联苯四甲酸及联吡啶构筑的一维链状银(Ⅰ)配合物的合成及晶体结构
2012-12-11王记江侯向阳曹培香高楼军张美丽任宜霞
王记江 侯向阳 曹培香 高楼军 张美丽 任宜霞
(延安大学化学与化工学院,陕西省化学反应工程重点实验室,延安 716000)
联苯四甲酸及联吡啶构筑的一维链状银(Ⅰ)配合物的合成及晶体结构
王记江*侯向阳 曹培香 高楼军 张美丽 任宜霞
(延安大学化学与化工学院,陕西省化学反应工程重点实验室,延安 716000)
采用水热法合成了一个新颖的配合物{[Ag(4,4′-bpy)][Ag2(H3btc)(H2btc)(4,4′-bpy)2]·3H2O}n(1)(H4btc=联苯-2,2′,4,4′-四甲酸;4,4′-bpy=4,4′-联吡啶),并对其进行了元素分析、红外光谱和X射线单晶衍射测定。配合物1属于三斜晶系,空间群为P1,a=1.12251(6)nm,b=1.55401(8)nm,c=1.77459(9)nm,β=91.6410(10)°,V=2.8308(3)nm3,Z=2,Dc=1.764 g·cm-3,μ=1.113 mm-1,F(000)=1508,R1=0.0450,wR2=0.0862(I>2σ(I))。在1中,一维线性[Ag(4,4′-bpy)]+阳离子链包含在一维{[Ag2(H3btc)(H2btc)(4,4′-bpy)2]-阴离子双链与游离水分子通过分子间氢键组装成的三维超分子结构中。研究了配合物的热稳定性和电化学性质。
银配合物;联苯四甲酸;晶体结构
Recently,the chemistry of Ag(Ⅰ)complexes has attracted interest for a number of reasons.The Ag(Ⅰ)is d10electronic configuration and can adopt different coordination numbers from 2 to 4.Moreover,the Ag complexes offer not only the fascinating structure,but only a wide range of potential application in many aspects,suchas optical,electrical conductivity,catalysis and even magnetic materials[1-4].Up to now,a large numbers of Ag(Ⅰ)complexes formed by Ag(Ⅰ)and various N-donor ligands have been successfully synthesized and characterized[5-14].However,the complex based on biphenyl-2,2′,4,4′-tetracarboxylic acid with Ag(Ⅰ)has never been reported before.
Inspired by our previous works[15],we employedbiphenyl-2,2′,4,4′-tetracarboxylic acid(H4btc)and Ag(Ⅰ)to synthesize a novel complex,namely,{[Ag(4,4′-bpy)][Ag2(H3btc)(H2btc)(4,4′-bpy)2]·3H2O}n(1),which provides the first example of complex based on biphenyl-2,2′,4,4′-tetracarboxylate-Ag(Ⅰ).
1 Experimental
1.1 Materials and methods
All reagents and solvents employed werecommercially available and used without furtherpurification. The C, H and N microanalyses werecarried out with a Vario EL elemental analyzer.Thermogravimetric analysis was performed on aNETZSCH STA 449F3 analyzer. The IR spectra wererecorded with a Nicolet Avatar 360 FTIR spectrometerusing the KBr pellet technique.
1.2 Syntheses of complex 1
A mixture of AgAc(0.15 mmol),H4btc(0.1 mmol),4,4′-bpy(0.15 mmol)and 10 mL H2O was stirred for 30 min.The mixture was then placed in a 23 mL Teflonlined stainless steel vessel and heated for 160℃for 4 d.Colorless crystals were obtained when the mixture was cooled to room temperature.Yield:ca.36%based on Ag.Calcd.for C62H47Ag3N6O19(%):C 49.52;H 3.15;N 5.59.Found(%):C 49.50;H 3.20;N 5.62.IR(KBr pellet,cm-1):3 430s,1 692m,1 595vs,1 576s,1 413m,1367m,1215w,1057w,804m,626w.
1.3 Crystal structure determination
Diffraction intensities for the complex 1 was collected at 296(2)K on a Bruker Smart APEXⅡCCD diffractometer equipped with a graphite-monochromated Mo Kα radiation(λ=0.071073 nm)using an ω-φ scan mode.A semiempirical absorption correction was applied using the SADABS program[16].The structure was solved by direct methods and refined by full-matrix least-squares on F2using the SHELXS 97 and SHELXL 97 programs,respectively[17-18].Non-hydrogen atoms were refined anisotropically and hydrogen atoms were placed in geometrically calculated positions.A total of 14 469 reflections of complex 1 were collected in the range of 1.93°<θ<25.25°(-13≤h≤13,-18≤k≤18,-17≤l≤21)and 10 171 were independent with Rint=0.0273,of which 6660 with I>2σ(I)(refinement on F2)were observed and used in the succeeding structure calculation.The final R1=0.0450,wR2=0.0862(w=1/[σ2(Fo2)+(0.035 6P)2+0.000P],where P=(Fo2+2Fc2)/3),(Δρ)max=527 e·nm-3and(Δρ)min=-408 e·nm-3.Selected bond lengths and bond angles are listed in Table 1.
CCDC:840549.
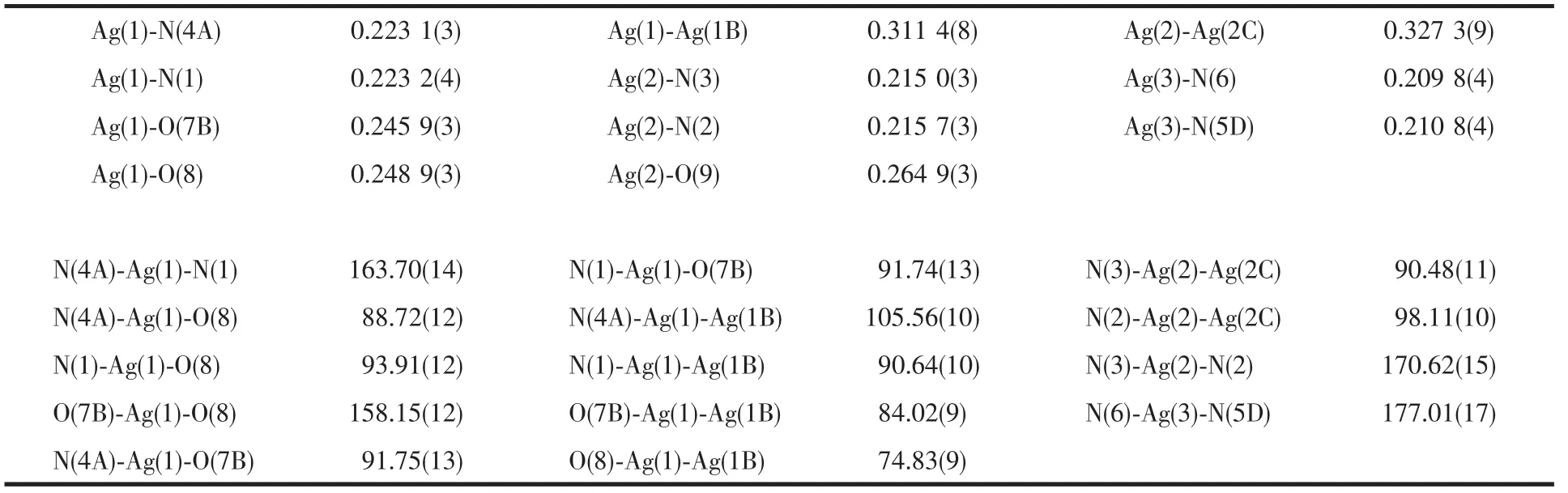
Table 1 Selected bond lengths(nm)and bond angles(°)for complex 1
2 Result and discussion
2.1 Description of crystal structures
Complex 1 features a complex{[Ag(4,4′-bpy)][Ag2(H3btc)(H2btc)(4,4′-bpy)2]·3H2O}ncontains 1D linear polymeric chains of[Ag(4,4′-bpy)]encapsulated within cavities built by 1D double-chains of[Ag2(H3btc)(H2btc)(4,4′-bpy)2].As depicted in Fig.1,the asymmetric unit of 1 contains three crystallographically independent Ag(Ⅰ)ions.Ag(1)is coordinated by two oxygen atoms fromtwo(H2btc)2-ligands(Ag-O 0.245 9(3)and 0.2489(3)nm)and two nitrogen atoms from two bpy ligands(Ag-N 0.223 2(4)and 0.223 1(3)nm)to furnish a distorted tetrahedral coordination.Different from those of Ag(1),Ag(2)adopts a distorted T-shaped coordination and is coordinated by an oxygen atom from one(H3btc)-ligands(Ag-O 0.264 9(3)nm)and two nitrogen atoms from two bpy ligands(Ag-N 0.215 7(3)and 0.215 0(3)nm).Ag(3)exhibits a linear coordination and is coordinated by two nitrogen atoms from two bpy ligands(Ag-N 0.209 8(4)and 0.210 8(4)nm).All the Ag-O and Ag-N bond lengths are in the normal ranges[13].

Fig.1 Coordination environments of Ag(Ⅰ)in complex 1
In the complex 1,Ag(1)and Ag(2)are linked together by μ2-bridged bpy ligands to form an infinite 1D chain depicted in Fig.2.The(H3btc)-ligands are located at the same side of the[Ag2(4,4′-bpy)2]chain.Two such neighboring[Ag2(H3btc)(4,4′-bpy)2]are joined together by(H2btc)2-anions facing opposite directions with bidentate bridging coordination mode to form a double-chain structure(Fig.2),which is further consolidated by the formation of inter-chain Ag…Ag interaction(Ag(1)…Ag(1B)0.311 4(8)nm,Ag(2)…Ag(2C)0.327 3(9)nm).The Ag…Ag distances are significantly shorter than the van der Waals contact distance 0.340 nm[19].Different from those of Ag(1)and Ag(2),Ag(3)is linked together by μ2-bridged bpy ligands to form 1D linear chain(Fig.3),which exists as a cation([Ag(4,4′-bpy)]+).

Fig.2 1D[Ag2(H3btc)(H2btc)(4,4′-bpy)2]-double-chain
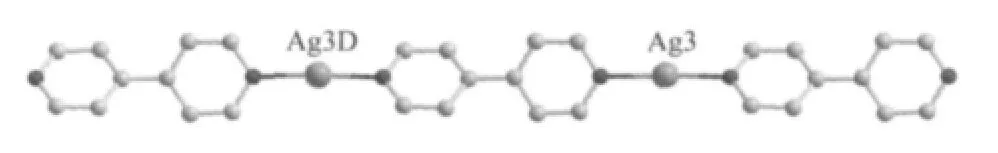
Fig.3 1D linear[Ag(4,4′-bpy)]+chain
Adjacent double-chains are connected together through intermolecular hydrogen bonds(distances O…O are in the range 0.254 8~0.294 6 nm)between the dissociative water molecules and uncoordinatedcarboxyl oxygen atoms, to generate a 3D supramolecular architecture.Interestingly,1D linear[Ag(4,4′-bpy)]+chains serving as the counter ions separated by dissociative water molecules reside in the cavities of 1D{[Ag2(H3btc)(H2btc)(4,4′-bpy)2]-double-chains(Fig.4).However,no obvious intermolecular interactions(hydrogen bonding,π-π stacking)have been found between the 1D linear chains and 1D double-chains in complex 1.It is likely that the electrostatic and van der Waals interactions play a crucial role in the assembly of this novel supramolecular architecture[10].

Fig.4 Clathrate-like structures of complex 1
2.2 Thermal analysis
The stability of the complex 1 was investigated by thermogravimetric analysis(Fig.5).The first weight loss of 3.76%for 1 is in the range from 26 to 116℃corresponding to the removal of H2O(calcd.3.59%).Upon further heating,an obvious weight loss(73.01%)occurs in the temperature range of 116~700℃,corresponding to the release of(H3btc)-,(H2btc)2-and 4,4′-bpy ligands(calcd.74.88%).After 700℃no weight loss is observed,indicating the complete decomposition of 1.The residual weight 23.23%(calcd.23.12%)corresponds to Ag2O.

Fig.5 TG of the complex 1
2.3 Electrochemistry
In the CV measurement,tri-electrode system was used with glass/C as working electrode,Pt as auxiliary electrode and SCE as reference electrode.The solvent is the mixture of methanol and water with complex condensation of 2.0×10-5mol·L-1.KCl was used as the supporting electrolyte was adopted.As depicted in Fig.6,one reduction peak(-0.856 V)corresponds to the Ag(Ⅰ)→Ag(0)single-electron reduction,the other oxidation peak(0.446 V)correspond to Ag(0)→Ag(Ⅰ)single-electron oxidation.Obviously,the transfer in the electrode reaction is irreversible.We can also deduce that the oxidizability of Ag(Ⅰ)in the title complex has been weakened.This study is significant for exploring the interrelation bet ween structure and property,developing the potential electric functional materials.

Fig.6 Cyclic voltammograms of complex 1
[1]Li C P,Chen J,Yu Q.Cryst.Growth Des.,2010,10:1623-1632
[2]Zheng S L,Zheng J P,Wong W T,et al.J.Am.Chem.Soc.,2003,125:6882-6883
[3]Natarajan S,Mandal S.Angew.Chem.Int.Ed.,2008,47:4798-4828
[4]HUANG Yan-Ju(黄艳菊),NI Liang(倪良).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(8):1649-1653
[5]Xiong K C,Wu M Y,Zhang Q F,et al.Chem.Commun.,2009:1840-1842
[6]Sun D,Luo G G,Zhang N,et al.Polyhedron,2010,29:1243-1250
[7]Sun D,Zhang N,Xu Q J,et al.Inorg.Chim.Acta,2011,368:67-73
[8]Sun D,Luo G G,Xu Q J,et al.Inorg.Chem.Commun.,2009,12:782-784
[9]Pramanik A,Das G.Cryst Eng Comm,2010,12:401-405
[10]Jiang J J,Li X P,Zhang X L,et al.Cryst Eng Comm,2005,7:603-607
[11]Xiong K C,Wu M Y,Zhang Q F,et al.Chem.Commun.,2009:1840-1842
[12]Li B,Zang S Q,Ji C,et al.Dalton Trans.,2011,40:788-792
[13]Ling Y,Chen Z X,Zhou Y M,et al.Cryst Eng Comm,2011,13:1504-1508
[14]Hao H J,Sun D,Li Y H,et al.Cryst.Growth Des.,2011,11:3564-3578
[15]Gao L J,Cao P X,Wang J J,et al.J.Coord.Chem.,2011,8:1299-1308
[16]Sheldrick G M.SADABS,A Program for Empirical Absorption Correction of Area detector Data,University of Göttingen,Germany,1997.
[17]Sheldrick G M.SHELXS 97,Program for Crystal Structure Solution,University of Göttingen,Germany,1997.
[18]Sheldrick G M.SHELXL 97,Program for Crystal Structure Refinement,University of Göttingen,Germany,1997.
[19]Jansen M.Angew.Chem.Int.Ed.,Engl.,1987,26:1098-1110
Synthesis and Crystal Structure of 1D Chainlike Ag(Ⅰ)Complex Assembled by Biphenyl-2,2′,4,4′-tetracarboxylic Acid and 4,4′-Bipyridine
WANG Ji-Jiang*HOU Xiang-Yang CAO Pei-XiangGAO Lou-Jun ZHANG Mei-LiREN Yi-Xia
(Department of Chemistry and Chemical Engineering,Shaanxi Key Laboratory of Chemical Reaction Engineering,Yan′an University,Yan′an,Shannxi 716000,China)
A novel Ag(Ⅰ)complex based on 4,4′-bipyridine(4,4′-bpy)and biphenyl-2,2′,4,4′-tetracarboxylic acid(H4btc),namely,{[Ag(4,4′-bpy)][Ag2(H3btc)(H2btc)(4,4′-bpy)2]·3H2O}n(1)has been hydrothermally synthesized and characterized by elemental analysis,IR spectroscopy and single-crystal X-ray diffraction analysis.The complex crystallizes in triclinic system,space group P1 with a=1.122 51(6)nm,b=1.554 01(8)nm,c=1.774 59(9)nm,β=91.6410(10)°,V=2.8308(3)nm3,Z=2,Dc=1.764 g·cm-3,μ=1.113 mm-1,F(000)=1508,and the final R1=0.0450,wR2=0.0862 for I>2σ(I).In complex 1,1D linear[Ag(4,4′-bpy)]+chains reside in 3D supramolecular structure of 1D{[Ag2(H3btc)(H2btc)(4,4′-bpy)2]-double-chains and dissociative water molecules assembled into by hydrogen bonds.In additional,thermal stability and electrochemistry of 1 have also been studied.CCDC:840549.
Ag(Ⅰ)complex;bipheny ltetracarboxylic acid;cryatal structure
O614.122
A
1001-4861(2012)04-0829-04
2011-10-30。收修改稿日期:2011-12-23。
国家自然科学基金(No.21103146)和陕西省教育厅科研基金(No.11JK0572)资助项目。
*通讯联系人。E-mail:yadxwjj@126.com,Tel(Fax):0911-2332037;会员登记号:S06N0331M1005。
