Quantitative characteristics of microorganisms in permafrost at different depths and their relation to soil physicochemical properties
2012-12-09WeiZhangXiaoPeiDongGuangXiuLiuGaoSenZhangXiuKunWuXiShengTaiHaoZhiLongBaoGuiZhang
Wei Zhang , XiaoPei Dong , GuangXiu Liu *, GaoSen Zhang , XiuKun Wu ,XiSheng Tai , HaoZhi Long , BaoGui Zhang
1. Key Laboratory of Desert and Desertification, Cold and Arid Regions Environmental Engineering Research Institute, Chinese Academy of Sciences, Lanzhou, Gansu 730000, China
2. School of Life Science, Lanzhou University, Lanzhou, Gansu 730000, China
Quantitative characteristics of microorganisms in permafrost at different depths and their relation to soil physicochemical properties
Wei Zhang1, XiaoPei Dong2, GuangXiu Liu1*, GaoSen Zhang1, XiuKun Wu1,XiSheng Tai1, HaoZhi Long1, BaoGui Zhang1
1. Key Laboratory of Desert and Desertification, Cold and Arid Regions Environmental Engineering Research Institute, Chinese Academy of Sciences, Lanzhou, Gansu 730000, China
2. School of Life Science, Lanzhou University, Lanzhou, Gansu 730000, China
Microorganisms in permafrost can live in cold environments due to coadapted physicochemical processes in this environment. In this paper, the relation between microbial number and soil physicochemical properties at the headwaters area of the Urumqi River is analyzed by using fluorescence microscopy counting and oligo-culture techniques. In total, 20 samples from a 200-cm permafrost core were used as study materials. The study reveals that the number of culturable bacteria has a significantly positive correlation with soil water content, total carbon and total nitrogen concentrations, and a significantly negative correlation with soil pH value. In addition, the ratio of culturable bacteria to total cell number decreases with depths. The results demonstrate that the number of culturable bacteria in permafrost is closely correlated with soil physicochemical properties and depositional age.
microorganisms in permafrost; culturable bacteria; physicochemical properties; correlation
1. Introduction
Permafrost regions account for almost 26% of the terrestrial ecosystems on the Earth. However, research into living organisms in permafrost, in particular microorganisms, has received little attention (Stevenet al., 2006). Even though the existence of culturable microorganisms in permafrost was indicated by research into mammoths in Siberia and the Far East at the end of the nineteenth century (Omelyansky,1911), there were hardly any key breakthroughs in the following century. Research into microorganisms and their adaptation mechanisms has received more attention since the 1990s. In 1997, the National Science Foundation Committee of America started a multidisciplinary integrated research project, named "Extreme Environment Life." The European Union financed a three-year project in 1996,called "The Biotechnology of Extremophiles," and then started a €7 million project in the next year, named "Extremophiles as Cell Factories" (Aguilaret al., 1998). In the same year, the journalExtremophileswas founded in Japan.These projects have greatly promoted the research of microorganisms in permafrost.
Microbial population structure and genetic evolution are sensitive to environmental conditions (Gilichinskyet al.,1989, 1992; Khlebnikovaet al., 1990; Bakermanset al.,2003; Wagneret al., 2005; Panikovet al., 2006; Rivkinaet al., 2006, 2007; Ganzertet al., 2007; Morozovaet al., 2007),and research into microbial distribution, hereditary variation,and natural selection in permafrost are useful in documenting past climate and environmental changes (Willerslevetal., 2003; Lydolphet al., 2005). Consequently, studies of microorganisms in permafrost are of great importance.
Low temperature can cause microbial death; consequently, this has restricted the microorganisms that can exist in permafrost. The microorganisms that do exist in permafrost can endure low temperatures but the mechanisms of cold tolerance remain unclear. Coadaptation between the microorganisms in permafrost and the permafrost environment is likely. In this study we analyze the microbial populations at different depths of permafrost, and have investigated the relationship between these populations and soil physicochemical properties. The results provide insight into optimal environmental conditions for soil microbial survival at the Urumqi River Head.
2. Materials and methods
2.1. Sampling
The Urumqi River Head (43°06′N, 86°49′E) is located in the east of Tianshan Mountain in Middle Asia. The average altitude is above 3,000 m. A 6 years continuous geothermal observation showed that the thickness of permafrost increased with the increase of altitude, and average earth temperature ranged from -0.7 °C at 3,348 m to -4.9 °C at 3,900 m (Liuet al., 2001; Ju and Liu, 2004).
The sample site was located near the Daxigou weather station (43°06′584′N, 86°50′301′E), the altitude was 3,586 m, and the average temperature was about -5.4 °C. A soil core 13 cm in diameter and 200 cm in depth was drilled. To avoid contamination, cores were obtained by drilling, using the column rotation method without washing with drilling fluid. Samples were taken at 10-cm intervals. The surface layer of the core was shaved with a sterilized knife and put into an aseptic aluminum tin, which was sealed and kept at-20 °C until further analysis.
2.2. Soil physicochemical properties analysis
Soil water content was measured by weight loss after 24 hrs at 90 °C. Soil pH value was measured in a 1:1 soil/deionized water slurry by using a digital pH meter(PT-10, Sartorius). After air-drying and grinding when passing through a 100-mesh sieve, total carbon and nitrogen of the soil were determined by using an elemental analyzer(Elementar Vario-EL, Germany).
2.3. Cultivation and total counts of bacteria
All samples were thawed at 4 °C. Soil samples were diluted by aseptically placing with 2 g of wet sediment in a flask containing 18 mL autoclaved 0.85% NaCl solution with glass beads, and shaken at 150 r/min for 15 min at 4 °C.The suspended cells and soil particles were serially diluted with autoclaved 0.85% NaCl solution. A 0.2-mL aliquot of dilution was plated on PYGV media and incubated aerobically at 25 °C. After seven days, the colony forming units(CFU) were calculated as averages of the triplicate plates.
Total counts of bacteria were determined by DAPI staining (4',6'-diamino-2-phenylindole, 1.5 μg/mL), as described by Margesinet al. (2010). Diluted samples were filtered through polycarbonate membrane filters and counted at 1,000× magnification under epifluorescence microscopy(BX51TRF, Olympus). Cell numbers present on random fields were counted (for at least 30 fields). Standard errors were calculated using at least three samples.
2.4. Statistical analysis
SPSS software (SPSS Inc., USA) was used for correlation analysis.
3. Results and discussion
Figure 1 shows that the culturable bacterial number varied from 2.00×104CFU/g to 6.00×105CFU/g in the study area, which is similar to counts for both the Antarctic permafrost region (0-105) (Parry and Marchant, 1992) and the Siberian permafrost region (0-108) (Gilichinskyet al., 1992;Vorobyovaet al., 1997), but higher than the Canadian permafrost region (101-103) (Stevenet al., 2007).
The results of correlation analysis showed that the culturable bacterial number was significantly correlated with water content, total C, total N, pH value, and depth (Table 1).In particular, the number of culturable bacteria was positively correlated with water content (r=0.854,P<0.01), total C (r=0.837,P<0.01), and total N (r=0.821,P<0.01), but negatively correlated with pH value (r=-0.782,P<0.01) and depth (r=-0.700,P<0.01). The depth of permafrost was positively correlated with pH value (r=0.715,P<0.01). The water content was positively correlated with total C(r=0.947,P<0.01) and total N (r=0.943,P<0.01), but negatively correlated with pH value (r=-0.574,P<0.01). The pH value was negatively correlated with total C (r=-0.579,P<0.01) and total N (r=-0.603,P<0.01). The total C was positively correlated with total N (r=0.994,P<0.01).
The liquid water film in permafrost is believed to be the principal survival habitat for microorganisms in permafrost.Since nutrients will concentrate in such a water film when the surrounding water is frozen (Deming, 2002), the film could protect the living bacteria. The liquid water film is thought to be the "nutrition medium" (Bitton, 2002). Such a water film could also let the nutrients exchange with metabolic end products, and this conversion would be important during cell growth. Based on this, the liquid water film is an important factor for the growth of microorganisms in permafrost.Therefore, the liquid water content is a critical factor determining the number and vitality of bacteria in permafrost, and in agreement with this, the number of culturable bacteria would be positively correlated with water content.
As the main nutrients for microorganisms in permafrost,the content of C and N could directly affect the microbial community (Zaket al., 1990, 1994). In addition, as a part of ecosystem, the soil microbiotas play important roles in the circulation of soil C and N (Jenkinsonet al., 1990; Jiang and Zhou, 2003). Our results indicated a positive correlation between culturable bacterial number and total C and N contents, as was expected.

Figure 1 Soil parameters changing with depths

Table 1 Correlation between culturable bacteria and physicochemical indexes
Figure 2 indicates that the ratio of culturable bacteria to total cell number was higher at the depth 50-60 cm, and decreased thereafter with increasing depth. These results indicated that the ability of microorganisms recovering in permafrost may not correlate with temperature, but with the permafrost depth. The reasons for this may be due to selection by low temperature, low water content, lack of nutrients,and geomagnetic radiation (Gilichinskyet al., 1989, 1992;Khlebnikovaet al., 1990). Our results also suggest that the viability of microbial populations living in the frost zone can be limited by exposure time to these conditions.
The freeze-thaw cycle is another factor determining microbial viability. Previous research indicated that the age of permafrost increased with the permafrost depth (Amatoet al., 2007). Zhanget al. (2007) found that the number of culturable bacteria decreased with an increase of permafrost age in the Qinghai-Tibet Plateau permafrost areas, and the number of culturable bacteria increased when the diversity of culturable bacteria decreased during permafrost melting. In a study of the Tianshan Mountain permafrost, Baiet al. (2006)also found that bacterial diversity decreased with the freeze-thaw cycle times. Presumably, the incidence of freeze-thaw cycles is higher with an increase of permafrost depth, and this correlates with lower microbial viability.
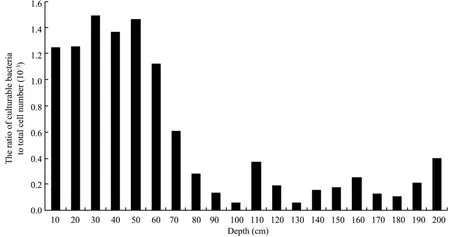
Figure 2 The ratio of culturable bacteria to total cell number at different depths
Organic acids are the main driving force for bacterial chemotaxis, and are important for absorption of nutrients,improvement of soil characteristics, and metal decontamination (Barbouret al., 1991; Grierson, 1992; Xia and Roberts,1994; Ryanet al., 1995). Chemotaxis is the directed movement of bacteria in response to changes in their chemical environment, especially in relation to localised carbon and nitrogen resources (Jianget al., 2005). Figure 1 indicates that the pH value increases with permafrost depth. As the pH value is principally determined by the organic acid content,the results indicate that the organic acid content decreases with permafrost depth. Previous research has demonstrated that there is only a minor contribution to soil organic acid content by atmospheric sedimentation or canopy transmittance, and the main source of organic acids in soil is from root exudates and soil microorganisms (Milletet al., 1997).Our data are consistent with a reduced bacterial population with increasing distance from the surface and plant rhizosphere that correlates with reduced organic acid supply and consequent increase of pH value with increasing permafrost depth that directly affects bacterial viability (Joneset al.,1996). Organic acids could not only improve the bacterial living environment, but also promote the usage of carbon and nitrogen resources by bacteria, influencing microbial viability and the number of culturable bacteria.
4. Conclusion
The viability of microorganisms in permafrost is due to coadaptation between these bacteria and physicochemical processes in the permafrost environment. It is exemplified by correlations between increasing permafrost depth and physicochemical factors such as decreasing water content,total C, total N, and increasing pH values associated with a decrease in the number of culturable bacteria. In other words,the number of culturable bacteria has a significantly positive correlation with water content, total C and total N, but a significantly negative correlation with permafrost depth and pH values. Our study reveals that the viability of microorganisms in the frost zone is closely linked with physicochemical properties of permafrost, and changes according to the physicochemical properties of permafrost.
The authors are very thankful to the Tianshan Glaciological Station, Cold and Arid Regions Environmental and Engineering Research Institute, Chinese Academy of Sciences.Thanks for their help during sampling procedure. This project is supported by the National Natural Science Foundation of China (Grant Nos. 30800154, 31170465, 31100365,40971034) and the China Postdoctoral Science Fund (Grant No. 20080430794).
Aguilar A, Ingemansson T, Magnien E, 1998. Extremophile microorganisms as cell factories: Support from the European Union. Extremophiles, 2(3):367-373.
Amato P, Hennebelle R, Magand O, Sancelme M, Delort A, Barbante C,Boutron C, Ferrari C, 2007. Bacterial characterization of the snow cover at Spitzberg, Svalbard. FEMS Microbiology Ecology, 59(2): 255-264.
Bai Y, Yang D, Wang J, Xu S, Wang X, An L, 2006. Phylogenetic diversity of culturable bacteria from alpine permafrost in the Tianshan Mountains,northwestern China. Research in Microbiology, 157: 741-751.
Bakermans C, Tsapin AI, Souza-Egipsy V, Gilichinsky DA, Nealson KH,2003. Reproduction and metabolism at -10 °C of bacteria isolated from Siberian permafrost. Environmental Microbiology, 5(4): 321-326.
Barbour WM, Hattermann DR, Stacey G, 1991. Chemotaxis ofBradyrhizobium japonicumto soybean exudates. Applied and Environmental Microbiology, 57(9): 2635-2639.
Bitton G, 2002. Encyclopedia of Environmental Microbiology. Wiley, New York.
Deming J, 2002. Psychrophiles and polar regions. Current Opinion in Microbiology, 25(1): 301-309.
Ganzert L, Jurgens G, Münster U, Wagner D, 2007. Methanogenic communities in permafrost-affected soils of the Laptev Sea coast, Siberian Arctic,characterized by 16S rRNA gene fingerprints. FEMS Microbiology Ecology, 59(2): 476-488.
Gilichinsky DA, Khlebnikova GM, Zvyagintsev DG, Fedorov-Davydov DG,Kudryavtseva NN, 1989. Microbiology of sedimentary materials in the permafrost zone. International Geology Review, 31(8): 847-858.
Gilichinsky DA, Vorobyova E, Erokhina LG, Fyordorov-Dayvdov DG,Chaikovskay NR, 1992. Long-term preservation of microbial ecosystems in permafrost. Advances in Space Research, 12(4): 255-263.
Grierson PF, 1992. Organic-acids in the rhizosphere ofBanksia integrifoliaL.f. Plant and Soil, 144: 259-265.
Horowitz NH, Cameron RE, Hubbard JS, 1972. Microbiology of the dry valleys of Antarctica. Advancement of Science, 176: 242-245.
Jenkinson DS, Andrew SPS, Lynch JM, Goss MJ, Tinker PB, Soulas G,Lagacherie B, 1990. The turnover of the organic carbon and nitrogen in soil. Philosophical Transactions of the Royal Society B, 329: 361-368.
Jiang J, Zhang R, He J, Zhang X, Cui Z, Li S, 2005. Bacterial chemotaxis to environmental pollutants and its significance in bioremediation. Acta Ecologica Sinca, 25(7): 1764-1771.
Jiang P, Zhou G, 2003. Changes in soil microbial biomass carbon and nitrogen under eroded red soil by vegetation recovery. Journal of Soil Water Conservation, 17(1): 112-114.
Jones DL, Prabowo AM, Kochian LV, 1996. Aluminum-organic acid interactions in acid soils. Plant and Soil, 182(2): 229-237.
Ju Y, Liu G, 2004. Climate and environment changes inferred from pollen records since 4000 BP in the headwaters of the Urümqi River, Tianshan.Journal of Glaciology and Geocryology, 26(2): 166-170.
Khlebnikova GM, Gilichinsky DA, Fedorov-Davydov DG, Vorobeva EA,1990. Quantitative evaluation of microorganisms in permafrost deposits and buried soils. Microbiology, 59(1): 106-112.
Liu G, Hu C, Zhang J, Shen Y, 2001. Microbial communities in permafrost of the Tibetan Plateau and their significance. Journal of Glaciology and Geocryology, 23(4): 419-422.
Lydolph MC, Jacobsen J, Arctander P, Gilbert MTP, Gilichinsky DA, Hansen AJ, Willerslev E, Lange L, 2005. Beringian paleoecology inferred from permafrost-preserved fungal DNA. Applied and Environmental Microbiology, 71(2): 1012-1017.
Margesin R, Płaza GA, Kasenbacher S, 2010. Characterization of bacterial communities at heavy-metal-contaminated sites. Chemosphere, 82:1583-1588.
Millet M, Wortham H, Sanusi A, 1997. Low molecular weight organic acids in fogwater in an urban area: Strasbourg (France). Science of the Total Environment, 206(1): 57-65.
Morozova D, Mohlmann D, Wagner D, 2007. Survival of methanogenic archaea from Siberian permafrost under simulated Martian thermal conditions. Origins of Life and Evolution of Biospheres, 37(2): 189-200.
Omelyansky VL, 1911. Bacteriological investigation of the Sanga mammoth and surrounding soil. Arkhiv Biologicheskikh Nauk, 16: 335-340.
Panikov NS, Flanagan PW, Oechel WC, Mastepanov MA, Christensen TR,2006. Microbial activity in soils frozen to below -39 °C. Soil Biology and Biochemistry, 38(4): 785-794.
Rivkina EM, Laurinavichius K, McGrath J, Tiedje J, Shcherbakova V,Gilichinsky D, 2006. Microbial life in permafrost. Advances in Space Research, 33(8): 1215-1221.
Rivkina EM, Shcherbakova V, Laurinavichius K, Petrovskaya L, Krivushin K, Kraev G, Pecheritsina S, Gilichinsky D, 2007. Biogeochemistry of methane and methanogenic archaea in permafrost. FEMS Microbiology Ecology, 61(1): 1-15.
Ryan PR, Delhaize E, Randall PJ, 1995. Malate efflux from root apices and tolerance to aluminum are highly correlated in wheat. Australian Journal of Plant Physiology, 22(4): 531-536.
Steven B, Leveille R, Pollard WH, Whyte LG, 2006. Microbial ecology and biodiversity in permafrost. Extremophiles, 10(4): 259-267.
Steven B, Briggs G, McKay CP, Pollard WH, Greer CW, Whyte LG, 2007.Characterization of the microbial diversity in a permafrost sample from the Canadian high Arctic using culture-dependent and culture-independent methods. FEMS Microbiology Ecology, 59(2):513-523.
Vorobyova E, Soina V, Gorlenko M, Minkovskaya N, Zalinova N, Mamukelashvili A, Gilichinsky D, Rivkina E, Vishnivetskaya T, 1997. The deep cold biosphere: Facts and hypothesis. FEMS Microbiology Reviews, 20(3-4): 277-290.
Wagner D, Lipski A, Embacher A, Gattinger A, 2005. Methane fluxes in permafrost habitats of the Lena delta: Effects of microbial community structure and organic matter quality. Environmental Microbiology, 7(10):1582-1592.
Willerslev E, Hansen AJ, Binladen J, Brand TB, Gilbert MTP, Shapiro B,Bunce M, Wiuf C, Gilichinsky DA, Cooper A, 2003. Diverse plant and animal genetic records from Holocene and Pleistocene sediments. Science, 300: 791-795.
Xia JH, Roberts JK, 1994. Improved cytoplasmic pH regulation, increased lactate efflux, and reduced cytoplasmic lactate levels are biochemical traits expressed in root-tips of whole maize seedlings acclimated to a low-oxygen environment. Plant Physiology, 105: 651-657.
Zak DR, Grigal DR, Gleeson S, Tilman D, 1990. Carbon and nitrogen cycling during old-field succession: Constraints on plant and microbial biomass. Biogeochemistry, 11(2): 111-129.
Zak DR, Tilman D, Parmenter RR, Rice CW, Fisher FM, Vose J, Milchunas D, Martin CW, 1994. Plant production and soil microorganisms in late-successional ecosystems: A continental scale study. Ecology, 75:2333-2347.
Zhang G, Ma X, Niu F, Dong M, Feng H, An L, Cheng G, 2007. Diversity and distribution of alkaliphilic psychrotolerant bacteria in the Qinghai-Tibet Plateau permafrost region. Extremophiles, 11: 415-424.
10.3724/SP.J.1226.2012.00127
*Correspondence to: Dr. GuangXiu Liu, Professor of Cold and Arid Regions Environmental and Engineering Research Institute, Chinese Academy of Sciences. No. 320, West Donggang Road, Lanzhou, Gansu 730000, China. Tel: +86-931-4967525;Email: liugx@lzb.ac.cn
September 21, 2011 Accepted: December 3, 2011
杂志排行
Sciences in Cold and Arid Regions的其它文章
- Research progress in monitoring and simulating stem radius growth: an overview
- A model test system with a dynamic load device for geotechnical engineering in cold regions
- Overall efficiency of a V-shaped nylon net fence in preventing sand damage to the Mogao Grottoes
- Analysis on mechanisms of anomalous variations of tropopause pressure over the Tibetan Plateau during summer in Northern Hemisphere
- Application study of the awning measure to obstruct solar radiation in permafrost regions on the Qinghai-Tibet Plateau
- Trend of snow cover fraction over East Asia in the 21st century under different scenarios
