一些含杂环配体的过渡金属配合物的微波合成、光谱、热化学性质和生物活性
2012-09-15JainRajendraKMishra
Jain Rajendra KMishra A P
(Department of Chemistry,Synthetic Inorganic&Coordination Chemistry Laboratories,Dr.H.S.Gour Central University,Sagar,Madhya Pradesh 470003,India)
一些含杂环配体的过渡金属配合物的微波合成、光谱、热化学性质和生物活性
Jain Rajendra K*Mishra A P
(Department of Chemistry,Synthetic Inorganic&Coordination Chemistry Laboratories,Dr.H.S.Gour Central University,Sagar,Madhya Pradesh 470003,India)
用5-氯邻羟苯甲醛与2-氨基-5-硝基噻唑(L1H)和4-氯苯甲醛与2-氨基-3-羟基吡啶(L2H)的反应,再用传统和微波方法将生成物与Cr(Ⅲ),Co(II),Ni(Ⅱ)和Cu(Ⅱ)合成了一些席夫碱配合物。用元素分析,FTIR,快原子轰击质谱,摩尔电导率,电子光谱,1HNMR,ESR,磁化率,热分析,电导率和XRD等对这些化合物进行了表征。这些配合物在空气中稳定并有颜色。分析数据表明所有配合物金属与配体的比均为1∶2,配位数为4或6。原子轰击质谱和热分析数据说明了这些配合物的降解方式。XRD图给出了这些配合物的结晶情况。席夫碱的上述金属配合物对革兰氏阳性菌:金黄色葡萄球菌和革兰氏阴性菌:大肠埃希菌(大肠杆菌)和真菌:黑曲霉和白色念珠菌显示出良好的抗菌活性。
微波方法;O,N供体;热分析;生物活性
0 Introduction
Transition metal complexes containing the Schiff base ligands have been of interest for many years because the transition metal complexes play a central role in the conduction of molecular materials,whichdisplay unusual conducting, magnetic, thermal properties and find application in materials chemistry and biochemistry[1].A large number of Schiff bases and their metal complexes have been found to possess important biological and catalytic activity[2].Various heterocycles,especially thiazole and pyridine,occupy an important place owing to their versatile activities due to the presence of multifunctional groups.Thiazoles and their derivatives play significant part in industry and biology[3].Pyridine derivatives play important role in many biochemical reactions.The molecular systems with chelating molecular designing and incorporating pyridine ring have been examined in the field of mycology for the development of metal based drugs.In several decades,efforts have been made to design and develop these drugs with pyridine based molecular devices[4].
Microwave-assisted synthesis is a branch of green chemistry.The application of microwave-assisted synthesis in organic,organometallic and coordination chemistry continues to develop at an astonishing pace[5-7].Microwave irradiated reactions under solvent free or less solvent conditions are attractive in offering reduced pollution,low cost and high yields with simplicity in processing and handling.The salient features of microwave approach are shorter reaction times,simple reaction conditions and enhancement in yields[8-10].
In this paper,we have described the synthesis,physicochemical characterization and biological activities of Cr(Ⅲ),Co(Ⅱ),Ni(Ⅱ) and Cu(Ⅱ) complexes with ligands derived from 5-chlorosalicylaldehyde with 2-amino-5-nitrothiazole (L1H) and 4-chlorobenzaldehyde with 2-amino-3-hydroxypyridine(L2H)(Fig.1).The reaction was carried out by both conventional and microwave methods.This ligand system coordinates with the metal ions in a bidentate manner through the oxygen and azomethine nitrogen.The metal complexes formed with these two new ligands may be used as precursors for the synthesis of new compounds.Some of them may exhibit interesting physical and chemical properties and potentially useful biological activities.
1 Experimental
All the chemicals and solvents used were of A R grade.All the reagents used for the preparation of the Schiff bases were obtained from Sigma-Aldrich.Metal salts were obtained from Loba Chemie.Elemental analyses were performed on an Elemental Vario EL III Carlo Erba 1108 analyzer.FAB-mass spectra were recorded on a JEOL SX 102/DA 6000 Mass Spectrometer using argon/xenon(6 kV,10 mA)as the FAB gas.
The accelerating voltage was 10 kV and the spectra were recorded at room temperature.Electronic spectra (in DMSO)were recorded on Perkin Elmer Lambda-2B-spectrophotometer.Molar conductance measurements were conducted using 10-3molL-1solution of the complexes in DMSO on Elico-CM 82 Conductivity Bridge at room temperature.Magnetic susceptibility measurements were carried out on a Gouy balance at room temperature using Hg[Co(SCN)4]as the calibrant.FTIR spectra were recorded using KBr pellet on a Perkin Elmer RX1 spectrophotometer in wave number region of 4 000~400 cm-1.1H NMR spectra were recorded on a JEOL AL300 FTNMR spectrometer employing TMS as the internal reference and CDCL3as the solvent.X-band EPR spectra were recorded on a Varian E-112 spectrometer at room temperature operating at the X-band region with 100 kHz modulation frequency,5 mW microwave power and 1 G modulation amplitude using TCNE as the internalstandard.Thermogravimetric analysiswas carried outunderatmospheric condition with a heating rate of 10℃·min-1on TGA Q500 Universal V4.5A TA Instrument.Powder X-ray diffraction(XRD)patterns were recorded on a RINT2000 wide angle goniometer.The solid state electrical conductivity has been measured by impedance spectroscopic method usingHIOKI3532-50 LCR Hitester.Microwave assisted synthesis were carried out in open glass vessel on a modified microwave oven model 2001 ETB with rotating tray and a power source of 230 V,microwave energy output of 800 W and microwave frequency of 2450 MHz.A thermocouple device used to monitor the temperature inside the vessel of the microwave.The microwave reactions were performed using on/off cycling to control the temperature.
1.1 Biological activity
The in vitro biological activity of the investigated Schiff base and its metal complexes was tested against the bacteria Escherichia coliand Staphylococcus aureus by disc diffusion method using nutrient agar as the medium and streptomycin as the control.The antifungal activities of the compounds were also tested bytheWelldiffusion method againstthefungi Aspergillus niger and Candida albicans,on potato dextrose agar as the medium and miconazole as the control.Each of the compounds was dissolved in DMSO and solutions with the concentration of 25,50 and 100 mg·L-1were prepared separately.In a typical procedure,a wellwasmade on agarmedium inoculated with microorganism.The well was filled with the test solution using a micropipette and the plate was incubated 24 h for bacteria at 37℃and 72 h for fungi at 30℃.During this period,the test solution diffused and the growth of the inoculated microorganism was affected.The inhibition zone was developed,at which the concentration was noted.
1.2 Conventional synthesis of Schiff bases
The Schiff bases have been synthesized by the condensation of equimolar ratio of 5-chlorosalicylaldehyde with 2-amino-5-nitrothiazole and 4-chlorobenzaldehyde with 2-amino-3-hydroxypyridine dissolved in ethanol.The resulting reaction mixture was stirred well,refluxed for 3~4 h and then allowed to cool overnight.The coloured solid precipitate of Schiff base obtained was filtered,washed with coldethanolseveraltimesand dried underreduced pressure in a desiccator.The purity of the synthesized compounds was checked by TLC using silica gel G(yield:72.5%~75.0%).
1.3 Microwavemethod forthesynthesisof Schiff bases
The equimolar (1∶1)ratio of 5-chlorosalicylaldehyde with 2-amino-5-nitrothiazole and 4-chlorobenzaldehyde (L1H) with 2-amino-3-hydroxypyridine were mixed thoroughly in a grinder.The reaction mixture was then irradiated by the microwave oven by taking 3 ~4 mL solvent.The reaction was completed in a short time(4~5 min)with higher yields.The resulting productwas then recrystallized with ethanol and finally dried under reduced pressure over anhydrous CaCl2in a desiccator.The progress of the reaction and purity of the product was monitored by TLC using silica gel G(yield:85.4%~87.0%).
1.4 Conventional synthesis of metal complexes
The metal complexes (Fig.2 and 3)have been prepared by mixing 50 mL ethanolic solution of CrCl3·6H2O/CoCl2·6H2O/NiCl2·6H2O/CuCl2·2H2O with 50 mL ethanolic solution of Schiff bases (L1H/L2H)in 1∶2(metal∶ligand)ratio.The resulting mixture was refluxed on water bath for 6~10 h.A coloured product appeared on standing and cooling the above solution.The precipitated complex was filtered,washed with ether and recrystallized with ethanol several times and dried under the reduced pressure over anhydrous CaCl2in a desiccator.It was further dried in electric oven at 50~70 ℃ (yield:59.5%~70.1%).
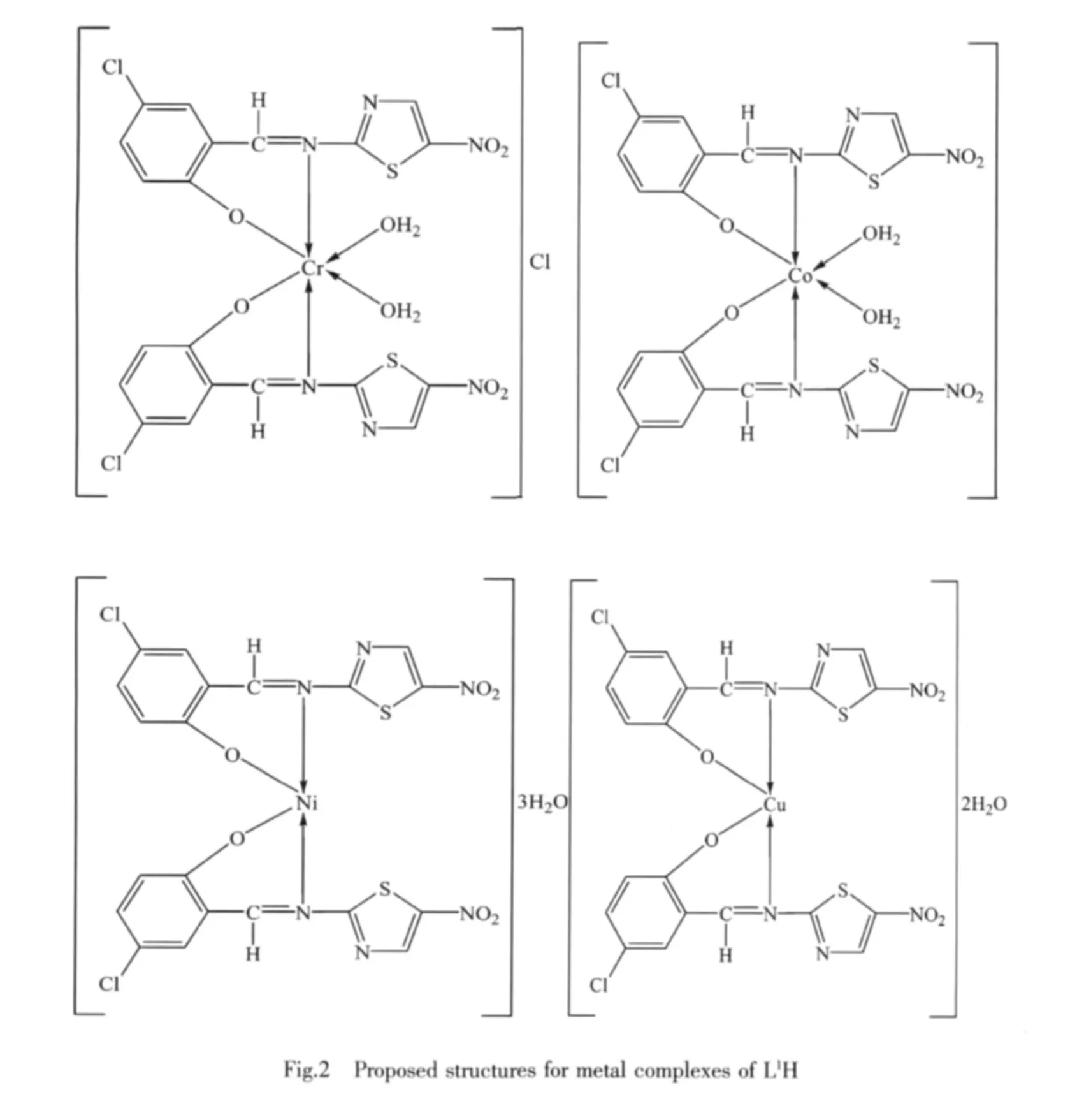
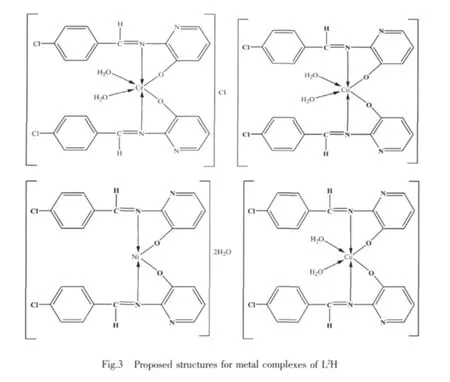
1.5 Microwave method for the synthesis of metal complexes
The ligand and the metal salts were mixed in 1∶2(metal∶ligand)ratio in a grinder.The reaction mixture was then irradiated by the microwave oven by taking 3~5 mL solvent.The reaction was completed in a short time (7~10 min)with higher yields.The resulting product was then recrystallized with ethanol and ether and finally dried under reduced pressure over anhydrous CaCl2in a desiccator.
The progress of the reaction and purity of the product was monitored by TLC using silica gel G(yield:78.0%~84.3%).
2 Results and discussion
As a result of microwave assisted synthesis,it was observed that the reaction was completed in a shorttime with higheryields compared to the conventionalmethod.In the microwave method homogeneity of the reaction mixture was increased by the rotating of reaction platform tray.The confirming of the results was also checked by the repeating of the synthesis process.Comparative study results obtained by microwave assisted synthesis versus conventional heating method is that some reactions required 3.2~9.2 h by conventional method was completed within 4.1~9.4 min.by the microwave irradiation technique,yields have been improved from 59.5% ~75.0%to 78.0%~87.0%.
All the metal complexes are coloured,solid and stable towards air and moisture at room temperature.They decompose on heating at high temperature,and are more or less soluble in common organic solvents.The comparative results of conventional and microwave methods and analyticaldata ofthe compounds,together with their physical properties consistentwith proposed molecularformula and magnetic moment values,are given in Table 1.All the metal chelates have 1∶2 (metal∶ligand)stoichiometry.The observed molar conductance of the complexes in DMSO at room temperature are consistent with the non-electrolytic nature of the complexes except Cr(Ⅲ)complex of both the Schiff base ligands,which was electrolytic nature.
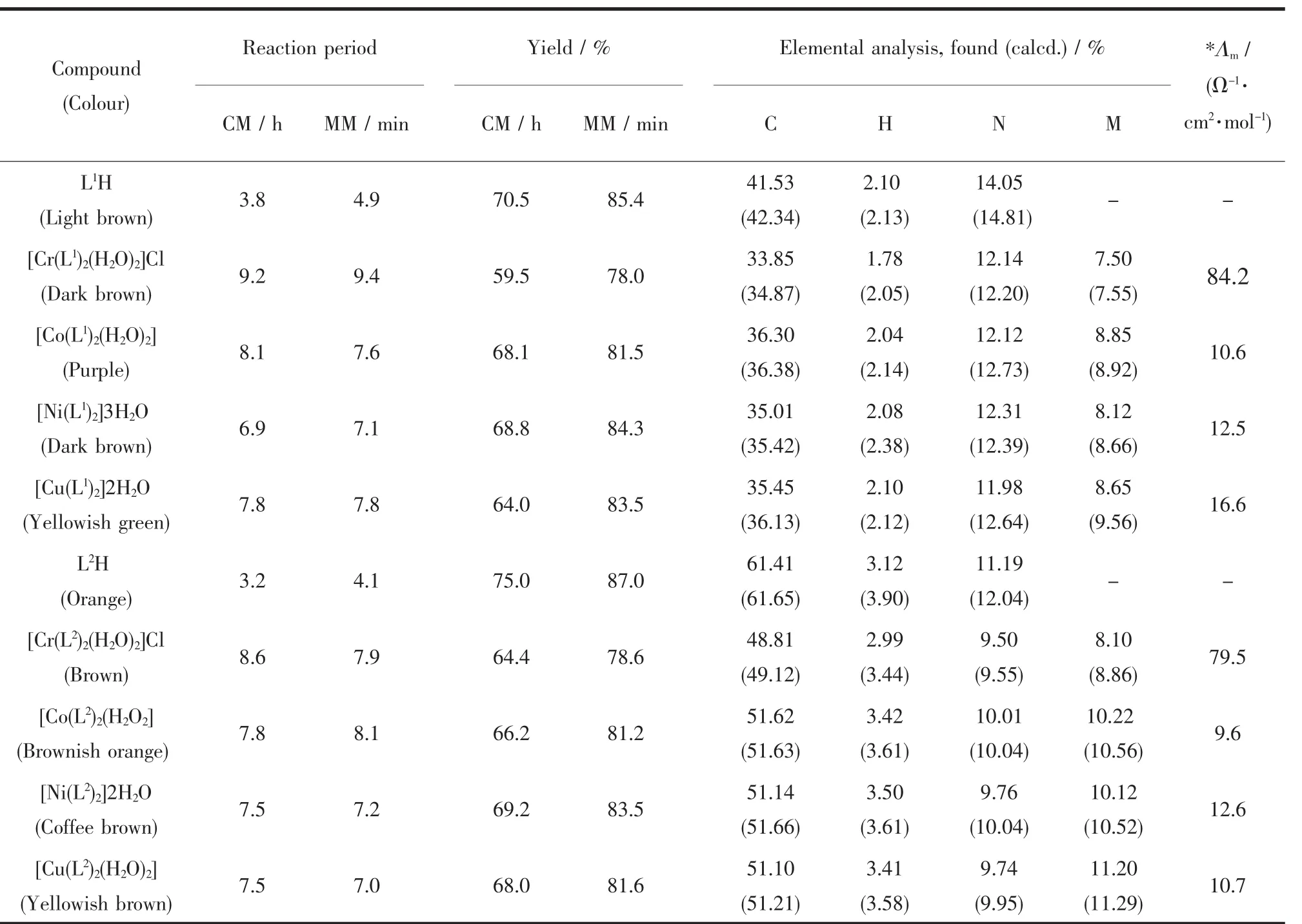
Table 1 Comparative results of conventional and microwave methods,analytical,and physical data of the compounds
2.1 FAB-mass spectra
The FAB-massspectrasuggestthatallthe complexes have a monomeric nature.These complexes show molecular ion peaks in good agreement with the empirical formula suggested by elemental analyses.The FAB mass spectra of the ligand(L1H)and its[Co(L1)2(H2O)2]complex,and(L2H)and its[Co(L2)2(H2O)2]complex were recorded and they are used to compare theirstoichiometry composition.The Schiffbases(L1H)and(L2H)show a molecular ion peak at m/z 285 and 230 respectively.The[Co(L1)2(H2O)2]and[Co(L2)2(H2O)2]complex shows a molecular ion peak at m/z 662 and 560, respectively, confirming the stoichiometry of Schiff bases and thier complexes as ML2type.Itis in good agreementwith the microanalytical data[11-12].
2.21H-NMR spectra
1H-NMR spectra of the Schiff bases were recorded in CDCL3solution using TMS as the internal standard.The1H-NMR spectra of the L1H ligand shows the signal at 7.250~7.668 ppm(m)for aromatic proton,6.986 ppm(s)for thiazole proton and 9.228 ppm(s)for azomethine proton.The peak at 12.279 (s)is attributed to the phenolic-OH group,disappeared upon shaken of D2O.The1H-NMR spectra of the L2H ligand shows the signal at 6.992~7.632 ppm(m)for aromatic proton and 8.783 ppm(s)for azomethine proton.The peak at 10.262 ppm(s)is attributed to-OH group present in pyridine moiety,disappeared upon addition of D2O[13-15].
2.3 IR spectra
The data of the IR spectra of Schiff base ligand and its metal complexes are listed in Table 2.The IRspectra of the complexes are compared with those of the free ligand in order to determine the involvement of coordination sites in chelation.Characteristic peaks in the spectra of the ligand and complexes are considered and compared.
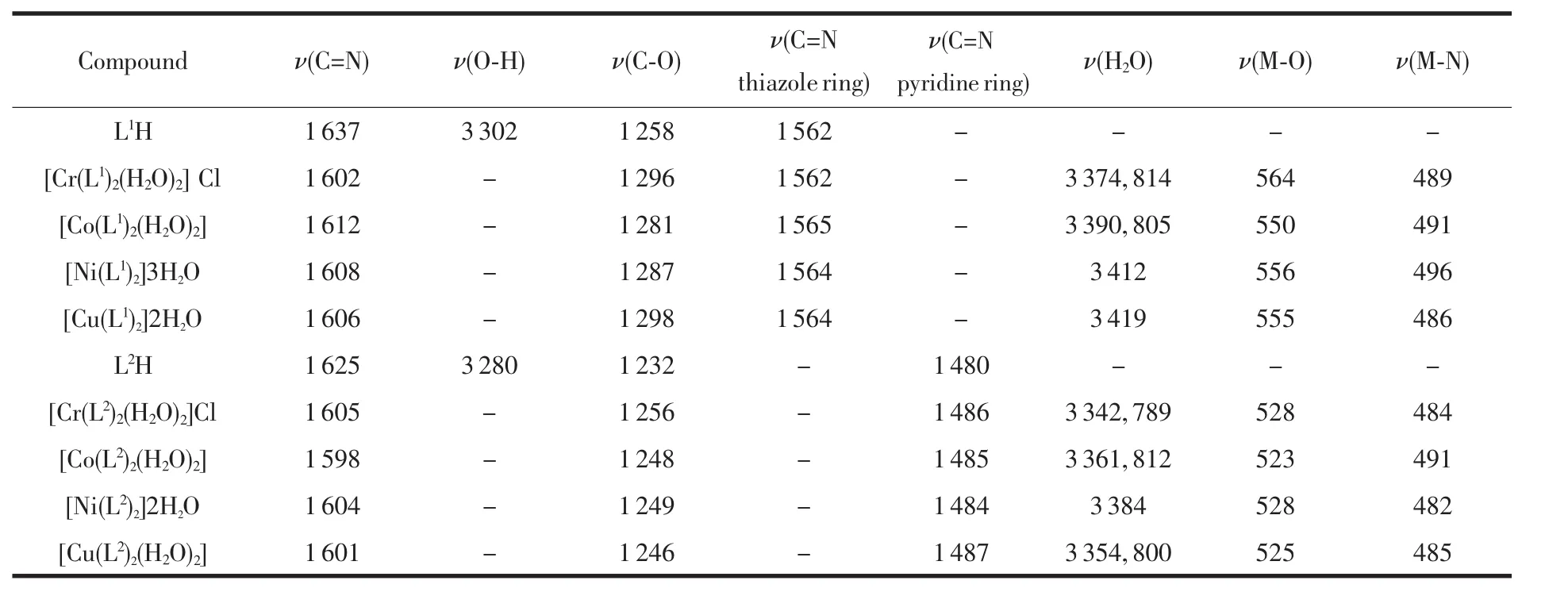
Table 2 IR bands of Schiff base ligands and their complexes
IR spectra of the L1H ligand exhibit the most characteristic bands at 3 302 cm-1Ⅴ(O-H),1 637 cm-1Ⅴ(C=N,azomethine),1 562 cm-1Ⅴ(C=N,thiazole),1 258 cm-1Ⅴ(C-O)and 754 cm-1Ⅴ(C-S-C).The formation of Schiff base, 5-chlorosalicylidene-2-amino-5-nitrothiazole is noted from the absence of C=O and NH2peaks in the ligand.All the metal complexes show a broad band at 3 374~3 419 cm-1which may be due to str(OH).A medium intensity band at 814 and 805 cm-1suggests the presence of coordinated water in Cr(Ⅲ) and Co(Ⅱ).The band at 1 637 cm-1due to the azomethine group of the Schiff base has shifted to lower frequency (1 602~1 612 cm-1)after complexation,indicating the bonding of nitrogen of the azomethine group to the metal ion and this can be explained by the donation of electrons from nitrogen to the empty d-orbital of the metal atom.The phenolic C-O stretching vibration appeared at 1 258 cm-1in Schiff base shifts towards higher frequencies (23~40 cm-1)in the complexes.This shift confirms the participation of oxygen in the C-O-M bond.The new bands at 550~564 cm-1and 486~496 cm-1have been assigned to M-O and M-N modes,respectively.The Ⅴ(C=N)at 1 563 cm-1and Ⅴ(C-S-C)at 754 cm-1of the thiazole ring remain unchanged,suggesting that thiazole group does not coordinate to metal by neither nitrogen nor sulpher atom[16-18].
IR spectra of the L2H ligand exhibit the most characteristic bands at 3 280 cm-1Ⅴ(O-H),1 625 cm-1Ⅴ(C=N,azomethine)and 1 480 cm-1Ⅴ(C=N,pyridine).The formation of Schiff base,4-chlorobenzylidene-2-amino-3-hydroxypyridine,is noted from the absence of C=O and NH2peaks in the ligand.All the metal complexes show a broad band at(3 342~3 384 cm-1)which may be due to Ⅴstr(OH).A medium intensity band at 789 cm-1,812 cm-1and 800 cm-1in Cr(Ⅲ),Co(Ⅱ) and Cu(Ⅱ),respectively,suggests the presence of coordinated water in these complexes.But this band is not presented in the Ni(Ⅱ)complex,indicating that the absence of coordinated water molecules in Ni(Ⅱ)complex.The band 1 625 cm-1due to the azomethine group of the Schiff base has shifted to lower frequency(1 598~1 605 cm-1)after complexation.This indicates the involvement of azomethine nitrogen to the metal ion.In the IR spectra of the complexes,the stretching vibration of the free ligand Ⅴ(O-H)3 280 cm-1is not observed,suggesting deprotonation of the hydroxyl group and formation of M-O bonds.In the low frequency region,the band of weak intensity observed for the complexes in the region 517~528 cm-1is attributed to(M-O)and in the region 482~491 cm-1to(M-N).The Ⅴ(C=N)at 1 480 cm-1in ligand,there is no appreciable change in the complexes spectrumwhich indicates that the pyridine ring nitrogen does not participate in coordination[16-18].
The IR data of both the Schiff base and its metal complexes show that the Schiff bases (L1H and L2H)behave as bidentates and are bonded to the metal ion through oxygen and imino nitrogen of azomethine group.
2.4 Electronic spectra and magnetic moment
The electronic spectral data along with ligand field parametersarepresented in Table3.The electronic spectra of[Cr(L1)2(H2O)2]Cl and[Cr(L2)2(H2O)2]Cl show bands in the range of 17 982~18 210 cm-1,23 940~24 440and 35981~35679 cm-1,which may be assigned to4A2g→4T2g(F)(Ⅴ1),4A2g→4T1g(F)(Ⅴ2)and4A2g→4T1g(P)(Ⅴ3)transitions,respectively.The values of magnetic moment for these complexes are 3.92 and 3.88 B.M.,respectively.Thus the octahedral geometry has been suggested for these complexes.
The electronic spectra of[Co(L1)2(H2O)2]and[Co(L2)2(H2O)2]exhibit three bands in the region of 9 631~ 9 281cm-1,14 751~15 540 cm-1and 20 025~20 480 cm-1which have tentatively been assigned to4T1g→4T2g(F)(Ⅴ1),4T1g→4A2g(F)(Ⅴ2)and4T1g→4T1g(P)(Ⅴ3)transitions,respectively.The magnetic moment values of the complexesare5.06 and 4.98 B.M.The observed transitions are consistent with an octahedral geometry.

Table 3 Electronic spectral and ligand field parameters data of complexes
The electronic spectra of the[Ni(L1)2]3H2O and[Ni(L2)2]2H2O show two bands at 13 811~13 212 cm-1and 19351~18620 cm-1assignable to1A1g→1Eg(1)and1A1g→1B2gtransitions, respectively. These are diamagnetic complexes; therefore square planar geometry has been suggested for these Ni(Ⅱ) complex.The electronic spectra of the Cu(Ⅱ)complex of L1H show two bands at 14 212 cm-1and 19 518 cm-1assignable to2B1g→2B2gand2B1g→2Egtransitions,respectively.Since the value of magnetic moment is 1.97 B.M.therefore square planar geometry has been suggested for Cu(Ⅱ) complex.The electronic spectrum of the Cu(Ⅱ)complex of L2H displays a single broad band at 14 480 cm-1corresponding to transition2Eg→2T2g.The value of magnetic moment for this complex is 1.82 B.M.;thus the octahedral geometry has been suggested for Cu(Ⅱ) complex.The parameters like B(Racah parameters),Dq,covalency factor β and LFSE have been calculated for the complexes[19-23].
2.5 ESR spectra
The ESR spectra of Cu(Ⅱ)provide information about the extent of the delocalization of unpaired electron.ESR spectra of both Cu(Ⅱ) complexes have been recorded on X-band and their g∥,g⊥,Δg,gavand G have been calculated.The values ofESR parameters g∥,g⊥,gav,Δg and G for Cu(Ⅱ) complex of L1H are 2.199 2,2.130 7,2.153 5,0.068 5 and 1.532 2,respectively.Similarly,the corresponding values for Cu(Ⅱ) complex of L2H are 2.2375,2.0665,2.1235,0.1710 and 3.6512,respectively.
ESR spectra of the complexes reveal two g values(g and g).Since the g∥and g⊥values are closer to 2,g∥>g⊥suggests a tetragonal distortion around the Cu(Ⅱ) ion.The trend g∥>g⊥>ge(2.0023)shows that the unpaired electron is localized in dX2-Y2orbital in the ground state of Cu(Ⅱ)and spectra are characteristic of axial symmetry.The g∥>2.3 is characteristic of an ionic environment and g∥<2.3 indicates a covalent environment in metal ligand bonding.The gvalues for the complexes are less than 2.3,suggesting the covalent environment.
The exchange coupling interaction between two Cu(Ⅱ) ions is explained by Hathaway expression G=(g∥-2.002 3)/(g⊥-2.002 3).According to Hathaway,if the value G is greater than four (G>4.0),the exchange interaction is negligible;whereas when the value of G is less than four (G <4.0)a considerable exchange coupling is presentin solid complex.The G values for the Cu(Ⅱ)complexes are less than four indicating considerable exchange interaction in the complexes[24-25].
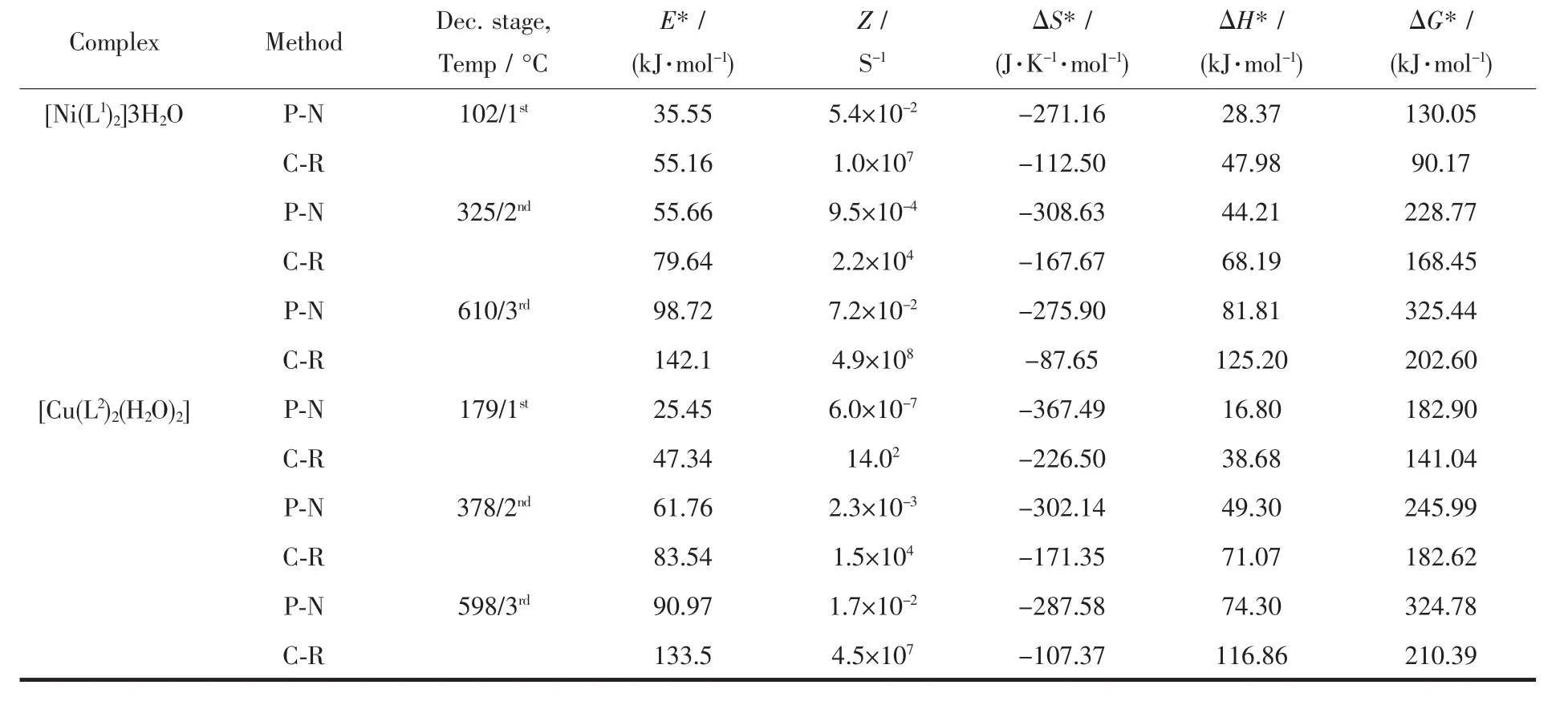
Table 4 Kinetic and thermodynamic parameters of complexes
2.6 Thermal analyses
The thermal behavior of metal complexes shows thatthe hydrated complexes lose molecules of hydration first;followed by decomposition of ligand molecules in the subsequent steps.
The thermal degradation behavior of the Ni(Ⅱ)complex of L1H has been studied by thermogravimetric analysis.The complex decomposes in three steps within the temperature range of 30~750 ℃.The TG curve of the complex shows that in first step the complex starts decomposition at 65℃.Elimination of lattice water molecules has been observed onincreasing the temperature up to 120℃(remaining wt%,obs./cald.,93.12/92.04).The complex does not show any weight loss between 120~210 ℃.After 210℃in second step,a weight loss has been observed in general up to 370℃corresponding to the loss of noncoordinated ligand part from the complex(remaining wt%,obs./cald.,62.12/59.32),which is considered to be the removal of thiazole moiety.Above 450 ℃ in third step,an inflection occurs in the curve and loss in weight goes up to 655℃.This indicates the elimination of coordinated part of the complexes.After 655℃,aplateauhasbeenobservedwhich corresponds to metal oxide as an ultimate pyrolysis product(remaining wt%,obs./cald.,23.42/19.54).The DTG curve of the complex shows peak at 102,325 and 610℃.
The thermal degradation behavior of the Co(Ⅱ)complex of L2H has been studied by thermogravimetric analysis.The complex decomposed in three steps.The thermogram of the Cu(Ⅱ)complex of L2H indicates that the complex is stable up to 110℃.Above 110℃ in first step,an elimination of two coordinated water molecules has been observed between the temperature range of 110 ~205 ℃ (remaining wt%,obs./calcd.,93.65/93.60).Above this temperature in second step a weight loss has been observed in general up to 410℃indicating the decomposition of the ligand moiety(remaining wt%,obs./calcd.,50.25/45.87),which is considered to be the removal of benzaldehyde moiety.The decomposition of remaining ligand moiety in third step occurs between 460~630℃,above 620℃ a horizontal curve has been obtained suggesting the ultimate pyrolysis product as metal oxide(remaining wt%,obs./calcd.,24.70/20.52).DTG curve of the Cu(Ⅱ) complex shows peak at 179,378 and 598 ℃[28-30].
Apart from evaluating the thermal stability of the metal complexes,this study also helps to characterize the metal complexes.
2.7 Kinetic study
The kinetic evaluations of the thermal decomposition of the complexes were carried out.All stages were selected for the study of kinetics of decomposition of complexes.The kinetic parameters data are summarized in Table 4.On the basis of thermal decomposition,the kinetic analysis parameter such as activation energy (E*),pre-exponential factor(Z),entropy of activation(ΔS*),enthalpy of activation(ΔH*)and free energy of activation (ΔG*)were calculated by using Piloyan-Novikova[26]and Coats-Redfern[27]equation.
Piloyan-Novikova:ln[α/T2]=ln[ZR/(βE*)]-E*/(RT)
Coats-Redfern:ln[g(α)/T2]=ln[ZR/(βE*)]-E*/(RT)
where α is the fraction of the reacted material,T is the absolute temperature,g(α)is integral mechan-ism function,E*is the activation energy in kJ·mol-1,Z is the pre-exponential factor,β is the heating rate and R is the gas constant.A straight line plot of the left hand side of the above equations against 1/T gives the value of E*and Z from the slope and the intercept,respectively.The entropy of activation (ΔS*),enthalpy of activation(ΔH*)and free energy of activation(ΔG*)were calculated using the following equation:
ΔS*=2.303(logZh/kT)R
ΔH*=E*-RT
ΔG*= ΔH*-TΔS*
where k and h are the Boltzmann and Planck constant,respectively.
The high values of activation energies reflect the thermal stability of the complexes.The complexes have negative entropy,which indicatesthatthe decomposition reactions proceed with a lower rate than the normal ones.The negative value of entropy also indicates that the activated complexes have a more ordered and more rigid structure than the reactants or intermediates.The negative values of the entropies of activation are compensated by the values of enthalpies of activation,leading to almost the same values for the free energy of activation[28-29].
2.8 X-ray diffraction study
X-ray diffraction was performed of metal complexes.The XRD patterns indicate crystalline nature for the complexes.X-ray powder diffractogram of the complexes were recorded using CuKα as source in the range 5°~70°(2θ).X-ray crystal system hasbeen worked out by trial and error methods for finding the best fit between observed and calculated sin2θ.The diffractogram of Ni(Ⅱ) complex of L1H has recorded 12 reflections with maxima at 2θ=7.97465 with interplanar distance d=11.077 75 nm.The complex crystallized in tetragonal system.Sin2θ and hkl values for different lattice planes have been calculated.Crystal data for complex,a=b=1.5671 nm,c=2.3244 nm,V=570.798 nm3,Z=6,Dobs=1.338 6 g·cm-3and Dcal=1.286 4 g·cm-3.The observed and calculated values of density and sin2θ show good agreement[30-31].
2.9 Electrical conductivity
The temperature dependence of the solid state conductivity(σ)of the compounds in their compressed pellet form have been measured at fixed frequency 1KHz in the temperature range of 297~413 K.The values of the solid state electrical conductivity of the Schiff base,its complexes increase with increasing temperature,decrease upon cooling over the studies temperature range indicating theirsemiconducting behavior.The general behavior of electrical conductivity follows the Arrehenius equation:
σ=σoexp[-Ea/(KT)]
where Eais the thermal activation energy of conduction,σois the conductivity constant,k is the Boltzman constant.The lots of σ vs 1000/T for all the compounds are found to be linear over a studies temperature range.The room temperature electrical conductivity of all the compounds lies in the range of 1.21×10-6~5.42×10-9Ω·cm-1.These values show their semi-conducting nature.The electrical conductivity at room temperature for the complexes of L1H are Cr>Co>Cu>Ni and for the metal complexes of L2H is Co>Cr>Cu>Ni.The activation energy of the compound lies in the range 0.248 ~0.785 eV[32-34].The confirming of the temperature dependence conductivity ofthe compounds was also checked by the repeating of the conductivity measurements.
2.1 0 Biological screening
The in-vitro Antimicrobial activity of the synthesized Schiff base ligands and their corresponding metal complexes on selected bacteria E.coli and S.aureus and two fungi A.niger and C.albicans was carried out.All of the tested compounds show good biological activity against microorganism.On comparing the biological activity of the Schiff base and its metal complexes with the standard bactericide and fungicide,it is shown that the metal complexes have moderate activity as compared to the standardbut all the complexes are more active than their respective ligands.The higher inhibition zone of metal complexes than those of the ligands can be explained on the basis of Overtones concept and Chelation theory.On chelation,the polarity of the metal ion will be reduced to greater extent due to the overlap of the ligand orbital and partial sharing of the positive charge of the metal ion with donor groups.Further,it increases the delocalization ofπ-electrons over the whole chelating ring and enhances the penetration of the complexes into lipid membranes and blocking of the metal binding sites in the enzymes of microorganisms.There are other factors which also increases the activity are solubility,conductivity and bond length between the metal and ligand[35-38].
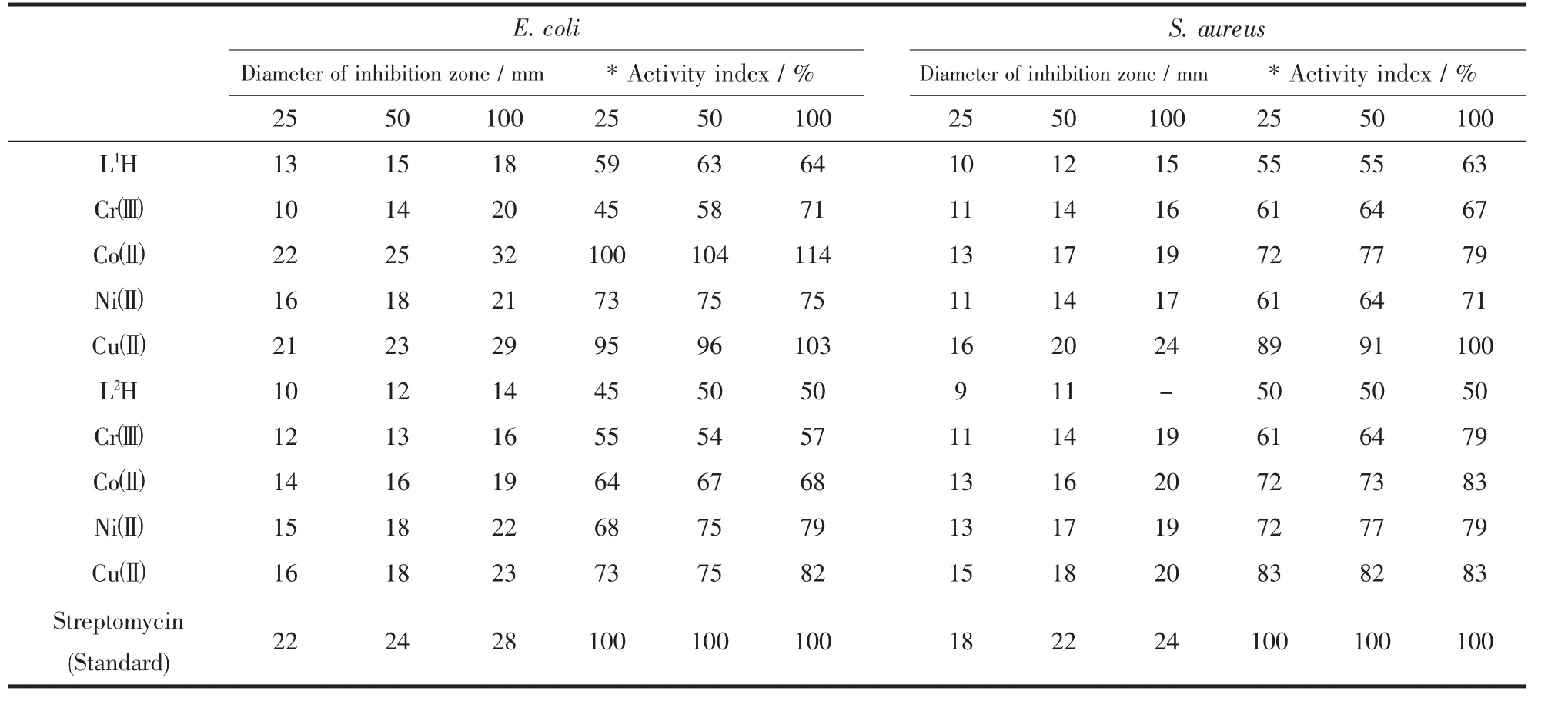
Table 5 Antibacterial screening data for the ligands and their complexes
The bactericidal and fungicidal investigation data of the compounds are summarized in Tables 5 and 6.The results of the investigations account for the antipathogenic behavior of the compounds and this efficacy is positively modified on complexation

Table 6 Antifungal screening data for the ligands and their complexes
3 Conclusions
In this report,we describe coordination chemistry of a Schiff base metal complexes obtained from the reaction of 5-chlorosalicylaldehyde with 2-amino-5-nitrothiazole (L1H)and 4-chlorobenzaldehyde with 2-amino-3-hydroxypyridine (L2H)have synthesized and characterized by various physicochemical and spectral analyses.In the result of microwave assisted synthesis,it has been observed that the reaction time is decreased from hours to minutes and the product within better yields is obtained compared to the classical method.FAB-mass and thermal data show degradation pattern of the complexes.Thermogravimetric studiesofthe complexes also help to characterize the complexes.The XRD patternsindicate crystalline nature ofthe complexes.Electrical conductivity data suggest that all the complexes fall in the semiconducting range.The findings from the bactericidal and fungicidal investigation of the compounds against the opportunistic pathogens reveal that the synthesized compounds have the antipathogenic activity.
Acknowledgement:We are thankful to I.I.T.Mumbai for ESR analysis.We also acknowledge SAIF,CDRI Lucknow for micro analysis and spectral analysis.Thanks are also due to the Head,Department of Chemistry,Physics and Botany Dr.Hari Singh Gour University,Sagar(M.P.)for Laboratory facilities.
[1]Chandra S,Kumar R.Trans.Met.Chem.,2004,29:269-275
[2]Shirodkar S G,Mane P S,Chondhekar T K.Indian J.Chem.,2001,40A:1114-1117
[3]Soliman A A,Mohamed G G.Thermochim Acta,2004,421:151-159
[4]Coombs R R,Westcott S A,Decken A,et al.Trans.Met.Chem.,2005,30:411-418
[5]Mahajan K,Fahmi N,Singh R V.Indian J.Chem.,2007,46A:1221-1225
[6]Sun Y,Machala M L,Castellano F N.Inorg.Chim.Acta 2010,363:283-287
[7]Garg R,Saini M K,Fahmi N,et al.Trans.Met.Chem.,2006,31:362-367
[8]Mahajan K,Swami M,Singh R V.Russ.J.Coord.Chem.,2009,35:179-185
[9]Sharma K,Singh R,Fahmi N,et al.Spectrochim.Acta,2010,75A:422-427
[10]Mohanan K,Kumari B S Rijulal G J.Rare Earths,2008,26,16-21
[11]Dubey R K,Dubey U K,Mishra C M.Indian J.Chem.,2008,47A:1208-1212
[12]Mishra A P,Soni M.Metal-Based Drug,2008.
[13]Raman N,Raja S J,Joseph J,et al.J.Chil.Chem.Soc.,2007,52:1138-1141
[14]Ourari A,Ourari K,Moumeni W,et al.Trans.Met.Chem.,2006,31:169-175
[15]Garg B S,Kumar D N.Spectrochim.Acta,2003,59A:229-234
[16]Nakamoto K.Infrared and Raman Spectra of Inorganic,Coordination Compounds.5thEd.New York:John Wiley&Sons,Part A&B,1998.
[17]Mishra A P,Mishra R K,Shrivastava S P.J.Serb.Chem.Soc.,2009,74:523-535
[18]Neelakantan M A,Marriappan S S,Dharmaraja J,et al.Spectrochim.Acta,2008,71A:628-635
[19]Lever A B P.Inorganic Electronic Spectroscopy.2ndEd.Amsterdam:Elsevier,1984.
[20]Chandra S,Jain D,Sharma A K,et al.Molecules,2009,14:174-190
[21]Abda llah S M,Zyed M A,Mohammed G G.Arabian J.Chem.,2010,3:103-113
[22]Jian-ning L I U,Bo-wan W U,Yongchun L I U.Turk J.Chem.,2006,30:41-48
[23]Dutta R L,Syamal A.Elements of Magneto Chemistry.2ndEd.New Delhi:Affiliated East West Press,1993.
[24]Hathaway B J.Comprehensive Coordination Chemistry.5thEd.U.K.:Wilkinson Pergamon Press,1987,534
[25]Mishra A P,Pandey L R.Indian J.Chem.,2005,44A:94-97[26]Piloyan G O,Novikova O S.Russ.J.Inorg.Chem.,1966,12:313-315
[27]Coats A W,Redfern J P.Nature,1964,201:68-69
[28]Mohamed G G,Omar M M,Ibrahim A A.Eur.J.Med.Chem.,2009,44:4801-4812
[29]Wang Y F,Liu J F,Xian H D,et al.Molecules,2009,14:2582-2593
[30]Sujamol M S,Athira C J,Sindhu Y,et al.Spectrochim.Acta,2010,75A,106-112
[31]Patange V N,Arbad B R.J.Indian Chem.Soc.,2007,84:1096-1103
[32]Mishra A P,Pandey L R.Indian J.Chem.,2005,44A:1800-1805
[33]Makode J T,Yaul A R,Bhadange S G,et al.Russ.J.Inorg.Chem.,2009,54:1372-1377
[34]Wahed M G,Bayoumi H A,Mohamme M I.Bull.Korean Chem.Soc.,2003,24:1313-1318
[35]Chohan Z H.Metal Based Drugs,1999,6:75-79
[36]Singh V P,Katiyar A.Singh S.,Bio Metals,2008.
[37]Chohan Z H,Munawar A,Supuran C T.Metal Based Drugs,2001,8:137-143
[38]Tweedy B G.Phytopathology,1964,55:910-918
Microwave Synthesis,Spectral,Thermal and Biological Significance of Some Transition Metal Complexes Containing Heterocyclic Ligands
Jain Rajendra K*Mishra A P
(Department of Chemistry,Synthetic Inorganic&Coordination Chemistry Laboratories,Dr.H.S.Gour Central University,Sagar,Madhya Pradesh 470003,India)
Some new Schiff base complexes of Cr(Ⅲ),Co(Ⅱ),Ni(Ⅱ) and Cu(Ⅱ) derived from 5-chlorosalicylaldehyde with 2-amino-5-nitrothiazole (L1H)and 4-chlorobenzaldehyde with 2-amino-3-hydroxypyridine (L2H)have been synthesized by conventional as well as microwave methods.These compounds have been characterized by elemental analysis,FTIR,FAB-mass,molar conductance,electronic spectra,1H-NMR,ESR,magnetic susceptibility,thermal,electrical conductivity and XRD analysis.The complexes are coloured and stable in air.Analytical data reveal that all the complexes exhibit 1∶2 (metal∶ligand)ratio with coordination number of 4 or 6.FAB-mass and thermal data show degradation pattern of the complexes.XRD patterns indicate crystalline nature for the complexes.The Schiff base and metal complexes show a good activity against the Gram-positive bacteria:Staphylococcus aureus and Gramnegative bacteria:Escherichia coli and fungi:Aspergillus niger and Candida albicans.
microwave method;O,N donor;thermal analyses;biological activities
O614.4
A
1001-4861(2012)08-1687-13
2011-10-02。收修改稿日期:2012-03-19。
*通讯联系人。E-mail:jainrajchem@gmail.com
