贵州普定灯盏河岩溶泉的硫同位素季节变化特征
2012-09-14刘再华
赵 敏,曾 成,杨 睿,刘再华
(1.中国科学院地球化学研究所环境地球化学国家重点实验室,贵州贵阳 550002;2.中国科学院地球化学研究所普定喀斯特生态综合试验站,贵州普定 562100)
贵州普定灯盏河岩溶泉的硫同位素季节变化特征
赵 敏1,2,曾 成1,2,杨 睿1,2,刘再华1,2
(1.中国科学院地球化学研究所环境地球化学国家重点实验室,贵州贵阳 550002;2.中国科学院地球化学研究所普定喀斯特生态综合试验站,贵州普定 562100)
通过对贵州普定后寨地下河补给区的灯盏河岩溶泉为期1年的泉水水文地球化学特征与水中SO2-4的硫同位素组成及季节性变化规律的分析,揭示灯盏河岩溶泉泉水中硫酸盐的来源及形成机理。结果表明:灯盏河岩溶泉的水化学类型主要为HCO-3·SO2-4-Ca2+型,具有很高的硫酸盐浓度,且变化幅度较大,SO2-4浓度为0.35~8.76 mmol·L-1;灯盏河泉水SO2-4的硫同位素组成为(3.80~25.76)×10-3,反映泉水的硫同位素组成主要受土壤有机硫氧化和石膏岩层溶解的控制;泉水硫同位素组成季节性变化明显,表现为旱季大于雨季,且旱季变化平缓,主要受石膏溶解的控制,而雨季变化幅度较大,反映雨季地下水硫酸盐土壤有机硫源贡献的增加及其季节性差异。
水文地球化学;硫同位素;溶解;氧化;有机硫;泉;岩溶;贵州
0 引 言
硫同位素作为一种有效的地球化学手段,已被广泛运用于自然生态系统中各种硫源的示踪和地球化学循环研究之中,如大气酸沉降研究[1-2]、矿坑废水湖泊研究[3-5]、湿地及江河湖泊硫源的示踪研究[6-11]、地下水研究[12-17]等。利用硫同位素可判别流域生态系统中硫的来源及其迁移转化途径。自20世纪80年代,国际上开始了河流体系的硫同位素地球化学研究[18],中国也逐步开始研究水体的硫同位素地球化学,如洪业汤等研究认为中国大气降水和地表水硫同位素组成具有区域分异的格局[1],储雪蕾研究发现北京地区地表水的硫同位素季节性差异可能主要受生物成因硫排放的控制[6]。地下水和地表水中SO24-的硫同位素组成(δ(34S))主要反映硫的来源和循环过程,不同物质来源的硫具有不同的硫同位素组成[6,12-17,19-20]。
贵州省位于西南岩溶区的中心,境内碳酸盐岩分布面积占全省面积的70%以上。岩溶地区地表和地下水中的δ(34S)随着硫的物质来源不同、地下水赋存环境的水文地球化学条件不同而存在很大的不同[21]。近年来,硫同位素已被广泛应用于地表水和地下水中含硫组分的来源及形态转化过程示踪研究等方面[16,19,22-24]。碳酸盐岩风化作用在很大程度上控制了岩溶地下水的化学组成;因此,对碳酸盐岩地区泉水的水文地球化学特征组成变化进行研究,将有助于弄清楚碳酸盐岩地区的化学风化作用与水文地球化学特征的关系以及控制泉水化学组成变化的多种因素[25-29]。
关于硫同位素的研究,前人主要集中在水化学特征及同位素区域差异上,而季节变化特征研究相对较少,尤其是碳酸盐岩地区地下水中SO24-来源及形成机理的研究鲜见报道。因此,笔者选择贵州普定典型的峰丛谷地区,以岩溶泉为代表,通过对岩溶泉水硫同位素及其季节变化规律的研究,弄清楚碳酸盐岩地区地下水中SO24-来源及形成机理,并探讨硫酸参与流域化学侵蚀的过程,为正确估算岩石风化碳汇通量提供参考。
1 研究区概况
灯盏河岩溶泉区域位于贵州省安顺市普定县境内,属长江流域的乌江水系,并位于著名的后寨岩溶地下河系统补给区。该区域属湿润亚热带气候,全年湿润多雨、冬温夏暖、雨热同期,年均气温15.1℃,多年平均降水量为1 314.6 mm,降水时空分布不均,每年5~10月为雨季,降水量约占全年的80%,每年11月至次年4月为旱季,降水量约占全年的20%[30-32]。岩溶泉区域内出露地层为中三叠统关岭组上段(T2g3),岩性为白云岩、泥质白云岩夹灰岩、石膏夹层,以地形分水岭为汇水边界。地貌类型由岩溶槽谷外围的岩溶丘陵及谷底的平坝组成。槽谷呈北东—南西向延伸,总体趋势是东北高、西南低,长约0.6 km,周围岩溶丘陵海拔为1 400~1 520 m,槽谷底部海拔为1 320 m。该岩溶槽谷底部地表覆盖第四系残坡积和冲洪积成因的松散土层,并且已经被开垦为水田和旱地。岩溶泉区域位于槽谷底部的开口处,流量变幅较大,为常年不干的长流泉。其土地利用状况详见文献[33]。
2 采样与分析方法
笔者于2008年8月至2009年8月采集1个完整水文年的灯盏河泉水样品。水样采集后经0.45μm醋酸纤维滤膜过滤,用于测定硫同位素的水样加入超纯盐酸将p H值调至小于2后,加入过量10%BaCl2溶液,使溶液中的SO2-4全部转化为BaSO4沉淀;将沉淀的样品带回实验室做进一步处理,其具体操作过程详见文献[34];然后将处理好的样品用连续硫同位素质谱仪(EA-Isoprime)测定。其结果用相对于国际标准CDT值的千分差值表示,具体为
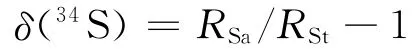
式中:RSa、RSt分别为样品及标准的硫同位素比值;测试精度优于±0.2×10-3。
用于测定水中溶解无机碳的碳同位素δ(13CDIC)样品采用直接沉淀法采集水样,具体过程详见文献[35]。带回实验室的野外水样δ(13CDIC)均在中国科学院地球化学研究所环境地球化学国家重点实验室测定。测定的δ(13CDIC)与国际标准PDB同位素比值相对应的计算式为式中:Rsample、RPDB分别为样品及标准的碳同位素比值;分析误差优于±0.15×10-3。
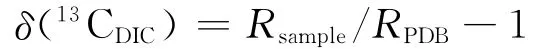
现场滴定使用德国Merck公司生产的碱度计和硬度计测定泉水的HCO3-和Ca2+浓度,精度分别为6、4 mg·L-1。在用于测定阳离子(K+、Na+、Ca2+、Mg2+)的水样中加入超纯HNO3酸化至p H值小于2条件下,测定阴离子(Cl-、、)的样品不作处理。将过滤样品进行酸化处理后,使用美国瓦里安(Varian)公司生产的ICP-OES电感耦合等离子体-光发射光谱(VISTA-MPX)测定阳离子质量浓度;使用美国戴安(DIONEX)公司的ICP-90型离子色谱仪测定阴离子质量浓度。
3 结果与讨论
3.1 水文地球化学特征
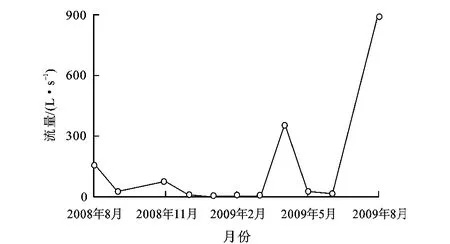
图1 灯盏河泉流量季节变化Fig.1 Seasonal Change of Flow for Dengzhanhe Spring
3.2 硫同位素特征及其来源分析
不同物质来源的硫具有不同的硫同位素组成。根据地下水中硫同位素组成变化,水体中硫酸盐物质来源的可能端元包括:①石膏岩层的溶解具有最高的δ(34S),但不影响水体中的δ(13CDIC),故δ(34S)与δ(13CDIC)很可能不存在相关关系;②受土壤有机质的氧化(或降解),可能有略微负偏的δ(34S)和最低的δ(13CDIC),两者成正相关关系。值得一提的是,灯盏河岩溶泉区域地下水中基本可以排除由地层中硫化矿床氧化而成的可能,主要因为在该区域地下水露头调查过程中,未发现p H值小于6的强酸性水体,而且在各采样点水样的化验结果中,Fe3+等金属离子从未检出[36]。
图2显示了硫同位素和碳同位素组成变化的相互关系。δ(13CDIC)<-10×10-3或δ(34S)<20×10-3时,δ(13CDIC)与δ(34S)成正相关关系;当δ(13CDIC)>-10×10-3或δ(34S)>20×10-3时,δ(13CDIC)与δ(34S)不相关;因此,灯盏河泉硫酸盐来源于土壤有机质的氧化和石膏岩层的溶解,但不存在硫化物的氧化。从图2还能看出δ(13CDIC)<-10×10-3或δ(34S)<20×10-3的情况发生在高流量时(即暴雨后),这进一步说明土壤有机质氧化来源的SO2-4主要发生在雨季,而在旱季,地下水中的来源于含水层中的石膏溶解(δ(34S)>20×10-3)。
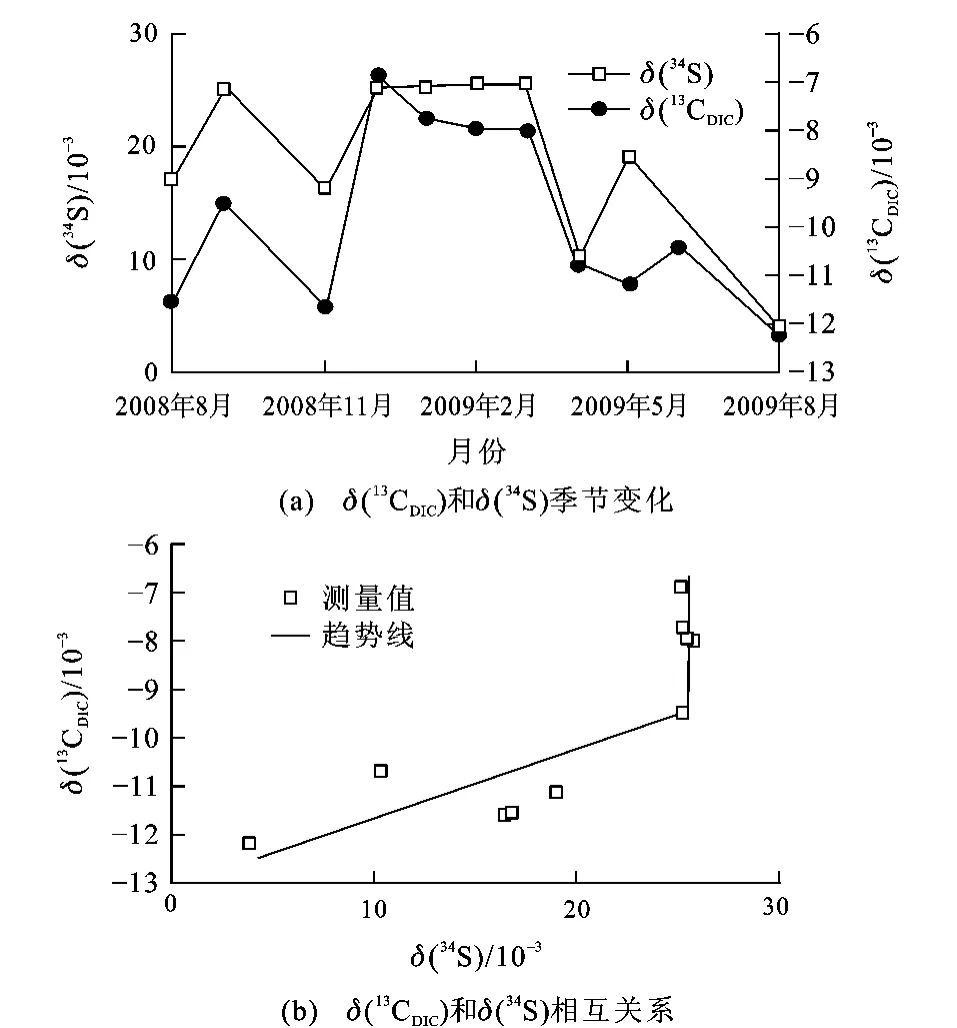
图2 灯盏河泉δ(13CDIC)和δ(34S)的相互关系及其季节变化Fig.2 Relationship Betweenδ(13CDIC)andδ(34S)of Dengzhanhe Spring and Its Seasonal Change
由图3可知,灯盏河地下水的δ(34S)与浓度具有相似的季节变化,灯盏河地下水体中硫酸盐同位素值为(3.8~25.76)×10-3,雨季降低,旱季升高。区域内出露地层为中三叠统关岭组上段,地下水流经含水岩层中膏岩层时,水-岩作用的结果使地下水具有较高的浓度和高的δ(34S),通常接近或分布在该区域海相蒸发硫酸盐(石膏)的δ(34S)范围((23.7~29.56)×10-3)内;基于前人关于西南喀斯特坡地土壤硫的生物地球化学循环研究,普定表层土壤的δ(34S)均为负值,为(-3.65~-1.34)×10-3;不考虑土壤类型的影响,普定表层土壤有机硫的δ(34S)为(-1.63~1.18)×10-3,明显高于表层土壤[37]。若取平均值26.63×10-3作为石膏来源的端元值[38],-0.23×10-3作为土壤有机硫来源的端元值[37],则通过同位素质量守恒定律,可计算出石膏来源的比例为15%~97%。由此可见,灯盏河硫酸盐及其δ(34S)的变化,旱季主要受石膏溶解的影响,而雨季则受土壤有机硫来源的影响显著增加。
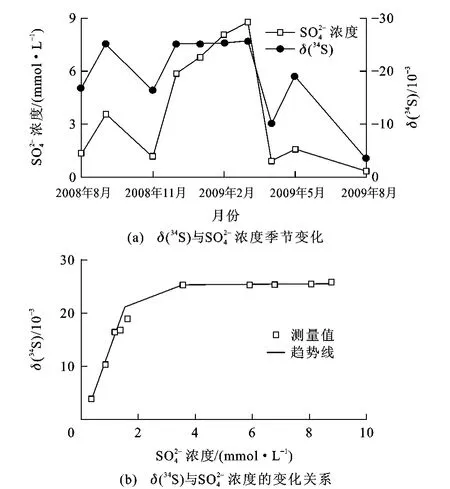
图3 灯盏河δ(34S)与浓度的相关关系及其季节变化Fig.3 Relationship Betweenδ(34S)andConcentration of Dengzhanhe Springand Its Seasonal Change
此外,从图3还可知,δ(34S)随着SO2-4浓度的增加而增加,高硫酸盐浓度对应的δ(34S)均大于25× 10-3。图3(b)中趋势线后面部分变幅不大,主要原因是这一阶段正好是旱季,受降水的影响较小,几乎没有土壤有机硫来源的影响,主要由石膏岩层的溶解所致。
4 结 语
(3)泉水δ(34S)季节性变化明显,表现为旱季大于雨季,且旱季变化平缓,主要受石膏溶解的控制,而雨季变化幅度较大,反映雨季地下水硫酸盐土壤有机硫源贡献的增加及其季节性差异。
References:
[1] 洪业汤,张鸿斌,朱咏煊,等.中国大气降水的硫同位素组成特征[J].自然科学进展,1994,4(6):741-745.HONG Ye-tang,ZHANG Hong-bin,ZHU Yong-xuan,et al.Sulfur Isotopic Composition of Rain in China[J].Progress in Natural Sciences,1994,4(6):741-745.
[2] 姚文辉,陈佑蒲,刘 坚,等.衡阳大气硫同位素组成环境意义的研究[J].环境科学研究,2003,16(3):3-5.
YAO Wen-hui,CHEN You-pu,LIU Jian,et al.The Research on the Environmental Significance of Atmospheric Sulfur Isotopic Composition in Hengyang[J].Research of Environmental Sciences,2003,16(3):3-5.
[3] KNOLLER K,STRSUCH G.Assessment of the Flow Dynamic of a Mining Lake by Stable Isotope Investigations[J].Isotopes in Environmental and Health Studies,1999,35(1/2):75-83.
[4] KNOLLER K,STRSUCH G.The Application of Stable Isotopes for Assessing the Hydrological,Sulfur,and Iron Balances of Acidic Mining Lake ML 111(Lusatia,Germany)as a Basis for Biotechnological Remediation[J].Water,Air and Soil Pollution:Focus,2002,2(3):3-14.
[5] PELLICORI D A,GAMMONS C H,POULSON S R.Geochemistry and Stable Isotope Composition of the Berkeley Pit Lake and Surrounding Mine Waters,Butte,Montana[J].Applied Geochemistry,2005,20(11):2116-2137.
[6] 储雪蕾.北京地区地表水的硫同位素组成与环境地球化学[J].第四纪研究,2000,20(1):87-97.
CHU Xue-lei.Sulfur Isotopic Compositions and Environmental Geochemistry of Surface Water in Beijing District[J].Quaternary Sciences,2000,20(1):87-97.
[7] SCHIFFS L,SPOELSTRA J,SEMKIN R G,et al.Drought Induced Pulses offrom a Canadian Shield Wetland:Use ofδ34S andδ18O into Determine Sources of Sulfur[J].Applied Geochemistry,2005,20(4):691-700.
[8] 蒋颖魁,刘丛强,陶发祥.贵州乌江水系枯水期河水硫同位素组成研究[J].地球化学,2006,35(6):623-628.
JIANG Ying-kui,LIU Cong-qiang,TAO Fa-xiang.Sulfur Isotopic Compositions of Wujiang River Water in Guizhou Province During Low-flow Period[J].Geochimica,2006,35(6):623-628.
[9] 蒋颖魁,刘丛强,陶发祥.贵州乌江水系河水硫同位素组成特征研究[J].水科学进展,2007,18(4):558-565.JIANG Ying-kui,LIU Cong-qiang,TAO Fa-xiang.Sulfur Isotope Composition Characters of Wujiang River Water in Guizhou Province[J].Advances in Water Science,2007,18(4):558-565.
[10] 王中良,刘丛强,朱兆洲.中国西南喀斯特湖泊硫酸盐来源的硫同位素示踪研究[J].矿物岩石地球化学通报,2007,26(增):580-581.
WANG Zhong-liang,LIU Cong-qiang,ZHU Zhaozhou.Stable Isotopic Research to Investigate Potential Sulfate Sources of Karst Lakes in Southwest China[J].Bulletin of Mineralogy,Petrology and Geochemistry,2007,26(S):580-581.
[11] BRENOT A,BRENOT M,CARIGAN J,et al.Geological and Land Use Control onδ34S andδ18O of River Dissolved Sulfate:the Moselle River Basin,France[J].Chemical Geology,2007,244(1/2):25-41.
[12] 顾慰祖,林曾平,费光灿,等.环境同位素硫在大同南寒武—奥陶系地下水资源研究中的应用[J].水科学进展,2000,11(1):14-20.GU Wei-zu,LIN Zeng-ping,FEI Guang-can,et al.The Use of Environmental Sulphur Isotopes in the Study of the Cambrian-Ordovician Aquifer System in the South of Datong[J].Advances in Water Science,2000,11(1):14-20.
[13] BOTTCHER M E.The Stable Isotopic Geochemistry of the Sulfur and Carbon Cycles in a Modern Karst Environment[J].Isotopes in Environmental and Health Studies,1999,35(1/2):39-61.
[14] PICHLER T.δ34S Isotope Values of Dissolved Sulfate()as a Tracer for Battery Acid(H2SO4)Contamination in Groundwater[J].Environmental Geology,2005,47(2):215-224.
[15] KNOLLER K,TRETTIN R,STRAUCH G.Sulphur Cycling in the Drinking Water Catchment Area of Torgau-Mockritz(Germany):Insights from Hydrochemical and Stable Isotope Investigations[J].Hydrological Processes,2005,19(17):3445-3465.
[16] TUTTLE M L W,BREIT G N,COZZARELLI I M.Processes Affectingδ34S andδ18O Values of Dissolved Sulfate in Alluvium Along the Canadian River,Central Oklahoma,USA[J].Chemical Geology,2009,265(3/4):455-467.
[17] YANG Y C,SHEN Z L,WEN D G,et al.Distribution ofδ34S andδ18O in SO2-4in Groundwater from the Ordos Cretaceous Groundwater Basin and Geological Implications[J].Acta Geologica Sinica:English Edition,2010,84(2):432-440.
[18] 李干蓉,刘丛强,陈 椽,等.丰水期乌江上游干流水库—河流体系硫同位素组成[J].长江流域资源与环境,2009,18(4):350-355.
LI Gan-rong,LIU Cong-qiang,CHEN Chuan,et al.Sulfur Isotope Composition of River Channel and Reservoir Water in Upper Reaches of Wujiang River in High Flow Season[J].Resources and Environment in the Yangtze Basin,2009,18(4):350-355.
[19] DAS A,PAWAR N J,VEIZER J.Sources of Sulfur in Deccan Trap Rivers:a Reconnaissance Isotope Study[J].Applied Geochemistry,2011,26(3):301-307.
[20] 张江华,梁永平,王维泰,等.硫同位素技术在北方岩溶水资源调查中的应用实例[J].中国岩溶,2009,28(3):235-241.
ZHANG Jiang-hua,LIANG Yong-ping,WANG Weitai,et al.A Practical Use of34S in the Investigation of Karst Groundwater Resource in North China[J].Carsologica Sinica,2009,28(3):235-241.
[21] 郎赟超.喀斯特地下水文系统物质循环的地球化学特征——以贵阳市和遵义市为例[D].贵阳:中国科学院地球化学研究所,2005.
LANG Yun-chao.Geochemical Characteristics of Cycling of Substances in Karstic Ground Water System—a Case Study from Guiyang and Zunyi Cities of China[D].Guiyang:Institute of Geochemistry,Chinese Academy of Sciences,2005.
[22] DOWUONA G N,MERMUT A R,KROUSE H R.Stable Isotope Geochemistry of Sulfate in Relation to Hydrogeology in Southern Saskatchewan,Canada[J].Applied Geochemistry,1993,8(3):255-263.
[23] DOGRAMACI S S,HERCZEG A L,SCHIFF S L,et al.Control onδ34S andδ18O of Dissolved Sulfate in Aquifers of the Murray Basin,Australia and Their Use as Indicators of Flow Processes[J].Applied Geochemistry,2001,16(4):475-488.
[24] KIRSTE D,DE CARITAT P,DANN R.The Application of the Stable Isotopes of Sulfur and Oxygen in Groundwater Sulfate to Mineral Exploration in the Broken Hill Region of Australia[J].Journal of Geochemical Exploration,2003,78/79:81-84.
[25] ROY S,GAILLARDET J,ALLEGRE C J.Geochemistry of Dissolved and Suspended Loads of the Seine River,France:Anthropogenic Impact,Carbonate and Silicate Weathering[J].Geochimica et Cosmochimica Acta,1999,63(9):1277-1292.
[26] TELMER K,VEIZER J.Carbon Fluxes,ρCO2and Substrate Weathering in a Large Northern River Basin,Canada:Carbon Isotope Perspectives[J].Chemical Geology,1999,159(1/2/3/4):61-86.
[27] YOSHIMURA K,NAKAO S,NOTO M,et al.Geochemical and Stable Isotope Studies on Natural Water in the Taroko Gorge Karst Area,Taiwan:Chemical Weathering of Carbonate Rocks by Deep Source CO2and Sulfuric Acid[J].Chemical Geology,2001,177(3/4):415-430.
[28] HELIE J F,HILLAIRE-MARCEL C,RONDEAU B.Seasonal Changes in the Sources and Fluxes of Dissolved Inorganic Carbon Through the St.Lawrence River:Isotopic and Chemical Constraint[J].Chemical Geology,2002,186(1/2):117-138.
[29] HAN G L,LIU C Q.Water Geochemistry Controlled by Carbonate Dissolution:a Study of the River Waters Draining Karst-dominated Terrain,Guizhou Province,China[J].Chemical Geology,2004,204(1/2):1-21.
[30] 蒙海花,王腊春.岩溶地区土地利用变化的水文响应研究——以贵州后寨河流域为例[J].中国岩溶,2009,28(3):227-234.
MENG Hai-hua,WANG La-chun.Hydrological Responses to Land-use Changes in Karst Area—a Case Study in Houzhai River,Guizhou Province[J].Carsologica Sinica,2009,28(3):227-234.
[31] 赵 敏,曾 成,刘再华.土地利用变化对岩溶地下水溶解无机碳及其稳定同位素组成的影响[J].地球化学,2009,38(6):565-572.
ZHAO Min,ZENG Cheng,LIU Zai-hua.Influence of Land Use Change on Dissolved Inorganic Carbon and Stable Isotopic Compositions of Karst Groundwater[J].Geochimica,2009,38(6):565-572.
[32] 束龙仓,柯婷婷,刘丽红,等.基于综合法的岩溶山区生态系统脆弱性评价——以贵州省普定县为例[J].中国岩溶,2010,29(2):141-144.
SHU Long-cang,KE Ting-ting,LIU Li-hong,et al.Assessment on Ecosystem Fragility of Karst Mountain Areas Based on Synthetic Method—a Case Study in Puding Country,Guizhou Province[J].Carsologica Sinica,2010,29(2):141-144.
[33] ZHAO M,ZENG C,LIU Z H,et al.Effect of Different Land Use/Land Cover on Karst Hydrogeochemistry:a Paired Catchment Study of Chenqi and Dengzhanhe,Puding,Guizhou,SW China[J].Journal of Hydrology,2010,388(1/2):121-130.
[34] YANAGLSAWA F,SAKAL H.Thermal Decomposition of Barium Sulfate-vanadium Pentoxide-silica Glass Mixtures for Preparation of Sulfur Dioxide in Sulfur Isotope Ratio Measurements[J].Analytical Chemistry,1983,55(6):985-987.
[35] LIU Z H,ZHANG M L,LI Q,et al.Hydrochemical and Isotope Characteristics of Spring Water and Travertine in the Baishuitai Area(SW China)and Their Meaning for Paleoenvironmental Reconstruction[J].Environmental Geology,2003,44(6):698-704.
[36] 曾 成.湿亚热带岩溶系统水文水化学对不同土地利用的响应研究[D].桂林:中国地质科学院岩溶地质研究所,2009.
ZENG Cheng.Study on the Response of Hydrology and Hydrochemistry of Karst Systems to Different Land Uses Under Humid Subtropical Climate[D].Guilin:Institute of Karst Geology,Chinese Academy of Geological Sciences,2009.
[37] 张 伟.西南喀斯特坡地土壤硫的生物地球化学循环研究[D].贵阳:中国科学院地球化学研究所,2009.
ZHANG Wei.Biogeochemical Cycle of Sulfur in Soils on Slopes of Karst Areas,Southwest China[D].Guiyang:Institute of Geochemistry,Chinese Academy of Sciences,2009.
[38] 郎赟超,刘丛强,SATAKE H,等.贵阳地表水-地下水的硫和氯同位素组成特征及其污染物示踪意义[J].地球科学进展,2008,23(2):151-159.
LANG Yun-chao,LIU Cong-qiang,SATAKE H,et al.δ37Cl andδ34S Variations of Cl-andin Groundwater and Surface Water of Guiyang Area,China[J].Advances in Earth Science,2008,23(2):151-159.
Seasonal Variation of Sulfur Isotope in Dengzhanhe Karst Spring of Puding,Guizhou
ZHAO Min1,2,ZENG Cheng1,2,YANG Rui1,2,LIU Zai-hua1,2
(1.State Key Laboratory of Environmental Geochemistry,Institute of Geochemistry,Chinese Academy of Sciences,Guiyang 550002,Guizhou,China;2.Puding Comprehensive Karst Ecological Experiment Station,
Institute of Geochemistry,Chinese Academy of Sciences,Puding 562100,Guizhou,China)
The concentration of sulfate is high in Dengzhanhe karst spring located in the recharge area of Houzhai underground river of Puding,Guizhou.Hydrogeochemistry characteristics of the spring,sulfur isotopic composition of SO2-4and their seasonal changes were analyzed during a hydrological year,and the source and formation mechanism of sulfate in the spring were revealed.The results showed that the hydrochemical type of Dengzhanhe karst spring was HCO-3·SO2-4-Ca2+,the concentration of sulfate was very high and the range of variation was significant,the concentration of SO2-4was 0.35-8.76 mmol·L-1;the sulfur isotopic composition of SO2-4in the spring was(3.80-25.76)×10-3,and indicated that the sulfur isotopic composition was mainly controlled by dissolution of gypsum and oxidation of organic sulfur in soil;seasonal variation of sulfur isotopic composition was remarkable,and indicated that sulfur isotopic composition in dry season was more than that in rainy season,and the change of composition in dry season was small and mainly controlled by the dissolution of gypsum,but the change in rainy season was large and reflected the increase of organic sulfur for underground sulfate soil and its seasonal difference.
hydrogeochemistry;sulfur isotope;dissolution;oxidation;organic sulfur;spring;karst;Guizhou
P595;P641.3
A
1672-6561(2012)03-0083-06
2011-10-16
国家自然科学基金项目(41103084,41003056);贵州省博士基金项目(2011GZ62743)
赵 敏(1980-),女,贵州兴义人,助理研究员,理学博士,E-mail:zhaomin@mails.gyig.ac.cn。
