Effects of Various Water Cultivation Regimes on Plankton Community in Grouper Epinephelus coioides Larviculture Ponds*
2012-05-09,,,,,
, , , , ,
(Institute of Aquaculture,Ocean Colledge∥ Key Lab of Tropical Biology Resources,Ministry of Education,Hainan University,Haikou 570228,China)
Groupers (Family: Serranidae),a very diverse family of carnivorous fish,are widely distributed throughout the tropical and subtropical seas of the world[1].As a member of grouper family,Epinepheluscoioidesis one of the most commonly cultured species[2],especially in China.Although groupers production are becoming more popular in worldwide markets[1],the aquaculture industry of this species is still poorly developed when compared to other marine fish species aquacultured such as stripped bass,cobia,gilthead seabream.Despite of other factors affecting the expansion of cultured area for groupers,the price and steady provision of larvae are important determinants.
Over the past few years,the hatchery technology for grouper has gotten improved,which resulted in a rapid increase in grouper aquaculture production in the Asia-Pacific region[1,3-4].However,mass production of grouper larvae is still encountering many difficulties,and high mortalities were often reported in grouper larvae culture[5-8].
The first-feeding is regarded as a critical point and improvement of the rearing conditions can play an important role in improving food intake,growth and survival of grouper larvae[9].Taking into account that it has been widely reported to improve fish larval growth,survival and feed ingestion[10-11],‘green water technique’ has been extensively used in larval culture of many marine fish species[3,12-14].Though the effect of microalgae on fish larvae performance is not completely understood[15],providing live prey and a colored water environment may be the main functions.Green water environment has been proved to be beneficial to larvae performance for many fish species[15-18].Apart from direct additions of microalgae,fertilization of ponds before stocking is also able to produce live prey and bring a green water environment[19-21].
Therefore,it is worth finding a new more effective approach to improving survival rates and growth of grouper larvae.Marine plankton communities are often looked as a stabilizer for fish larvae[22].Hence,plankton composition and populations density play an important role in fish larvae culture[21,23-24].Effective microorganisms have been reported to play a great role in natural and man-made aquatic ecosystems such as adjusting algal population,speeding up decomposition of organic matter[25-26],but it is unknown whether effective microorganisms will have an effect on plankton growth performance after they are added to grouper experiment ponds.The purpose of this study was to compare and discuss response of plankton composition,populations’ density,respiration and primary productivity in grouperEpinepheluscoioidesexperiment ponds under various additions of effective microorganisms’ solution (EMS),shrimp chip (SC)andPlatymonussppsolution (PS).
1 Materials and methods
1.1 Preparation of effective microorganisms and Platymonus spp.solutions and experiment ponds
Before the experiment ponds’ filling,ten litre of commercially available effective microorganisms (Dongfang Ocean Biology Corporation,Ltd,Haikou city,China)were co-cultured with 20 L clean seawater and 2 kg brown sugar in an enclosed polyethylene bucket.Effective microorganisms were comprised ofClostridiums,Photosyntheticbacterium,Lactobacillus,SaccharomycesandNitrobacteriaspecies,and total concentration of active bacterium contained was 1.0 × 108cells·mL-1.ThePlatymonusspp.were produced by standard protocols in an alga lab of Ocean Colledge of Hainan University,and the concentration ofPlatymonusspp.solution used was 8.0 × 106cells·mL-1.The prepared effective microorganisms’solution (EMS),shrimp chip (SC)(Chengdian Feed and Oil Corporation,Shaoan County of Haikou City,China)andPlatymonusspp.solution (PS)was used as materials for water cultivation.
All experimental ponds were totally drained,sun-dried and flooded,and water was flushed out to clean the pond bed.After disinfected by calcium hypochlorite (60% chlorine)at 20 mg·L-1,twelve 11 m3concrete grouper experiment ponds (2.5 m ×4.0 m ×1.1 m,W×L×H)were randomly distributed to different treatments,and each treatment had three replicates.Each of the experiment ponds was provided with 12 air stones connected to low-pressure electrical blowers,and dissolved oxygen (DO)levels were maintained at saturation.Water temperature was measured daily and was maintained at (27±1.0)℃.The salinity kept at a 23.5~24.5 g·L-1level.Light was applied 24 h a day by fluorescent light tubes.
For PS groups,experiment ponds were filled with clean sand-filtered seawater to an 80 cm depth and incubatedPlatymonusspp.solution equivalently twice daily at 08:00 and 15:00 one day before hatching until the end of the experiment.For EMS+SC groups,experiment ponds were added clean seawater to the same depth as PS groups one week before hatching,and subsequently,prepared effective microorganisms’ solution (EMS)and shrimp chip (SC)were added equivalently by hand twice daily at 08:00 and 15:00 until the end of the experiment.The details of different water cultivation regimes are presented in Table 1.

Table 1 Details of the daily adding schedule of different water cultivation regimes
1)Effective Microorganisms comprised ofClostridiums,Photosyntheticbacterium,Lactobacillus,SaccharomycesandNitrobacteriaspecies,with a total active bacterium concentration of 1.0 × 108cells·mL-1;
2)Platymonusspp.solution with a concentration of 8.0 × 106cells·mL-1
1.2 Larval feed
After experiment ponds’filling,eggs from a grouper-spawning cage in Hongsha Bay of Shanya city of China were hatched at a density of 1.0× 104ind·m-3.One day after hatching,rotifers (Brachionusplicatilis)were daily added to reach a density of 2× 104ind·L-1till the end of the experiment.
1.3 Water sampling and measurements
Water was sampled at two locations in each experiment pond on 2,7,12 and 17 DPH,respectively.Soluble reactive phosphate (SRP)was analyzed by colorimetry after reaction with ammonium molybdate and stannous chloride[27].Total NH3-N of analyses were conducted according to methods described byAPHA[28].
Water samples for phytoplankton were collected with a 100 mL plastic bottle,and samples were preserved with 1% Lugol’s iodine solution.Quantitative analysis of phytoplankton was done using a haemacytometer and a compound microscope.Phytoplankton was identified under a compound light microscope using keys and illustrations by Stafford[29]and Prescott[30]and other phycological taxonomic books.
Zooplankton samples were collected by taking a standard plankton net of 1 m length and mesh size of 63 μm fitted with a flowmeter (Hydrobios,Kiel)at two locations in each experiment pond.Samples were preserved in 240 mL of buffered formalin-sucrose solution prior to enumeration by light microscopy[31].All organisms in three 1 mL subsamples from each pond were counted by using a Sedgwick-Rafter counting cell as described by Geiger & Turner[31]and identified with the taxonomic keys of Thorp & Covich[32].
1.4 Statistic analysis
All data are presented as means ± S.D.and subjected to one-way analysis of variance (ANOVA)to test the effects of experiment treatments using the software of the SPSS (version 11.5)for windows.Duncan’s multiple range test was used to resolve the differences among treatment means[33].Differences among means were considered significant atP≤0.05.
2 Results
2.1 Response of water quality in grouper experiment ponds
Changes in water quality were presented in Table 2.On 17 DPH,total NH3-N level (3.41 μmol·L-1)in G2 was significantly higher than that in other groups (P≤0.05).In all groups,total NH3-N value increased with an increasing of larvae culture time.G4 exhibited significantly higher SRP levels than other groups did during the whole experiment period (P≤0.05).SRP levels were significantly improved by an increment of EMS + SC or PS (P≤0.05).
2.2 Response of plankton composition and populations density in grouper experiment ponds
Phytoplankton and zooplankton compositions observed in all groups were shown in Table 3 and Table 4,respectively.G2 had more abundant and complex plankton composition than other groups did.Thirteen different phytoplankton species and 16 different zooplankton species were observed in G2.On 2 DPH,phytoplankton populations density in G3 (381.85 × 104cell·L-1)and G4 (563.25 × 104cell·L-1)were significantly higher than values in G1 (66.10 × 104cell·L-1)and G2 (141.35 × 104cell·L-1)(p≤0.05)(Fig.1).On 7,12 and 17 DPH,differences in phytoplankton populations density between G2,G3 and G4 were not significant (p>0.05).G2 showed the highest zooplankton populations density (138.74 × 103ind·L-1on 2 DPH,155.90 × 103ind·L-1on 7 DPH,178.50 × 103ind·L-1on 12 DPH and 208.62 × 103ind·L-1on 17 DPH,respectively)among all groups (Fig.2),and differences were significant (P≤0.05)compared to values in G3 and G4 during the whole experiment period.

Table 2 Changes in water quality in grouper larviculture ponds under different water cultivation regimes1)
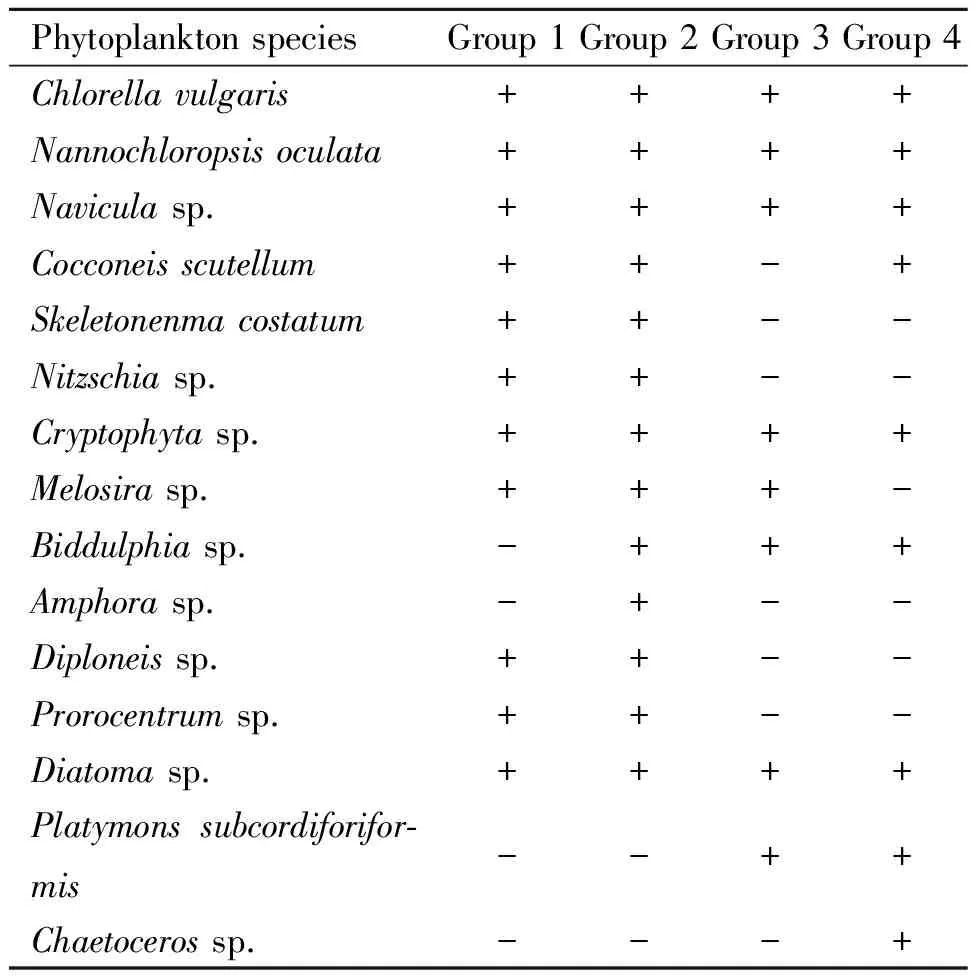
Table 3 Changes in phytoplankton composition in grouper larviculture ponds under different water cultivation regimes1)
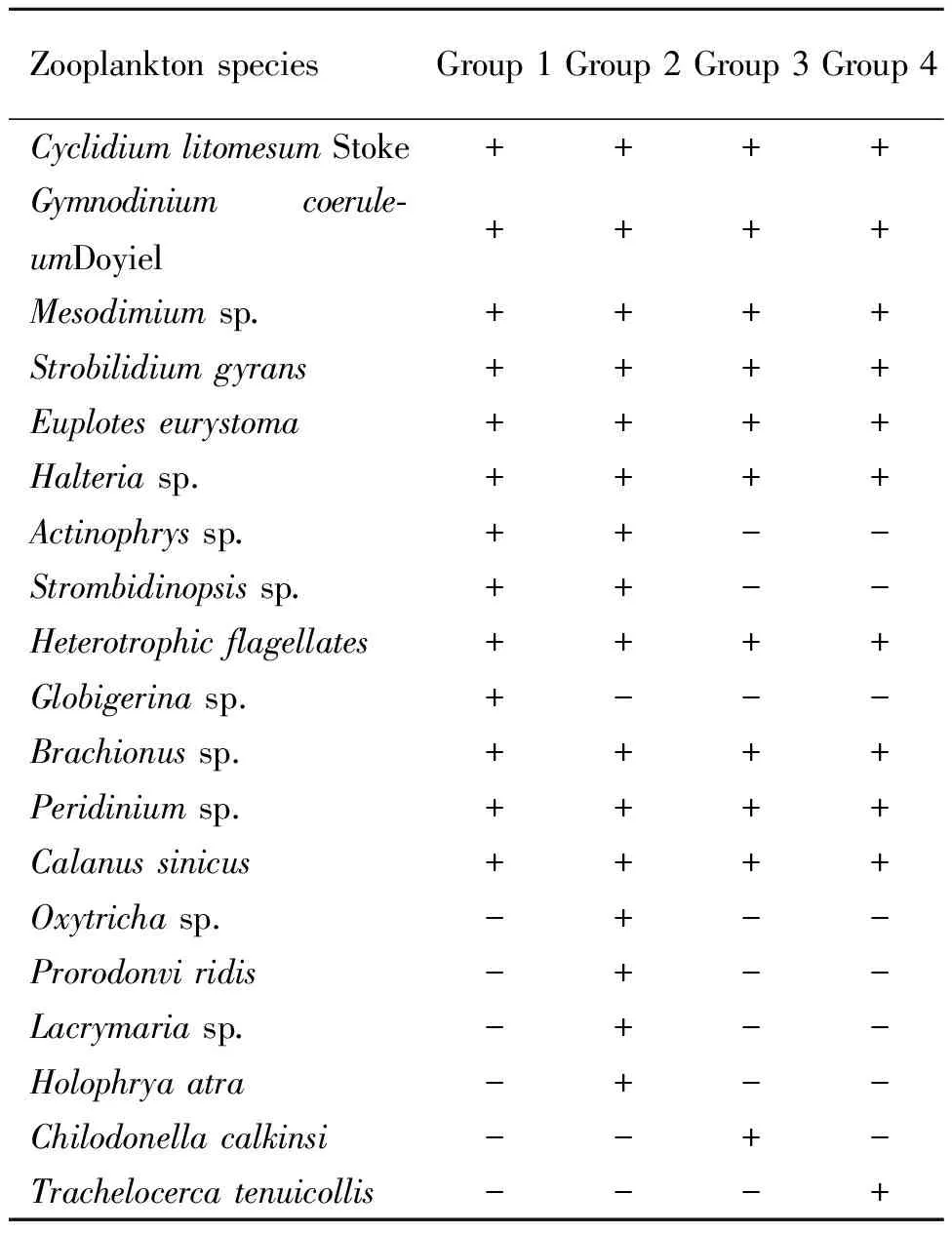
Table 4 Changes in zooplankton composition in grouper larviculture ponds under different water cultivation regimes1)
3 Discussion
In concrete grouperEpinepheluscoioidesexperiment ponds,abundant plankton community,especially zooplankton community,would be produced by additions of EMS and together with SC.Water cultivation to attaining abundant plankton community has been widely regarded as a necessary procedure for fish larvae culture[21].Effects of effective microorganisms on aquatic animals’ immunity and water quality have been studied by some researchers[25-26,34],but in fish experiment ponds,plankton growth performance responsible for effective microorganisms is less concerned.In the present study,high density of zooplankton populations observed in G2 indicates that effective microorganisms do have a positive role in improving zooplankton biomass of grouper experiment ponds.


Fig.1 Changes in phytoplankton density in grouper larviculture ponds under different water cultivation regimes.Values within the same number of DPH with different letters are significantly different (P ≤ 0.05)

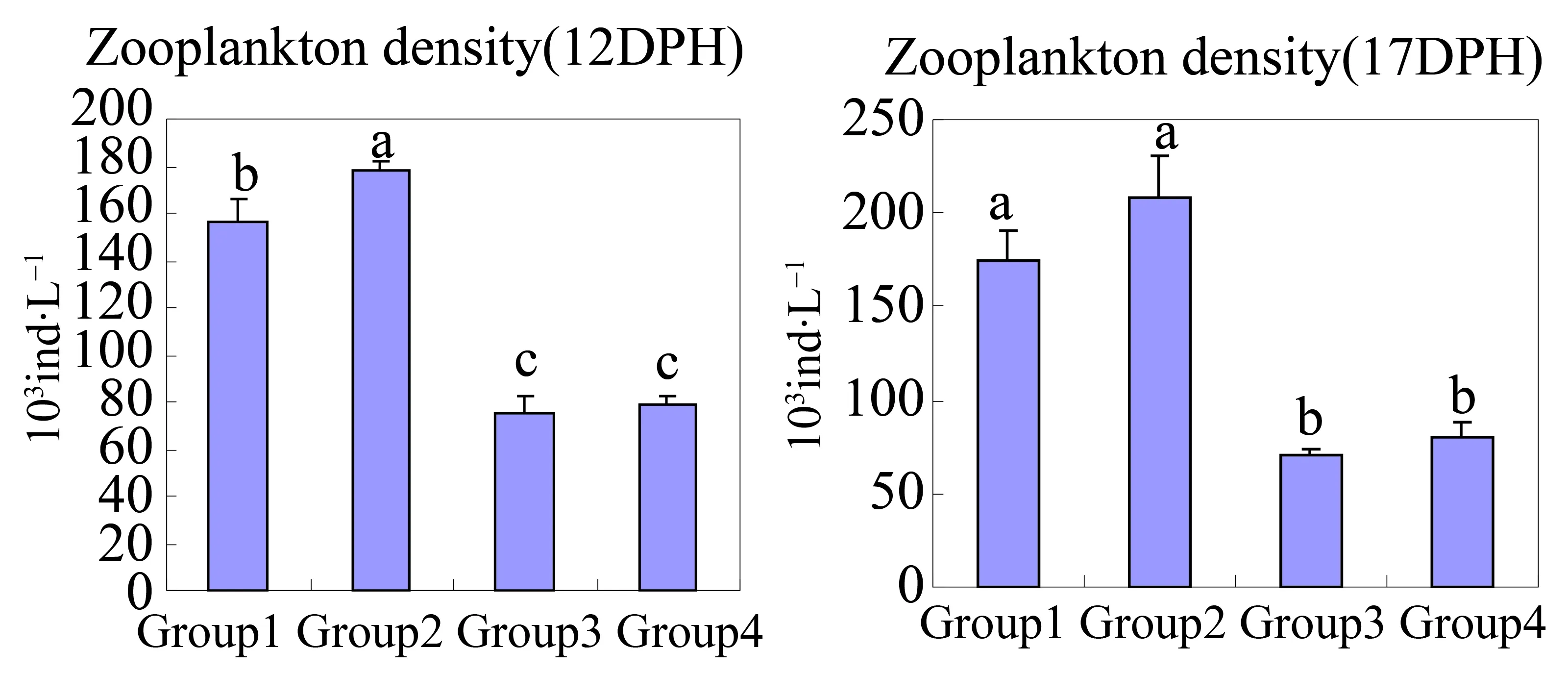
Fig.2 Changes in zooplankton density in grouper larviculture ponds under different water cultivation regimes.Values within the same number of DPH with different letters are significantly different (P ≤ 0.05)
It has been reported that early survival of aquacultured grouper larvae is very low compared to other finfish[35-37],which may be due to the poor first-feeding conditions such as live prey deprivation or environmental stress[38].Live zooplankton has been regarded as the main source of larval food for marine fish[24,39].In this study,G2 had more complex plankton composition and significantly higher zooplankton populations’ density than other groups did.This indicates that water cultivation regimes of G2 are more beneficial to grouper larvae survival and growth compared to other regimes.Additionally,a brown water environment came into being in ponds which been added shrimp chip and brown sugar.This may play a role in alleviating possible external stress to grouper larvae.
From 7 DPH,the phytoplankton populations’ density of G2 reached a high level as G4.This shows that effective microorganisms’ solution would also enhance phytoplankton biomass.These phytoplankton may provide enough live feed for zooplankton thriving.During the larval culture period,zooplankton populations’ density observed in G3 and G4 were low,which indicates thatPlatymonusspp.solution did not have an obvious effect on zooplankton biomass.
The increased total NH3-N and soluble reactive phosphate (SRP)concentrations observed in all groups may indicate that there have N and P accumulations in experiment ponds.In this study,there was a positive relationship between water SRP levels and phytoplankton population density.These results show that SRP is one of the limiting factors affecting water phytoplankton biomass,which was in accordance with the report of[31].
In conclusions,for concrete grouperEpinepheluscoioidesexperiment ponds,abundance of phytoplankton and zooplankton community could be attained by additions of EMS and together with SC.We suggest filling concrete grouper experiment ponds a week before hatching and adding EMS and SC at daily levels of 80 mL·m-3and 8 g·m-3,respectively.It is possible that regimes of water cultivation for grouperEpinepheluscoioidesproposed in the present study are also of practical use for other marine fish larvae culture.Further works should be required to consider the mechanism of foodchain variations in fish-culturing ponds.
Acknowledgements The authors would like to appreciate Xingye Aquaculture Corporation Ltd.for providing grouper experiment ponds.
:
[1]WILLIAMS K C.A review of feeding practices and nutritional requirements of postlarval groupers[J].Aquaculture,2009,292: 141-152.
[2]CHENG A C,CHENG S A,CHENG Y Y,et al.Effects of temperature change on the innate cellular and humoral immune responses of orange-spotted grouperEpinepheluscoioidesand its susceptibility toVibrioalginolyticu[J].Fish & Shellfish Immunology,2009,26: 768-772.
[3]LIAO I C,SU H M,CHANG E Y.Techniques in finfish larviculture in Taiwan[J].Aquaculture,2001,200: 1-31.
[4]RIMMER M A,McBRIDE S,WILLIAMS K C.Advances in Grouper Aquaculture [M].Australian Centre for International Agriculture Research,Canberra.ACIAR Monograph,vol.110.2004:137.
[5]DURAY M N,ESTUDILLO C B,ALPASAN L G.Larval rearing of the grouperEpinephelussuillusunder laboratory conditions[J].Aquaculture,1997,150: 63-76.
[6]BOMBEO-TUBURAN I,CONIZA E B,RODRIGUEZ E M,et al.Culture and economics of wild grouper (Epinepheluscoioides)using three feed types in ponds[J].Aquaculture,2001,201: 229-240.
[7]HSEU J R,HWANG P P,TING Y Y.Morphometric model and laboratory analysis on intracohort cannibalism in giant grouperEpinepheluslanceolatusfry[J].Fisheries Science,2004,70: 482-486.
[8]HSEU J R,SHEN P S,HUANG W B,et al.Logistic regression analysis applied to cannibalism in the giant grouperEpinepheluslanceolatusfry[J].Fisheries Science,2007,73: 472-474.
[9]YOSEDA K.Studies on early mass mortality during hatchery rearing of three grouper species,Malabar grouperEpinephelusmalabaricus,red spotted grouperEpinephelusakaara,and leopard coral grouperPlectropomusleopardus[J].Bulletin of Fisheries Research Agency,2008,23: 91-144.
[10]ØIE G,MAKRIDIS P,REITAN K I,et al.Protein and carbon utilization of rotifers (Brachionusplicatilis)in frst feeding of turbot larvae (ScophthalmusmaximusL.)[J].Aquaculture,1997,153: 103-122.
[11]REITAN K I,RAINUZZO J R,ØIE G,et al.A review of the nutritional effects of algae inmarine fish larvae[J].Aquaculture,1997,155: 207-221.
[12]FUSHIMI H.Production of juvenile marine finfish for stock enhancement in Japan[J].Aquaculture,2001,200: 33-53.
[13]LEE C S,OSTROWSKI A C.Current status of marine finfish larviculture in the United States[J].Aquaculture,2001,200: 89-109.
[14]SHIELDS R.Larviculture of marine finfish in Europe[J].Aquaculture,2001,200: 55-88.
[15]MEEREN T C D,MANGOR-JENSEN A,PICKOVA J.The effect of green water and light intensity on survival,growth and lipid composition in Atlantic cod (Gadusmorhua)during intensive larval rearing[J].Aquaculture,2007,265: 206-217.
[16]CAHU C L,ZAMBONINO INFANTE J L P A,Quazuguel et al.Algal addition in sea bass (Dicentrarchuslabrax)larvae rearing: effect on digestive enzymes[J].Aquaculture,1998,161: 479-489.
[17]BENGTSON D A,LYDON L,AINLEY J D.Green-water rearing and delayed weaning improve growth and survival of summer flounder[J].North American Journal of Aquaculture,1999,61: 239-242.
[18]PAPANDROULAKIS N,DIVANACH N,KENTOURI M.Enhanced biological performance of intensive sea bream (Sparusaurata)larviculture in the presence of phytoplankton with long photophase[J].Aquaculture,2002,204: 45-63.
[19]TUCKER C S,ROBINSON E H.ChannelCatfishFarmingHandbook[M].New York:Van Nostrand Reinhold,1990:454 .
[20]LUDWIG G M,STONE N M,COLLINS C.Fertilization of Fish Fry Ponds[M].SRAC Publication,vol 469.Southern Regional Aquaculture Center.Stoneville,Mississippi,1998:323.
[21]MISCHKE C C,ZIMBA P V.Plankton community responses in earthen channel catfish experiment ponds under various fertilization regimes[J].Aquaculture,2004,233: 219-235.
[22]YOSEDA K,YAMAOTO K,ASAMI K,et al.Influence of light intensity on feeding,growth,and early survival of leopard coral grouper (Plectropomusleopardus)larvae under mass-scale rearing conditions[J].Aquaculture,2008,279: 55-62.
[23]LUDWIG G M.The effects of increasing organic and inorganic fertilizer on water quality,primary production,zooplankton,and sunshine bassMoronechrysops×M.saxatilis,fingerling production[J].Journal of Applied Aquaculture,2002,12: 1-29.
[24]JAMES A,PITCHFORD J W,BRINDLEY J.The relationship between plankton blooms,the hatching of fish larvae,and recruitment[J].Ecological Modelling,2003,160: 77-90.
[25]MORIARTY D J W.The role of microorganisms in aquaculture ponds[J].Aquaculture,1997,151: 333-349.
[26]ZHOU Q L,LI K M,JUN X,et al.Role and functions of beneficial microorganisms in sustainable aquaculture[J].Bioresource Technology,2009,100: 3780-3786.
[27]EBINA J,TSUTSUI T,SHIRAI T.Simultaneous determination of total nitrogen and total phosphorus in water using peroxodisulfate oxidation[J].Water Research,1983,17: 1721-1726.
[28]APHA (American Public Health Association),American Water Works Association and Water Pollution Control Federation.Standard Methods for the Examination of Water and Wastewater[M].17th ed.American Public Health Association.Washington,DC,1989:1268.
[29]STAFFORD C.A Guide to Phytoplankton of Aquaculture Ponds[M].Collection Analysis and Identification.Department of Primary Industries.Queensland,1999:59.
[30]PRESCOTT G W.Algae of the Western Great Lakes Area with an Illustrated Key To the Genera of Desmid Sand Fresh Water Diatoms[M].Brown,Dubuque,IA ,1962:977.
[31]GEIGER J G,TURNER C J.Pond Fertilization and Zooplankton Management Techniques for Production of Fingerling Striped Bass and Hybrid Striped Bass[M]//HARRELL R M,KERBY J H,MINTON R V,eds.Culture and Propagation of Striped Bass and its Hybrids.American Fisheries Society.Bethesda,MA,1990: 79-98.
[32]THORP J H,COVICH A P.Ecology and Classification of North American Freshwater Invertebrates[M].San Diego,CA:Academic Press,1991:911.
[33]DUNCAN D.B.Multiple-range and multiple F tests[J].Biometrics,1955,11: 1-42.
[34]NAYAK S K.Probiotics and immunity: A fish perspective[J].Fish & Shellfish Immunology,2010,29: 2-14.
[35]YAMAOKA K,NANBU T,MIYAGAWA M,et al.Water surface tension-related deaths inprelarval red-spotted grouper[J].Aquaculture,2000,189: 165-176.
[36]YOSEDA K,DAN S,SUGAYA T,et al.Effects of temperature and delayed initial feeding on the growth of Malabar grouper (Epinephelusmalabaricus)larvae[J].Aquaculture,2006,256: 192-200.
[37]BOGLIONE C,MARINO G,GIGANTI M,et al.Skeletal anomalies in dusky grouperEpinephelusmarginatus(Lowe 1834)juveniles reared with different methodologies and larval densities[J].Aquaculture,2009,291: 48-60.
[38]KOHNO H.Early Life History Features Influencing Larval Survival of Cultivated Tropical Finfish[M]//De SILVA S S,ed.Tropical Mariculture.London: Academic Press,1998,:72-110.
[39]HAMRE K,MOREN M,SOLBAKKEN J,et al.The impact of nutrition on metamorphosis in Atlantic halibut (HippoglossushippoglussusL.)[J].Aquaculture,2005,250: 555-565.
