Novel functional proteins interact with midkine in hepatic cancer cells
2012-07-07
Hangzhou, China
Novel functional proteins interact with midkine in hepatic cancer cells
Qiang Yan, Hui-Lian Huang, Xing Yao, Jing Li, Li-Qin Li, Jing Zhong, Li-Shan Min, Li-Cheng Dai and Shu-Sen Zheng
Hangzhou, China
BACKGROUND: Midkine is a heparin-binding growth factor that promotes the proliferation, survival, migration and differentiation of various target cells. Midkine plays an important role in tumorigenesis and tumor progression, and is overexpressed in many human malignant tumors. Patients with high tumor midkine expression frequently have a worse prognosis than those with low expression. The present study was designed to investigate the interaction network of midkine in hepatic cancer cells, and to elucidate its role in hepatocellular carcinoma.
METHODS: DNA encoding full-length midkine was cloned into pDBLeu vector to serve as bait in yeast two-hybrid screening to identify interacting proteins. Candidate proteins were examined on SC-Leu-Trp-His+3-AT (20 mmol/L) plates and assayed for X-gal activity, then sequenced and classified according to the GenBank. Finally, identified proteins were expressed by thein vitroexpression system pCMVTnT, and protein interactions were confirmed by co-immunoprecipitation.
RESULTS: Using the yeast two-hybrid system, we found 6 proteins that interacted with midkine: NK-kappa-B inhibitor alpha (I-κ-B-α), Dvl-binding protein naked cuticle 2, granulin, latent active TGF-β binding protein 3, latent active TGF-β binding protein 4, and phospholipid scramblase 1.In vitroco-immunoprecipitation demonstrated that all identified proteins directly interacted with midkine.
CONCLUSION: The identification of midkine-interacting proteins in hepatic cancer cells indicates that midkine is a multifunctional factor that may participate in cell migration, differentiation, and proliferation, and is also associated with the multicellular response feedback during the development of hepatocellular carcinoma.
(Hepatobiliary Pancreat Dis Int 2012;11:272-277)
midkine; yeast two-hybrid; interaction; hepatic cancer cell
Introduction
Midkine is a member of the heparin-binding growth factor family and has been identified as the product of a retinoic acid-responsive gene.[1]It is a cytokine that is highly expressed in the mid-gestation period during embryogenesis.[2]Midkine enhances the proliferation, differentiation, survival and migration of various target cells,[3,4]and is involved in cancer development, inflammation, reproduction, and wound repair.[5,6]It is overexpressed in many human malignant tumors including hepatocellular carcinoma (HCC),[7]gastric carcinoma,[8]colon carcinoma,[9]lung carcinoma,[10]urinary bladder carcinoma,[11]prostate carcinoma,[12]breast carcinoma,[13]ovarian carcinoma,[14]neuroblastoma[15]and astrocytoma.[16]It acts as an angiogenic, fibrinolytic, and anti-apoptotic factor in carcinoma cell lines.[17]Patients with high midkine tumor expression frequently have a worse prognosis than those with low expression.
Midkine is overexpressed in HCC and can promote HCC cell proliferation and invasion. It is also involved in the angiogenesis and tumorigenesis of HCC,[18]but the cellular signaling receptors for midkine have not yet been identified and characterized in hepatic cancer cells. Study of the molecular basis of midkine signal transduction pathways in hepatic cancer cells would further enhancethe understanding of their roles in the development and growth of cancer. To date, receptor-type protein tyrosine phosphatase ζ (PTPζ),[19]anaplastic lymphoma kinase (ALK),[20]syndecans,[21]low-density-lipoprotein (LDL) receptor-related protein,[22]glypican-2,[23]PG-M/ versican,[24]neuroglycan C,[25]α4β1-integrin and α6β1-integrin[26]have been proposed to have a strong affinity for midkine, and may function as midkine receptors. Therefore, in this study we investigated the protein interactions with midkine in hepatic cancer cells by screening with the yeast two-hybrid system.
Methods
Plasmid constructs
The yeast expression vectors pDBLeu and pEXPAD502 (both from Invitrogen, Carlsbad, CA) are fusion vectors for the linkage of proteins to the GAL4 DNA binding domain and the GAL4 transactivation domain, respectively. Full-length midkine was amplified by PCR from the cDNA of hepatic cancer cells and cloned inframe into the Sal I/EcoR I sites of pDBLeu (pDBLeu-MK) to serve as bait in the screening assay.
Screening interaction proteins using the yeast two-hybrid system
Plasmid pDBLeu-midkine was used as the bait in the yeast two-hybrid system to screen a cDNA library from human hepatic cancer cells (Invitrogen) using the ProquestTMtwo-hybrid system, according to the manufacturer's protocol (Invitrogen). Briefly, the cDNA library was inserted into pEXP-AD502 vector (pEXP-cDNA), and was co-introduced into the MAV203 yeast strain along with pDBLeu-midkine. Yeast cells containing pDBLeu-midkine and the pEXP-cDNA library were spread onto SC-Leu-Trp-His (SC-LTH)+3-AT (20 mmol/L) plates. After culture for 48-72 hours at 30 ℃, positive colonies were acquired. Replicate plates were obtained by gently pressing the autoclaved filter discs onto SC-LTH plates to transfer clones to yeast extract peptone adenine sulfate dextros (YPAD) plates and selection plates, SC-Leu-Trp plates and SC-Leu-Trp-Ura plates. The former were incubated for X-gal assays to screen positive clones, and the latter were incubated for further screening through absence of uracil. Meanwhile, parallel controls prepared from fresh clones of 5 yeast control strains from glycerol stocks were plated onto SCLeu-Trp plates.
Screening and validation of positive clones
Individual clones were chosen and inoculated into SC-Leu-Trp culture medium. After culture at 30 ℃ for 24 hours, yeast plasmids that contained pDBLeu-midkine and low copies of pEXP-candidate protein were prepared by alkali lysis. Yeast plasmids were electroporated intoE. coliDH5α cells and incubated at 37 ℃ on luria broth (LB) plates (Amp+) for 12 hours. Due to the kanamycin resistance of pDBLeu vector and the ampicillin resistance of pEXP-AD502 vector, pEXP-X could be selected using LB plates (Amp+), resulting in high copies of pEXP-candidate protein plasmid. Then, the candidate protein-coding plasmids and pDBLeu were co-introduced into yeast cells to identify self-activity and to confirm the activity.
DNA sequencing
Candidate protein-coding plasmids were digested by Sal I and Nco I, and the insertion fragments were recorded. Positive colonies were sequenced by Invitrogen, and the genes were classified according to sequence homology analysis in the GenBank (http://blast.ncbi. nlm.nih.gov/).
Reverse identification using the yeast two-hybrid system
Midkine was cloned into the pEXP-AD502 vector, and the cDNA sequences of the potential interacting proteins were cloned into pDBLeu. Yeast cells were cointroduced with both pEXP-midkine and pDBLeu-X, thus identifying the interaction between midkine and the potential protein identified (reverse identification). The self-activity of the system was also identified.
Interaction identification using thein vitro protein expression system
The midkine gene was cloned into pET28a+ vector and fused with His-tag, while the candidate interacting protein genes were cloned into thein vitroprotein expression vector pCMVTnT (Promega). Proteins were expressed in the TnTin vitroprotein expression system and stained by 35S methionine. Coimmunoprecipitation analysis was performed according to the protocol for pCMVTnTTMvector (Promega). Briefly, solutions containing midkine protein and the candidate proteins were slowly mixed by rolling in binding buffer for 1 hour at 4 ℃. Ni2+-NTA beads were added and slowly mixed by rolling for 1 hour at 4 ℃, then centrifuged at 7000 rpm for 20 seconds. The supernatant was discarded, the residual liquid was thoroughly removed, and then the beads were carefully aspirated off. The complexes were suspended in 2× sample buffer (60 mmol/L Tris-HCl pH 6.8, 3% sodium dodecyl sulfate (SDS), 10% glycerol, 0.05% bovinefetal brain (BFB), 10% β-mercaptoethanol (β-Me), and boiled in a water bath for 5 minutes, then separated by SDS-PAGE followed by transfer onto polyvinylidene fluoride (PVDF) membranes. Anti-progranulin antibody (R&D Systems, Inc., Minneapolis, MN), anti-IKBA antibody (Abcam, Cambridge, MA), anti-PLSCR1 antibody (Abcam), anti-NKD2 antibody (Abcam), and anti-mouse IgG HRP antibody (R&D Systems) were used for immunoblotting. Proteins were detected by enhanced chemiluminescence.
Results
Yeast two-hybrid screening identification of midkine-interacting proteins
The midkine gene was cloned into the pDBLeu vector to form pDBLeu-midkine, and then assayed by X-gal to demonstrate that it lacked self-regulating activity in the yeast two-hybrid system. pDBLeu-midkine and the pEXP-cDNA library were co-introduced into competent yeast cells, then cultured on SC-LTH+3-AT (20 mmol/L) plates (Leu-, Trp-, His-) for 48-72 hours at 30 ℃, resulting in 56 positive clones. These were selected and re-selected using X-gal to obtain positive clones (Table 1). Of 44 positive clones, 22 displayed high, 8 moderate, and 14 weak X-gal activity. Thirty X-gal-positive highly and moderately active clones were cultured overnight in LB medium. Yeast plasmids were prepared and introducedintoE. coli. The amplified plasmid was then digested by Sal I and Nco I to identify the inserted fragment (Fig. 1), and the plasmids were sequenced. The coding proteins were identified by homology alignment in the GenBank (Table 2). The self-regulating activity of different coding protein colonies were identified and re-identified to exclude false-positive clones (Fig. 2).
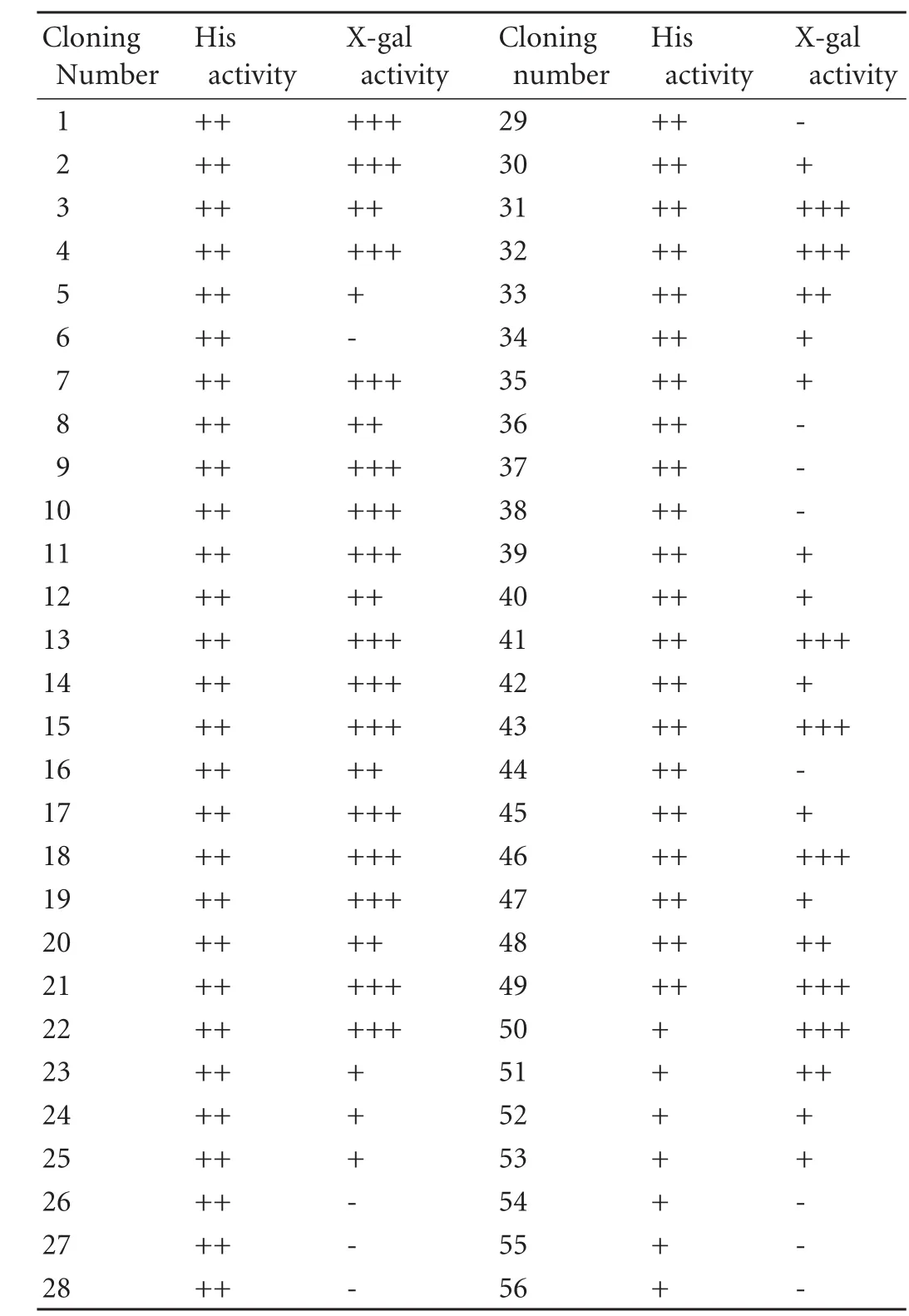
Table 1. X-gal activity of positive clones selected from SC-LTH+3-AT (20 mmol/L) plates (Leu-, Trp-, and His-)

Table 2. Plasmid cloning number, sequence number and BLAST results

Fig. 1. Positive clones with X-gal activity were cultured overnight, and the plasmids were prepared and then introduced intoE. coli.The plasmids were digested by Sal I and Nco I to acquire the insertion fragments.

Fig. 2. The left panel shows the self-activity of proteins interacting with midkine. A, B, C, D and E are comparison bacteria, with increasing blue intensity representing stronger activity. The results showed that the 6 interacting proteins had no self-regulating activity. The right panel shows confirmation of the activity of midkine-interacting proteins. The result showed that all 6 identified proteins have X-gal activity, and all were identified to interact with midkine.
After screening and identification from the human hepatic cDNA library, we found that NK-kappa-B inhibitor alpha (I-κ-B-α, IKBA), Dvl-binding protein naked cuticle 2 (NKD2), granulin (PGRN), latent active TGF-β binding protein 3 (LTBP3), LTBP4, and PLSCR1 interacted with midkine.
Protein interaction verification by co-immunoprecipitation

Fig. 3. Co-immunoprecipitation analysis was performed to confirm the individual interactions between midkine and IKBA, NKD2, PLSCR1 and PGRN. Empty vector and protein X served as the negative and positive controls, respectively.
In order to confirm interactions with midkine, the proteins acquired from yeast two-hybrid screening were tested byin vitroco-immunoprecipitation. The full-length cDNA of genes coding midkine-interacting proteins were individually cloned into the pCMVTnT vector (Promega). Protein X was labeled by [35S]. Nickel beads specifically bound with midkine protein fused with His-tag were then used to co-precipitate protein X. Empty pCMVTnT vector served as a control group. Midkine individually interacted with IKBA, NKD2, PLSCR1 and PGRN (Fig. 3).
Discussion
HCC is one of the most malignant diseases in the world with a high incidence and mortality.[27]No satisfactory treatment is currently available for patients with HCC, and the outcome of chemotherapy has been extremely disappointing. Recent insights into the biology of HCC suggest that certain pathways are likely to play essential roles in its development, such as Wnt-β-catenin,[28]TGF-β,[29]EGF[30]and PI3 kinase/Akt signaling.[31]But how these protein cascades relate to HCC is not clear, and any molecular alterations are likely to have important effects on signaling.
Our previous study demonstrated that midkine is overexpressed in HCC.[32]Antisense oligonucleotide (ASODN) targeting midkine has been reported to suppress the growth of tumors in nude mice.[33]In addition, siRNA or ASODN that targets midkine inhibits neointimal hyperplasia formation[34]and renal injury after ischemia.[35]However, the cellular signaling receptors for midkine in hepatic cancer cells urgently need to be identified and characterized.
Known cellular signaling receptors of midkine include PTPζ,[19]ALK,[20]syndecans,[21]LDL receptorrelated protein,[22]glypican-2,[23]PG-M/versican,[24]neuroglycan C,[25]α4β1-integrin and α6β1-integrin.[26]Among these, PTPζ, LDL receptor-related protein, glypican-2, PG-M/versican and neuroglycan C are involved in neuronal systems, ALK is involved in tumorigenesis,[36]α4β1-integrin and α6β1-integrin are involved both in embryonic neurons and osteoblastlike cells, and syndecans are involved in epithelialmesenchymal interactions during fetal development and organogenesis.[21]The six proteins identified in our experiment by the yeast two-hybrid screening of a hepatic cancer cell library, IKBA, NKD2, PLSCR1, PGRN, LTBP3 and LTBP4, might be involved in important intracellular signaling pathways as well as functioning as extracellular matrix components. IKBA inhibits NF-kappaB[37]and plays a role in cell migration, the immune response, inflammation primary stage reaction, apoptosis, differentiation, growth and other activities.[38]NKD2 is a dishevelled binding protein, and is a negative regulatory factor in Wnt-β-catenin-TGF signaling.[39]PLSCR1 is a multi-palmitoyl, Ca2+-binding endothelial membrane protein. It participates in cell proliferation, maturation, and apoptosis kinase (including c-Abl, c-Src, and PKC) phosphorylation.[40]PGRN is a newly-discovered growth factor, which activates PI3K, AKT/protein kinase B and p70S6 kinase in the ERK pathway.[41]Thus, it plays important roles in accelerating cell proliferation and migration.[42]Finally, LTBP is an extracellular matrix protein, and is involved in TGF-β signaling, with functions such as cell migration, extracellular matrix degradation and cell cycle regulation.[43]
In conclusion, the proteins identified in our study participate in cell migration, differentiation, proliferation, apoptosis, multicellular response feedback and cell cycle regulation. They have important biological functions and are closely associated with tumorigenesis as well as multiple signaling pathways, including the Wnt-β-catenin, TGF-β, EGF and PI3 kinase/Akt cascades, and are associated with HCC development possibly through complex mechanisms. From our study, the PGRN and midkine interaction could promote the proliferation and invasion of hepatic cancer cells, and affect tumor angiogenesis (data not shown). And the role of other protein interactions with midkine during HCC development will also be investigated in our future research.
The identification of midkine-interacting proteins indicates that midkine is a multifunctional factor, and unveils its role in HCC tumorigenesis and progression. The future challenges are to demonstrate and characterize the interactions and regulatory mechanisms of midkine with these functional proteins, from which novel therapeutic strategies for HCC could be developed.
Contributors: YQ and HHL wrote the main body of the article under the supervision of DLC and ZSS. YX provided advice on medical aspects. All authors contributed to the design and interpretation of the study and to further drafts. DLC and ZSS are the guarantors.
Funding: This study was supported by grants from the National Natural Science Foundation of China (30772534) and the Natural Science Foundation of Huzhou (2010YS05).
Ethical approval: Not needed.
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Kadomatsu K, Muramatsu T. Midkine and pleiotrophin in neural development and cancer. Cancer Lett 2004;204:127-143.
2 Muramatsu T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem 2002;132:359-371.
3 Ota K, Fujimori H, Ueda M, Shiniriki S, Kudo M, Jono H, et al. Midkine as a prognostic biomarker in oral squamous cell carcinoma. Br J Cancer 2008;99:655-662.
4 van der Horst EH, Frank BT, Chinn L, Coxon A, Li S, Polesso F, et al. The growth factor Midkine antagonizes VEGF signalingin vitroandin vivo. Neoplasia 2008;10:340-347.
5 Kerzerho J, Adotevi O, Castelli FA, Dosset M, Bernardeau K, Szely N, et al. The angiogenic growth factor and biomarker midkine is a tumor-shared antigen. J Immunol 2010;185:418-423.
6 Webb TR, Slavish J, George RE, Look AT, Xue L, Jiang Q, et al. Anaplastic lymphoma kinase: role in cancer pathogenesis and small-molecule inhibitor development for therapy. Expert Rev Anticancer Ther 2009;9:331-356.
7 Koide N, Hada H, Shinji T, Ujike K, Hirasaki S, Yumoto Y, et al. Expression of the midkine gene in human hepatocellular carcinomas. Hepatogastroenterology 1999;46:3189-3196.
8 Rawnaq T, Kunkel M, Bachmann K, Simon R, Zander H, Brandl S, et al. Serum midkine correlates with tumor progression and imatinib response in gastrointestinal stromal tumors. Ann Surg Oncol 2011;18:559-565.
9 Ye C, Qi M, Fan QW, Ito K, Akiyama S, Kasai Y, et al. Expression of midkine in the early stage of carcinogenesis in human colorectal cancer. Br J Cancer 1999;79:179-184.
10 Sone M, Muramatsu H, Muramatsu T, Nakashima T. Morphological observation of the stria vascularis in midkine and pleiotrophin knockout mice. Auris Nasus Larynx 2011; 38:41-45.
11 Terao S, Shirakawa T, Kubo S, Bishunu A, Lee SJ, Goda K, et al. Midkine promoter-based conditionally replicative adenovirus for targeting midkine-expressing human bladder cancer model. Urology 2007;70:1009-1013.
12 Konishi N, Nakamura M, Nakaoka S, Hiasa Y, Cho M, Uemura H, et al. Immunohistochemical analysis of midkine expression in human prostate carcinoma. Oncology 1999;57: 253-257.
13 Perez-Pinera P, Garcia-Suarez O, Menendez-Rodriguez P, Mortimer J, Chang Y, Astudillo A, et al. The receptor protein tyrosine phosphatase (RPTP)beta/zeta is expressed in different subtypes of human breast cancer. Biochem Biophys Res Commun 2007;362:5-10.
14 Nakanishi T, Kadomatsu K, Okamoto T, Tomoda Y,Muramatsu T. Expression of midkine and pleiotropin in ovarian tumors. Obstet Gynecol 1997;90:285-290.
15 Nakagawara A, Milbrandt J, Muramatsu T, Deuel TF, Zhao H, Cnaan A, et al. Differential expression of pleiotrophin and midkine in advanced neuroblastomas. Cancer Res 1995;55: 1792-1797.
16 Mishima K, Asai A, Kadomatsu K, Ino Y, Nomura K, Narita Y, et al. Increased expression of midkine during the progression of human astrocytomas. Neurosci Lett 1997;233:29-32.
17 Qi M, Ikematsu S, Ichihara-Tanaka K, Sakuma S, Muramatsu T, Kadomatsu K. Midkine rescues Wilms' tumor cells from cisplatin-induced apoptosis: regulation of Bcl-2 expression by Midkine. J Biochem 2000;127:269-277.
18 Muramatsu T. Midkine: a promising molecule for drug development to treat diseases of the central nervous system. Curr Pharm Des 2011;17:410-423.
19 Maeda N, Ichihara-Tanaka K, Kimura T, Kadomatsu K, Muramatsu T, Noda M. A receptor-like protein-tyrosine phosphatase PTPzeta/RPTPbeta binds a heparin-binding growth factor midkine. Involvement of arginine 78 of midkine in the high affinity binding to PTPzeta. J Biol Chem 1999;274:12474-12479.
20 Stoica GE, Kuo A, Powers C, Bowden ET, Sale EB, Riegel AT, et al. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J Biol Chem 2002;277:35990-35998.
21 Mitsiadis TA, Salmivirta M, Muramatsu T, Muramatsu H, Rauvala H, Lehtonen E, et al. Expression of the heparin-binding cytokines, midkine (MK) and HB-GAM (pleiotrophin) is associated with epithelial-mesenchymal interactions during fetal development and organogenesis. Development 1995;121:37-51.
22 Muramatsu H, Zou K, Sakaguchi N, Ikematsu S, Sakuma S, Muramatsu T. LDL receptor-related protein as a component of the midkine receptor. Biochem Biophys Res Commun 2000;270:936-941.
23 Kurosawa N, Chen GY, Kadomatsu K, Ikematsu S, Sakuma S, Muramatsu T. Glypican-2 binds to midkine: the role of glypican-2 in neuronal cell adhesion and neurite outgrowth. Glycoconj J 2001;18:499-507.
24 Zou K, Muramatsu H, Ikematsu S, Sakuma S, Salama RH, Shinomura T, et al. A heparin-binding growth factor, midkine, binds to a chondroitin sulfate proteoglycan, PG-M/ versican. Eur J Biochem 2000;267:4046-4053.
25 Ichihara-Tanaka K, Oohira A, Rumsby M, Muramatsu T. Neuroglycan C is a novel midkine receptor involved in process elongation of oligodendroglial precursor-like cells. J Biol Chem 2006;281:30857-30864.
26 Muramatsu H, Zou P, Suzuki H, Oda Y, Chen GY, Sakaguchi N, et al. alpha4beta1- and alpha6beta1-integrins are functional receptors for midkine, a heparin-binding growth factor. J Cell Sci 2004;117:5405-5415.
27 Alves RC, Alves D, Guz B, Matos C, Viana M, Harriz M, et al. Advanced hepatocellular carcinoma. Review of targeted molecular drugs. Ann Hepatol 2011;10:21-27.
28 Wang XH, Meng XW, Sun X, Liu BR, Han MZ, DU YJ, et al. Wnt/β-catenin signaling regulates MAPK and Akt1 expression and growth of hepatocellular carcinoma cells. Neoplasma 2011;58:239-244.
29 Baek HJ, Pishvaian MJ, Tang Y, Kim TH, Yang S, Zouhairi ME, et al. Transforming growth factor-β adaptor, β2-spectrin, modulates cyclin dependent kinase 4 to reduce development of hepatocellular cancer. Hepatology 2011;53:1676-1684.
30 Ueda S, Basaki Y, Yoshie M, Ogawa K, Sakisaka S, Kuwano M, et al. PTEN/Akt signaling through epidermal growth factor receptor is prerequisite for angiogenesis by hepatocellular carcinoma cells that is susceptible to inhibition by gefitinib. Cancer Res 2006;66:5346-5353.
31 Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Koch A, et al. PI-103 and sorafenib inhibit hepatocellular carcinoma cell proliferation by blocking Ras/Raf/MAPK and PI3K/AKT/ mTOR pathways. Anticancer Res 2010;30:4951-4958.
32 Dai LC, Wang X, Yao X, Lu YL, Ping JL, He JF. Antisense oligonucleotide targeting midkine suppressesin vivoangiogenesis. World J Gastroenterol 2007;13:1208-1213.
33 Dai LC, Wang X, Yao X, Min LS, Ping JL, He JF. Antisense oligonucleotides targeting midkine inhibit tumor growth in an in situ human hepatocellular carcinoma model. Acta Pharmacol Sin 2007;28:453-458.
34 Takei Y, Kadomatsu K, Goto T, Muramatsu T. Combinational antitumor effect of siRNA against midkine and paclitaxel on growth of human prostate cancer xenografts. Cancer 2006; 107:864-873.
35 Katsuno M, Adachi H, Minamiyama M, Waza M, Tokui K, Banno H, et al. Reversible disruption of dynactin 1-mediated retrograde axonal transport in polyglutamine-induced motor neuron degeneration. J Neurosci 2006;26:12106-12117.
36 Cunha SI, Pardali E, Thorikay M, Anderberg C, Hawinkels L, Goumans MJ, et al. Genetic and pharmacological targeting of activin receptor-like kinase 1 impairs tumor growth and angiogenesis. J Exp Med 2010;207:85-100.
37 Ihekwaba AE, Broomhead DS, Grimley RL, Benson N, Kell DB. Sensitivity analysis of parameters controlling oscillatory signalling in the NF-kappaB pathway: the roles of IKK and IkappaBalpha. Syst Biol (Stevenage) 2004;1:93-103.
38 Yang X, Lu H, Yan B, Romano RA, Bian Y, Friedman J, et al. ΔNp63 versatilely regulates a Broad NF-κB gene program and promotes squamous epithelial proliferation, migration, and inflammation. Cancer Res 2011;71:3688-3700.
39 Zhang S, Cagatay T, Amanai M, Zhang M, Kline J, Castrillon DH, et al. Viable mice with compound mutations in the Wnt/Dvl pathway antagonists nkd1 and nkd2. Mol Cell Biol 2007;27:4454-4464.
40 Nanjundan M, Sun J, Zhao J, Zhou Q, Sims PJ, Wiedmer T. Plasma membrane phospholipid scramblase 1 promotes EGF-dependent activation of c-Src through the epidermal growth factor receptor. J Biol Chem 2003;278:37413-37418.
41 Monami G, Gonzalez EM, Hellman M, Gomella LG, Baffa R, Iozzo RV, et al. Proepithelin promotes migration and invasion of 5637 bladder cancer cells through the activation of ERK1/2 and the formation of a paxillin/FAK/ERK complex. Cancer Res 2006;66:7103-7110.
42 Vehviläinen P, Koli K, Myllärniemi M, Lindholm P, Soini Y, Salmenkivi K, et al. Latent TGF-β binding proteins (LTBPs) 1 and 3 differentially regulate transforming growth factor-β activity in malignant mesothelioma. Hum Pathol 2011;42: 269-278.
43 Kantola AK, Ryynänen MJ, Lhota F, Keski-Oja J, Koli K. Independent regulation of short and long forms of latent TGF-beta binding protein (LTBP)-4 in cultured fibroblasts and human tissues. J Cell Physiol 2010;223:727-736.
October 18, 2011
Accepted after revision March 26, 2012
Author Affiliations: Department of Hepatobiliary and Pancreatic Surgery, Huzhou Central Hospital (Yan Q and Yao X); Huzhou Key Laboratory of Molecular Medicine, Affiliated Central Hospital of Huzhou Teachers College (Huang HL, Li J, Li LQ, Zhong J, Min LS and Dai LC), Huzhou 313000, China; Division of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Yan Q and Zheng SS)
Shu-Sen Zheng, MD, PhD, FACS, Division of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Tel: 86-571-87236601; Fax: 86-571-87236601; Email: shusenzheng@zju.edu.cn); Li-Cheng Dai, Professor, Huzhou Key Laboratory of Molecular Medicine, Affiliated Central Hospital of Huzhou Teachers College, Huzhou 313000, China (Tel/Fax: 86-572-2033020; Email: dlc@hzhospital.com)
© 2012, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(12)60160-X
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Management of splenic artery aneurysm associated with extrahepatic portal vein obstruction
- Pancreas-preserving segmental duodenectomy for gastrointestinal stromal tumor of the duodenum and splenectomy for splenic angiosarcoma
- Induction, modulation and potential targets of miR-210 in pancreatic cancer cells
- Quantitative analysis of intestinal gas in patients with acute pancreatitis
- Can the biliary enhancement of Gd-EOB-DTPA predict the degree of liver function?
- Quality control measures for lowering the seroconversion rate of hemodialysis patients with hepatitis B or C virus
