含双咪唑配体的锌配位聚合物的晶体结构、荧光性质及抑菌性能研究
2012-09-15周先礼张秉君王萃娟帅邱光美王尧宇
周先礼张秉君王萃娟*,黄 帅邱光美王尧宇
(1西南交通大学生命科学与工程学院化学化工系,成都 610031)
(2西北大学化学与材料学院,合成与天然功能分子化学教育部重点实验室,西安 710069)
含双咪唑配体的锌配位聚合物的晶体结构、荧光性质及抑菌性能研究
周先礼1张秉君1王萃娟*,1黄 帅1邱光美1王尧宇2
(1西南交通大学生命科学与工程学院化学化工系,成都 610031)
(2西北大学化学与材料学院,合成与天然功能分子化学教育部重点实验室,西安 710069)
本文报道了一个基于双咪唑配体的锌配位聚合物[Zn(oxa)(bbi)]n(1)(oxa=草酸,bbi=1,1′-(1,4-丁二基)双咪唑),并对该化合物进行元素分析、红外和X-射线单晶衍射分析。结果表明标题化合物通过配位键和π-π堆积作用力形成了一个由(4,4)拓扑形状的二维网络组成的具有大孔洞的三维金属有机框架结构。除此之外,测定了配合物1的荧光发射光谱并对其微生物抑菌性能进行了初步研究。
1,1′-(1,4-丁二基)双咪唑;配位聚合物;荧光光谱;抑菌性
0 Introduction
Design,synthesis and structural characterization of novel multidimensional coordination polymers are of great interest due to their interesting architectures and promising applications in magnetism, gas separation,optoelectronics and biomimetic materials[1].Studies in this field have been focused on the design and preparation,as well as the structure-property relationships.As we know,a large variety ofcoordination complexes has been prepared through taking certain factors into account,such as the coordination nature of the metal ions and functionality,flexibility and symmetry of ligands as well as the unique reaction methods[2],which may be a key for the rational design of coordination complexes.Therefore,by judicious choice of the organic spacers and the central metals,it is possible to produce different types of coordination networks with novel topologies.Up To date,N-heterocyclic compounds have been extensively studied in coordination chemistry research for their excellent bridging ability[3-4].At this stage, lately, a large number of coordination complexes based on flexible bis(imidazole)ligands have been constructed[5-6].Such ligands bearing alkyl spacers are a good choice of a secondary N-donor bridging ligand,and the flexible nature of spacers allows the ligands to bend and rotate when they coordinate to metal centers for meeting the steric requirement upon metal binding,and this often causes the structural diversity[7],attributed to the fact that long ligands usually result in large voids which may result in numerous fascinating interpenetrated topological structures[8].Furthermore,as is widely known,bisbenzimidazoles have prominent antibacterial activities.Therefore,the biological activities of the imidazolyl compounds enhance the function ofthe metal complexes with imidazoles ligands.With this background in mind,we were devoted to the rational design and synthesis of relevant unreported coordination polymers based on flexible bis(imidazole)ligands,and further testing their bioactivity.
On the other hand,we introduced the dicarboxylic acids as second ligand,where the acids were chosen due to their strong coordination ability as well as rich coordination modes,such as terminal monodentate,chelating to one metal center,bridging bidentate in a syn-syn,syn-anti,and anti-anti configuration to two metal centers,and bridging tridentate to two metal centers[9], so as to construct various dimensions of coordination polymers.Moreover,d10metal ions present variable coordination numbers and geometries,and their complexes generally exhibit luminescent properties[10].In particular,the d10center metals and the conjugated π systems containing aromatic rings favor the development of fluorescent materials[11].Hence,in light of this statement,we used the derivative of bis(imidazole)in this work,namely 1,1′-(1,4-butanediyl)bis(imidazole)(bbi),and oxalic acid(H2oxa)as linker to obtain a luminescent metal-organic coordination polymer derived from d10metal salt (Zn(Ⅱ)),whose fluorescent property and preliminary biological tests will be represented and discussed.
1 Experimental
1.1 Materials and physical measurements
All reagents and solvents employed were commercially available and used as received without further purification.Infrared spectra on KBr pellets were recorded on a Nicolet Nexus 670 FT-IR spectrophotometer in the range 4 000 ~400 cm-1.Elemental analyses were determined with an Elementar Vario EL model instrument.Luminescence spectrum of the solid sample was recorded with a Hitachi 850 fluorescence spectrophotometer.
1.2 Synthesis of[Zn(oxa)(bbi)]n(1)
Complex 1 was obtained by the reaction of Zn(NO3)2,H2oxa and bbi in molar ratio 1∶1∶1 in watermethanol(1∶1)solvent at room temperature in a sealed glass tube of type H.Colorless crystals of 1 were collected in 74%yield(based on Zn).Anal.Calcd.for C12H14N4O4Zn(%):C 41.98,H 4.08,N 16.33;found(%):C 42.18,H 3.91,N 16.31.IR data (KBr,cm-1):3741(w),3110(s),2858(w),1613(s),1560(vs),1516(vs),1 358(m),1 229(m),1 109(s),829(w),784(m),663(s),659(s),487(w).
1.3 Crystal structure determination
Diffraction experiment for 1 was carried out with Mo Kα radiation(λ=0.071 073 nm)using a BRUKER SMART APEX CCD diffractometer at 298(2)K.A summary of the crystallography data and structure refinement is given in Table 1,and selected bond lengths and angles of complex 1 is listed in Table 2.Structure was solved by direct methods and refined with the full-matrix least squares technique on F2using the SHELXS-97[12]and SHELXL-97[13]program.All non-hydrogen atoms present were anisotropically refined and the other hydrogen atoms were treated as riding method.
CCDC:842497.
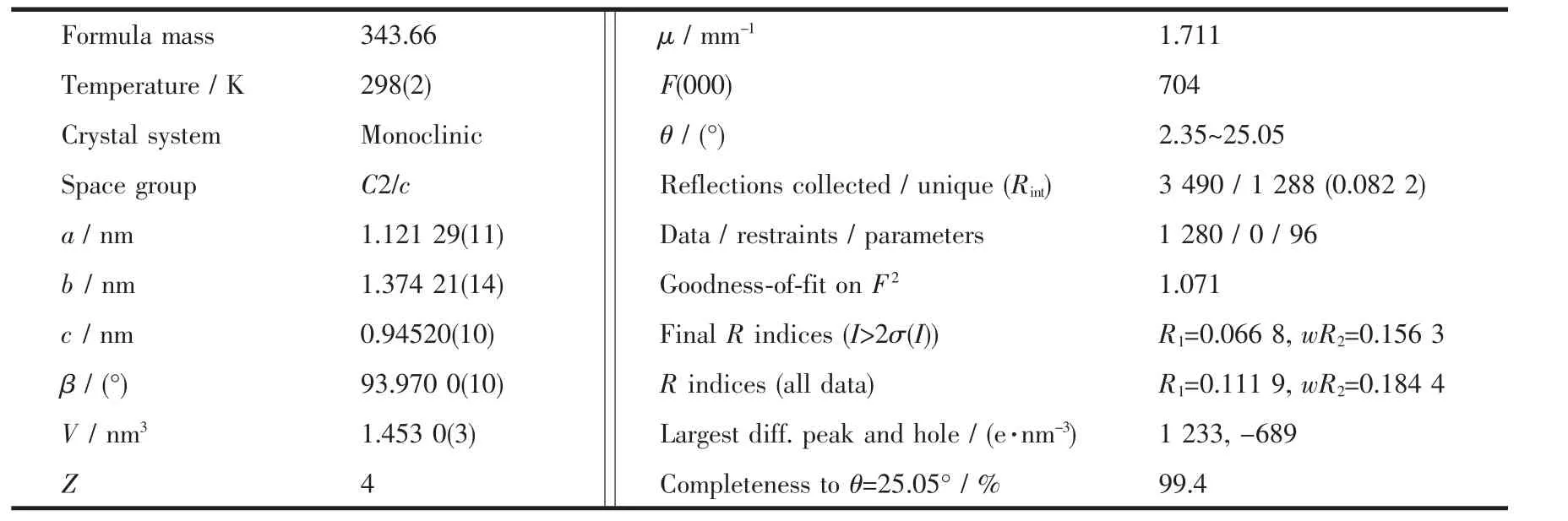
Table 1 Crystal data and structure refinements for 1
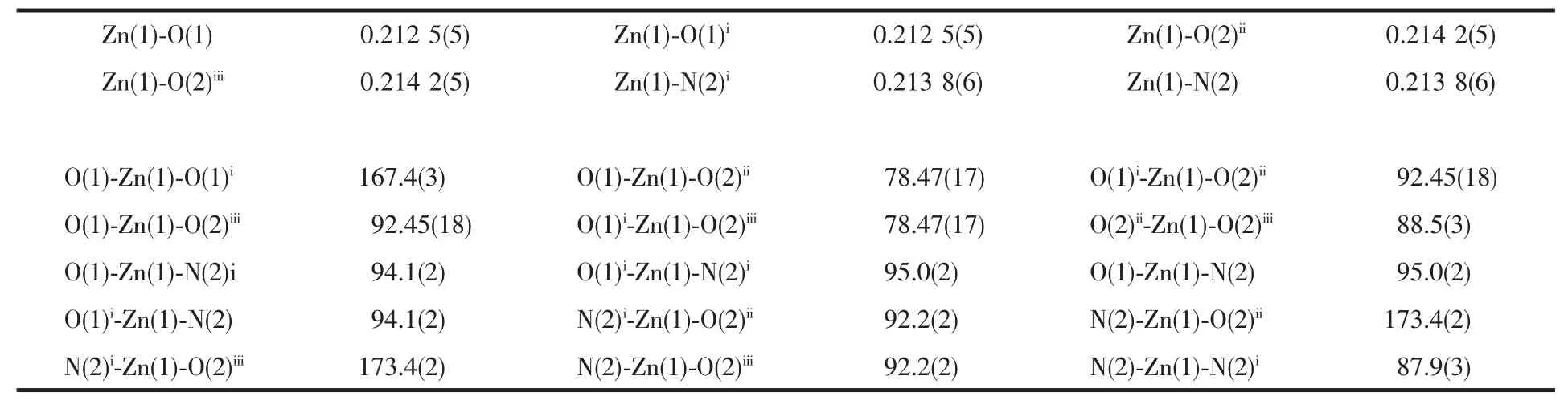
Table 2 Selected bond lengths(nm)and angles(°)for 1
1.4 Biological activity test
As a preliminary screening for antimicrobial activity,using agar diffusion method described in the previous paper[14],bbi and compound 1 dissolved in DMF were tested against standard strains of Escherichia coli, Staphylococcus aureus, and Enterobacter aerogenes,which belong to Gram-negative bacteria,Gram-positive bacteria and Gram-negative bacteria,respectively.The turbidities of these cultures were adjusted to equal that of a 0.5 McFarland turbidity standard using a spectrophotometer (600 nm).The amount of strains corresponded to about 1.5 ×108CFU·mL-1[15].After test strains were spread on the solid nutrient agar surface,stainless steel tubes (6 mm×8 mm×10 mm)were placed vertically on the surface.0.1 mL samples with certain concentration were injected to the steel tubes before 24 h incubation at 37℃.The inhibition zone around the disc was calculated as zone diameter in millimeters.An inhibition zone with a diameter less than 6 mm corresponds to lack of activity (6 mm is the diameter of the spot).Control experiments with solvents show that the solvents have no activity.Under the same cultivation conditions,selected duplicates were run to ensure reproducibility.
2 Results and discussion
2.1 Description of structure
Single-crystal X-ray diffraction reveals that the compound 1 adopts structure with space group C2/c,and the asymmetric unit consists of one Zn1 ion,one oxalate anion,and one bbi ligand.As shown in Fig.1,Zn1 ion adopts a distorted octahedral geometry,and is hexa-coordinated by two nitrogen atoms from two different bbi ligands and four carboxylate oxygen atoms from two different oxalate anions,completing the [Zn1O4N2]coordination sphere.The Zn1-N bond lengths in the crystal are equal(Zn1-N,0.213 8(6)nm),which are usual and well consistent with those of known zinc-imidazole complexes[16].The Zn1-O bond distances in the range of 0.212 5(5)~0.214 2(5)nmare normal and also comparable to those of reported zinc complexes having octahedral coordination geometry[17].Notably,Oxalate anion acts as a bidentate ligand and forms a five-membered ring with the central metal ion.It is interesting that dihedral angle between two five-membered rings linking the same central metal Zn1 ion is about 89.80°,which suggests the planes derived from different oxalate anions are mutually vertical to each other.In addition,the eight coordinating oxygen atoms of the two fussed chelate rings are cis-positioned with respect to the two imidazolyl nitrogen atoms.

As displayed in Fig.2,each oxalate anion links two neighboring Zn1 centers via a chelating bisbidentate coordination mode and serves as a bridging tetradentate ligand to form infinite one-dimensional(1D)wave-like metal chains with a Zn1…Zn1 separation of 0.5535 nm,while neighboring metal Zn1 centers located in adjacent metal-oxalate chains are bridged together by bbi ligands via Zn1-N coordination bonds,affording extraordinary mesohelical chains structure extended along the c axis with a Zn1…Zn1 separation of 1.184 4 nm.These chains contain both left-and right-handed helical loops in each chain,and display a “∞”shape.To date,only a few one-dimensional (1D) meso-helices among imidazole-based coordination polymershave been reported,which can be regarded as a threedimensional (3D)presentation ofa lemniscate.Furthermore,the bbi ligands as bidentate bridging ligandsconnectthe neighboring wave-like metal chains,thus resulting in the formation of a twodimensional layer structure with (4,4)topology which consist of thirty-membered rings constituted two bbi ligands,two oxalate anions and three Zn1 ions.It is noteworthy that the two distinct types of 1D chain structures,metal-oxalatechainsand metalmesohelical chains,are alternatively stacked in an-ABAB-sequence and are intercalated with each other which are shown in Fig.3.

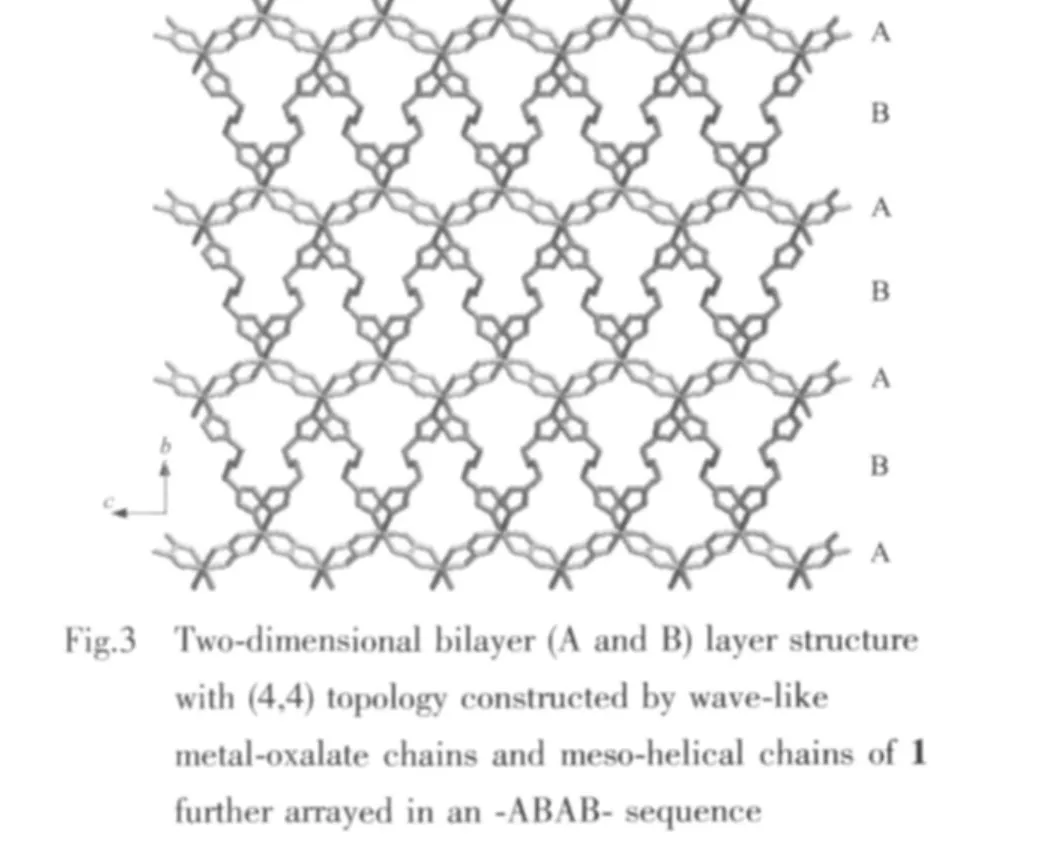
In such a coordination mode,the 2D layers are further stacked through intermolecular π-π stacking interactions(0.372 0(5)nm for centroid-centroid distance)of two equivalent imidazole rings,yielding three dimensional network structures.It is obvious that the flexibility of the bbi ligand has a great influence to the framework.Owing to the presence of a flexible-CH2CH2CH2CH2-spacer,the bbi ligand can link discrete clusters into an extended network and is agood candidatetoform highlyconnected 3D frameworks.Also,in structure of 1,as the methylene groups can bend freely relative to each other due to the C atoms connected via single bonds,the bbi ligand forms an S-shaped conformation,which makesthe voids in the three-dimensional network distorted,resulting in the formation of channels.The channels run approximately along the crystallographic c axis.
2.2 Fluorescence properties
The fluorescence spectrums of 1 and free bbi ligand in the solid state were investigated at ambient temperature,as depicted in Fig.4.The free bbi ligand exhibits a broad fluorescent emission at 338 nm upon excitation at 297 nm[18].Relative to bbi,1 exhibits a large red shift of emission with a main peak at 466 nm when irradiated at 280 nm.The fluorescent emission of 1 belongs neither to metal-to-ligand charge transfer (MLCT)nor ligand-to-metal charge transfer(LMCT),since Zn1 ions with d10configuration are difficult to be oxidized or to be reduced[19].Therefore,in comparison with those of the reported zinc complexes with N-donor ligands[20],the band is assigned to the intraligand(π*-π)fluorescent emission.The enhancement and red shift of the emission for 1 compared to bbi may be attributed to the coordination of Zn1 ions to bbi ligands and the use of second ligand because the photoluminescence behavior is closely associated with the local environments around the metal ions[21].The ancillary ligands and the counter anions play an important role in the fluorescence properties of d10metal complexes.

2.3 Biological results and discussion
The antimicrobial activities of bbi and 1 against bacteria (E.coli,S.aureus,and E.aerogenes)were examined by the inhibition zone test method.In this model,it could turn out that the samples exhibit moderate antibacterial activity against the selected strains.The ability of the samples to inhibit growth of the tested strains is listed in Table 3.From the viewpoint of the test,the inhibition activity of bbi and 1 increase with increase in the concentration of the test solution containing the samples.In addition,the antibacterial activity of bbi and 1 against the same strain give distinctness.Comparatively,it seems that compound 1 presents show higher activity against bacteria than the ligand bbi,especially in the activity against E.aerogenes and E.coli,whereas compound 1 exhibits only poor activity against E.aerogenes.The variation in the activity of different complexes against different organisms depends either on the impermeability ofthe cellsofthe microbesor differences in ribosomes in microbial cells[22].
These results indicate that bbi and compound 1 possess selective antimicrobial properties for the tested objects.Consequently,bbi and its compound are expected to have higher special bio-activity through further survey.
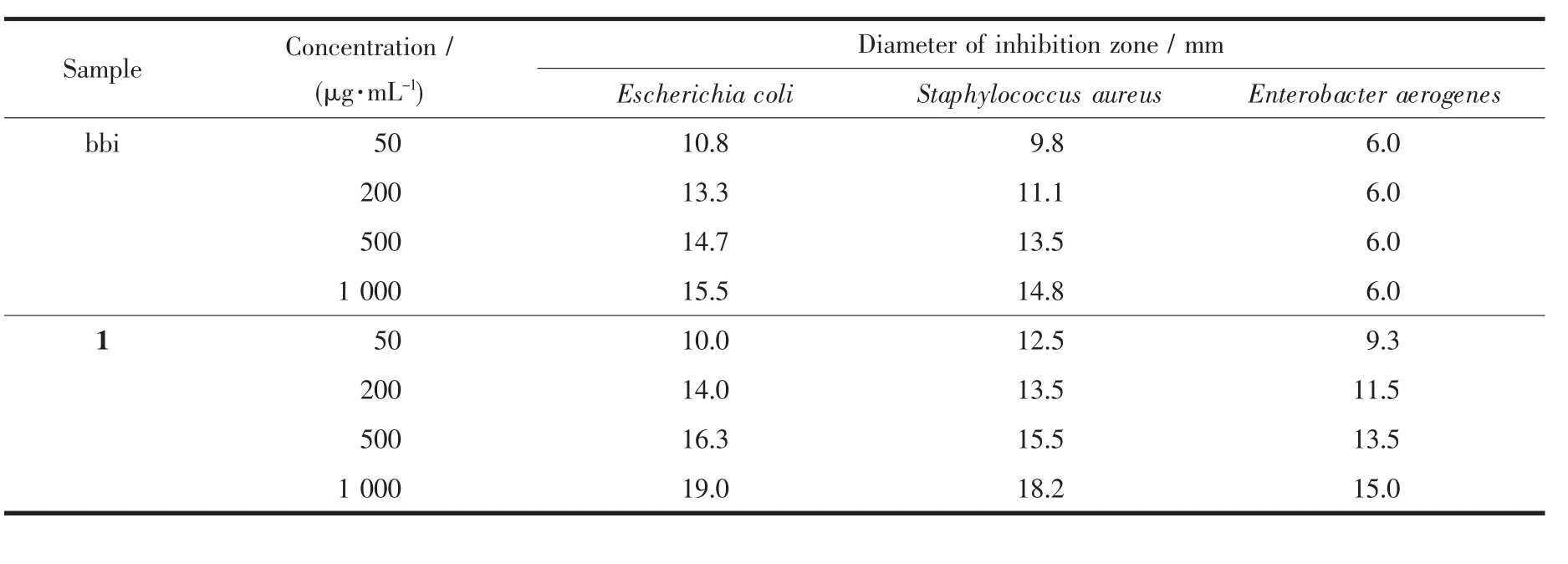
Table 3 Diameter of inhibition zone
References:
[1](a)Zhai Q G,Li S N,Gao X,et al.Inorg.Chem.Commun.,2010,13:211-214(b)Henninger S K,Habib H A,Janiak C,J.Am.Chem.Soc.,2009,131:2776-2777(c)Habib H A,Hoffmann A,Hppe H A,et al.Dalton Trans.,2009,10:1742-1751(d)Li D S,Fu F,Zhao J,et al.Dalton Trans.,2010,39:11522-11525(e)Zeng M H,Wang Q X,Tan Y X,et al.J.Am.Chem.Soc.,2010,132:2561-2563
[2](a)Braga D,Maini L,Polito M,et al.Coord.Chem.Rev.,2003,246:53-71(b)Tong M L,Wu Y M,Ru J,et al.Inorg.Chem.,2002,41:4846-4848(c)Zhang Y M,Wang L Y,Li B L,et al.J.Mol.Struct.,2008,875:527-539
[3](a)Meng C X,Li D S,Zhao J,et al.Inorg.Chem.Commun.,2009,12:793-795(b)Liu J Q,Zhang Y N,Wang Y Y,et al.Dalton Trans.,2009,27:5365-5378(c)Yan L,Li C B,Zhu D S,et al.J.Mol.Struct.,2011,1002:172-178(d)Fu F,Li D S,Gao X M,et al.Cryst.Eng.Commun.,2010,12:1227-1237
[4](a)Pan P B,Sun C F,Chen S M,et al.Inorg.Chem.Commun.,2011,14:1333-1336(b)Duan X Y,Cheng X,Lin J G,et al.Cryst.Eng.Commun.,2008,10:706-714(c)Li X J,Cao R,Guo Z G,et al.Polyhedron,2007,26:3911-3919(d)Mondal R,Basu T,Sadhukhan D,et al.Cryst.Growth Des.,2009,9:1095-1105
[5](a)Liu Y,Qi Y,Su Y H,et al.Cryst.Eng.Commun.,2010,12:3283-3290(b)Wang X L,Li J,Lin H Y,et al.J.Mol.Struct.,2010,983:99-103(c)Ma L F,Meng Q L,Wang L Y,et al.Inorg.Chim.Acta,2010,363:4127-4133(d)Shi X J,Wang X,Li L K,et al.Cryst.Growth Des.,2010,10:2490-2500>(e)LIU Tong-Fei(刘同飞),CUI Guang-Hua(崔广华),JIAO Cui-Huan(焦翠欢),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(7):1417-1422.
[6](a)Duan X Y,Li Y Z,Wang F M,et al.Inorg.Chem.Commun.,2010,13:1239-1243(b)Liu Q X,Shi M C,Wang Z Q,et al.Polyhedron,2010,29:2121-2126(c)KONG Zhi-Guo(孔治国),LU Ting-Feng(芦廷峰),WANG Qing-Wei(王庆伟),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(11):2271-2274(d)XIE Jing(谢静),CHEN Xuan(陈轩),LIU Guang-Xiang(刘光祥),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2007,7(23):1295-1298
[7](a)Wei G H,Liu Y Y,Yang J,et al.Inorg.Chem.Commun.,2008,11:1155-1158(b)Zhang W L,Liu Y Y,Ma J F,et al.Polyhedron,2008,27:3351-3358(c)Liu J Q,Wang Y Y,Jia Z B,et al.Inorg.Chem.Commun.,2011,14:519-521
[8](a)Liu M,Yang Z P,Sun W H,et al.Inorg.Chim.Acta,2009,362:2884-2889(b)Lu Y M,Lan Y Q,Xu Y H,et al.J.Solid State Chem.,2009,182:3105-3112(c)Huang X Y,Yue K F,Jin J C,et al.Inorg.Chem.Commun.,2010,13:338-341
[9](a)Policar C,Lambert F,Cesario M,et al.Eur.J.Inorg.Chem.,1999:2201-2207(b)Levstein P R,Calvo R.Inorg.Chem.,1990,29:1581-1583(c)Colacio E,Dominguez-Vera J M,Kiveks R,et al.Inorg.Chim.Acta,1993,212:115-121(d)Rueff J M,Masciocchi N,Rabu P,et al.Eur.J.Inorg.Chem.,2001:2843-2848(e)Li D S,Wu Y P,Zhang P,et al.Cryst.Growth Des.,2010,10:2037-2040
[10](a)Dong B X,Peng J,Gomez-Garca C J,et al.Inorg.Chem.,2007,46:5933-5941
(b)Cavero E,Uriel S,Romero P,et al.J.Am.Chem.Soc.,2007,129:11608-11618
(c)Shi X,Zhu G S,Wang X H,et al.Cryst.Growth Des.,2005,5:207-213
[11](a)Wen L L,Dang D B,Duan C Y,et al.Inorg.Chem.,2005,44:7161-7170(b)Wen L L,Li Y Z,Lu Z D,et al.Cryst.Growth Des.,2006,6:530-537(c)Wen L L,Lu Z D,Lin J G,et al.Cryst.Growth Des.,2007,7:93-99
[12]Sheldrick G M.SHELXS-97,Programs for X-ray Crystal Structure Solution,Universitt Göttingen,Germany,1997.
[13]Sheldrick G M.SHELXL-97,Programs for X-ray Crystal Structure Refinement,Universitt Göttingen,Germany,1997.
[14]GAO Jian(高健),CHEN Jun(陈军),Xu Xing-You(许兴友),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2004,20(11):1320-1324
[15]Aydin O N,Eyigor M,Aydin N.Eur.J.Anaesth.,2001,18:687-694
[16]Zhu H F,Fan J,Okamura T,et al.Cryst.Growth Des.,2005,5:289-294
[17]Jin S W,Wang D Q,Chen W Z.Inorg.Chem.Commun.,2007,10:685-689
[18]Ren Y X,Meng C X,Zhang M L,et al.J.Inorg.Organomet.Polym.(DOI:10.1007/s10904-011-9479-5)
[19](a)Yuan W B,Liu T F,Guo Z G,et al.J.Mol.Struct.,2010,965:82-88
(b)Yersin H,Vogler A.Photochemistry and Photophysics of Coordination Compounds I.Berlin:Springer,1987.
[20](a)Yang Z H,Xiong X F,Hu H M,et al.Inorg.Chem.Commun.,2011,14:1406-1409
(b)Zhang L H,Liu Y Y,Ma J F,et al.Polyhedron,2011,30:764-777
[21]Fu Z Y,Wu X T,Dai J C,et al.Eur.J.Inorg.Chem.,2002:2730-2735
[22]Sengupta S K,Pandey O P,Srivastava B K,et al.Transition Met.Chem.,1998,23:349-353
Structural Characterization,Luminescence Behavior and Antimicrobial Study of a Zinc Coordination Polymer Based on Bis(imidazole)Ligand
ZHOU Xian-Li1ZHANG Bing-Jun1WANG Cui-Juan*,1
HUANG Shuai1QIU Guang-Mei1WANG Yao-Yu2
(1Department of Chemistry and Chemical Engineering,School of Life Science and bioengineering,Southwest JiaoTong University,Chengdu 610031,China)
(2Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education,Department of Chemistry,Northwest University,Xi′an 710069,China)
A coordination polymer based on zinc(Ⅱ)and mixed ligands[Zn(oxa)(bbi)]n(1)(oxa=oxalate anion,bbi=1,1′-(1,4-butanediyl)bis(imidazole)),has been prepared and characterized by single-crystal X-ray diffraction.The structure of polymer 1 presents an unique two-dimensional(2D)layer structure with(4,4)topology in the presence of meso-helical chains and a three-dimensional(3D)network distorted with large channels.In addition,moderate fluorescent emission in the solid state at room temperature was observed in the Zn1-containing polymeric compound 1.And antimicrobial activity of the compound has been investigated.CCDC:842497.
1,1′-(1,4-Butanediyl)bis(imidazole);coordination polymer;fluorescence spectrum;antimicrobial activity
O614.24+1
A
1001-4861(2012)08-1736-07
2011-12-30。收修改稿日期:2012-05-06。
国家自然科学基金(No.20771090,No.21142004,No.31171695),新世纪优秀人才基金(No.NECT-08-0820),中央高校基本科研业务费专项资金(No.SWJTU2010ZT09,SWJTU12CX048),西南交通大学科学研究基金(No.2008A17)资助项目。
*通讯联系人。E-mail:ejuan6046@163.com
