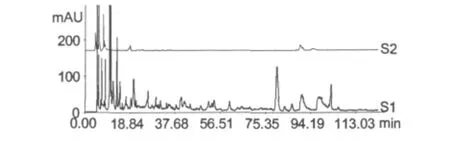
Fig.3 The chromatograms of drug-containing urine (S1)and blank urine(S2)extracted by SPE
Investigation of the best collection time of urine sample
Four serial urine samples(0-4,4-8,8-16,16-24 h after the dose)were treated with solid phase exatraction (Fig.4).HPLC fingerprint revealed that the 4-8 h urine samples have more metabolites of Bazhen decoction.So the optimum sampling time was 4-8 h.

Fig.4 The chromatograms of drug-containing urine between 0-4 h(S1),4-8 h(S2),8-16 h(S3)and 16-24 h(S4)
Discussion
Three sample preparation methods were compared in this paper.The extraction with n-butanol and ethyl acetate were incomplete due to some components were lost and fewer components were detected.SPE was chosen for extraction of urine due to its high selectivity,the results indicated that SPE was a suitable method for purification of urine after intragastric administration Bazhen decoction.
On this basis,the urine samples containing Bazhen decoction obtained in different time were pretreated by the SPE micro-column and analyzed by HPLC.The results showed that the urine samples after administration of 4-8 h have the most components of Bazhen decoction.The higher concentration was suitable to analyze,so selected 4-8 h as the best collecting time in rat urine.
In summary,pretreatment method of urine samples after oral Bazhen decoction was determined.It was that the blank and treated groups samples(4-8 h)were thawed at room temperature,then was centrifuged for 10 min at 3000 r/min.1 mL of the supernatant was loaded on a solid-phase extraction cartridge.The cartridge was wash with 1 mL of distilled water firstly,and then eluted with 2 mL of methanol and 1 mL of acetonitrile successively.The eluents were combined and concentrated to dry in vacuo,and the residue was dissolved with 1 mL acetonitrile for analysis by HPLC.
1 Pan HP.Pharmacological study and clinical application on Bazhen Tang.Chin Tradit Patent Med,2003,25(11):22-24.
2 Jiang N,Luo X,Chen DH.The effect of Bazhen decoction on cytokines in mice.J Sichuan Univ,2003,40:159-162.
3 Guo DP,Xie RL,Liang QB.Clinical observation of bazhen decoction in treating stable SLE anemia.J Emerg Tradit Chin Med,2008,33:58-62.
4 Wen HM,Guo KJ,Xing WL,et al.A study into components of ingested into blood of rats and rabbits.J Nanjing Tradit Chin Med Univ,2007,23(2):96-99.
5 Yang L,Xu SJ,Zeng X,et al.In vivo rat metabolism studies of ginsenoside-Rb1.Chem J Chin Univ,2006,6:1042-1044.
6 Yang L,Xu SJ,Zeng X,et al.Determination of ginsenoside Rd and itsmetabolites in rat urine by LC-MS.Acta Pharm Sin,2006,41:742-746.
7 Guo FQ,Huang LF,Zhou SY.Headspace solid-phase microextraction-gas chromatography-mass spectrometry for analysis of volatile components from Atractlodes macrocephala Koidz.Chin J Chromatogr,2007,25:43-47.
8 Liu Y,Shi RB,Liu B,et al.Variation of chemical compositions in body fluid of rats after taking decoction of Rhizoma Chuanxiong medicinal slices orally.J Beijing Univ Tradit Chin Med,2008,31:334-337.
9 Zhang L,Xu DS,Feng Y.Study on adscription of plasma effective constituents of rat after administrated with Paeonia lacliflora and Glycyrrhiza uralensis compound.China J Chin Mater Med,2007,32:1789-1791.
