Research on purification of metallurgical grade silicon(MG-Si)by acid leaching
2012-02-08LUODaweiLUYipingLIUNingLITingjuZHANGGuoliang
LUO Da-wei, LU Yi-ping, LIU Ning, LI Ting-ju,2, ZHANG Guo-liang
(1.School of Materials Science and Engineering,Dalian University of Technology,Dalian 116024,China;2.Key Laboratory of Materials Modification by Laser,Electron and Ion Beams,Ministry of Education,Dalian University of Technology,Dalian 116024,China)
0 Introduction
The global PV industry has rapidly developed over the past decade which leads to a large demand for silicon materials.Solar cells are currently fabricated from a variety of siliconbased materials.However,current market is difficult to ensure a steady supply for this material[1].Development of a new process to produce silicon at low cost is definitely necessary.MG-Si(metallurgical grade silicon)with the purity of 98%which is produced by carbothermic reduction in electric arc furnaces has been considered as a cheap starting material for conversion to 99.99%of purity[2].Many alternative methods for purifying MG-Si to solar grade silicon(SoG-Si)have been explored,for example,(1)pyrometallurgical processes[3];(2)hydrometallurgical processes[4];(3)electrochemical methods[5]. Among above methods,hydrometallurgical process is recognized as the best pre-treatment process for silicon refining because metallic impurities can be removed from MG-Si by this process without large amounts of energy consumption and capital investment of equipments.
The investigation of acid leaching as a pretreatment procedure in metallurgical route started in the 1920s[6].A number of studies had illustrated the feasibility of this process[7-9].For example,Norman,etal.obtained 99.9% silicon by leaching in three successive steps with aqua regia,hydrofluoric acid and hydrochloric acid[10].More recently,Juneja,etal.reported that the best leaching yield was obtained by using hydrofluoric acid at 50℃,150μm MG-Si.The purity of silicon achieved 99.95%[11].Zhang,etal.improved the purifying effect significantly using an ultrasonic field in the acid leaching process[12].Although many reports have been published on the MG-Si acid leaching,there were a lot of large diversity of experimental conditions,results and frequently contradictory results between the different literatures.In the meantime,many process parameters are given at a range of value.Therefore,it is difficult to find out the effective process parameters for large scale pickling.The objective of the present study is to illustrate the effect laws of different pickling parameters on removal effect for impurities(such as aluminum,iron and calcium)and attempt to find the optimum processing parameters for MG-Si pickling.Impurity contents and microstructures are confirmed by inductively coupled plasma atomic emission spectrometer(ICP-AES)and metallographic microscope(Olympus-GX51).
1 Experiments
1.1 Characterization of MG-Si
Impurity contents and their segregation coefficients in MG-Si are listed in Tab.1.The major metallic impurities are aluminum(0.271%),iron(0.234%)and calcium(0.058%).
Microstructures of MG-Si are shown in Fig.1.The bright phases at the grain boundaries are the segregation of metal impurities.The qualitative composition of bright phases and their relative content in MG-Si are listed in Tab.2.The sensitivity of these phases to the acids has been studied by observation of the samples after exposure to hydrochloric acid and hydrofluoric acid.The phases which were not attacked by hydrochloric acid are only Si-Fe and Si-Fe-Ti,but all of them are completely removed by the attack of hydrofluoric acid.According to the sensitivity of these phases to the acids in Tab.2,hydrochloric acid and hydrofluoric acid are selected as acid agents in this paper.The removal efficiency of aluminum,iron and calcium after the acid leaching by hydrochloric acid and hydrofluoric acid are checked.
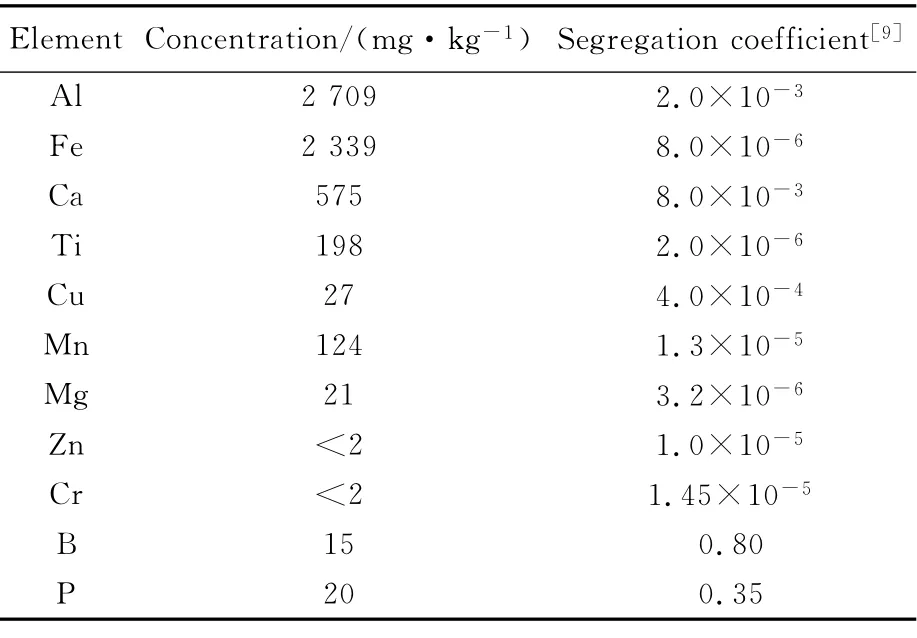
Tab.1 Impurity contents and their segregation coefficients in MG-Si

Tab.2 The main precipitated phases and their sensitivity to hydrochloric acid and hydrofluoric acid in MG-Si[2]
1.2 Experimental procedures
MG-Si is crushed by the crushing mill and grinded with the attritor which is composed of adamantine spar container and Al2O3sintered hard ceramic balls before the leaching experiments.And then,silicon particles are sieved to different particle sizes for leaching trials.Different particle sizes silicon is disposed by acetone solution under ultrasonic wave and washed by the deionized water.Leaching trials are done with aqueous thermostat after the silicon powders are dried.The mass of silicon powders employed at one time is 10 g and the liquid-solid mass ratio is 10∶1.After each experiment,silicon powders are filtered and washed with deionized water.The contents of aluminum,iron and calcium under different acid leaching conditions are analyzed by ICP-AES,respectively.
2 Results and discussion
2.1 Effect of various acid leaching parameters on the impurity content

Fig.2 Final impurity content after leaching with hydrochloric acid and hydrofluoric acid at different concentrations
A larger number of leaching trials are performed in a systematic way in order to study the effects of the acid agent,acid concentration,time,temperature and silicon particle size on the removal efficiencies of impurities element.Specific results are described in the following sections in more details.
2.1.1 Acid agent and acid concentration The change of impurity content with the increasing of the concentration of the hydrofluoric and hydrochloric acids is plotted in Fig.2.The contents of aluminum and iron gradually decrease with the increasing of concentration of hydrochloric acid,but the change regularity of calcium content is inequable.Calcium content reduces firstly and then grows little as the concentration of hydrochloric acid increases.The minimum value achieves at 15%.This is because the silicon powders generate passivation when the concentration of hydrochloric acid exceeds 15%.The change tendencies for the content of aluminum,iron and calcium after the hydrofluoric acid treatment are very different.Aluminum content increases and iron content gradually decreases with the increasing of concentration of hydrofluoric acid.Calcium content reduces firstly and then grows,the minimum value achieves at 2%.For the unusual change regularity of the aluminum and calcium,the reason most probably is the formation of insoluble fluorides.
2.1.2 Temperatures The effects of temperatures on the impurity contents are demonstrated in Fig.3.The content changes of aluminum,iron and calcium with temperatures are coincident after leaching with hydrochloric acid.They all gradually decrease with temperatures.But the results are diverse after leaching with hydrofluoric acid.With the increasing of temperature,the content of iron gradually decreases,but the contents of aluminum and calcium reduce firstly,and then grow a little.The minimum value achieves at 40℃and 60℃,respectively.The reason for above phenomenon is most probably the formation of insoluble fluorides.
2.1.3 Time The effect of the leaching time on the impurity contents is shown in Fig.4.The content changes of aluminum,iron with the increasing leaching time are the same after leaching with hydrochloric acid.They all reduce firstly and then grow a little,and the minimum value achieves at 10 h.The content changes of iron and calcium with time after hydrofluoric acid leaching are the same as that after hydrochloric acid leaching.The minimum value achieves at 2 h.But the aluminum content gradually decreases with the increasing of time.
2.1.4 Particle sizes The effects of the particle sizedon the impurity contents are demonstrated in Fig.5.The content changes of aluminum and calcium with particle sizes are alike after leaching with hydrochloric and hydrofluoric acids.The contents of aluminum and calcium gradually decrease with the increasing of particle sizes.
The total contents of aluminum,iron and calcium impurities reach the minimum at 100μm.This result is opposite to that of some previous research[4].In order to explain this consequence,the content of major impurities in MG-Si at different particle sizes is analyzed by ICP-AES before acid leaching.The measurement result is illustrated in Fig.6.The content of major impurities in silicon particles with 30μm is higher than that with 80μm.It is suggestsed that more friable phases including more impurities are aggregated to the finer fraction during sieving.A similar grain size effect is found for other minor impurities.Impurities,such as the doping elements boron and phosphorus with unfavorable segregation coefficients,are not greatly affected by acid leaching.However,they must be removed by pyrometallurgical treatment or a similar method[3].
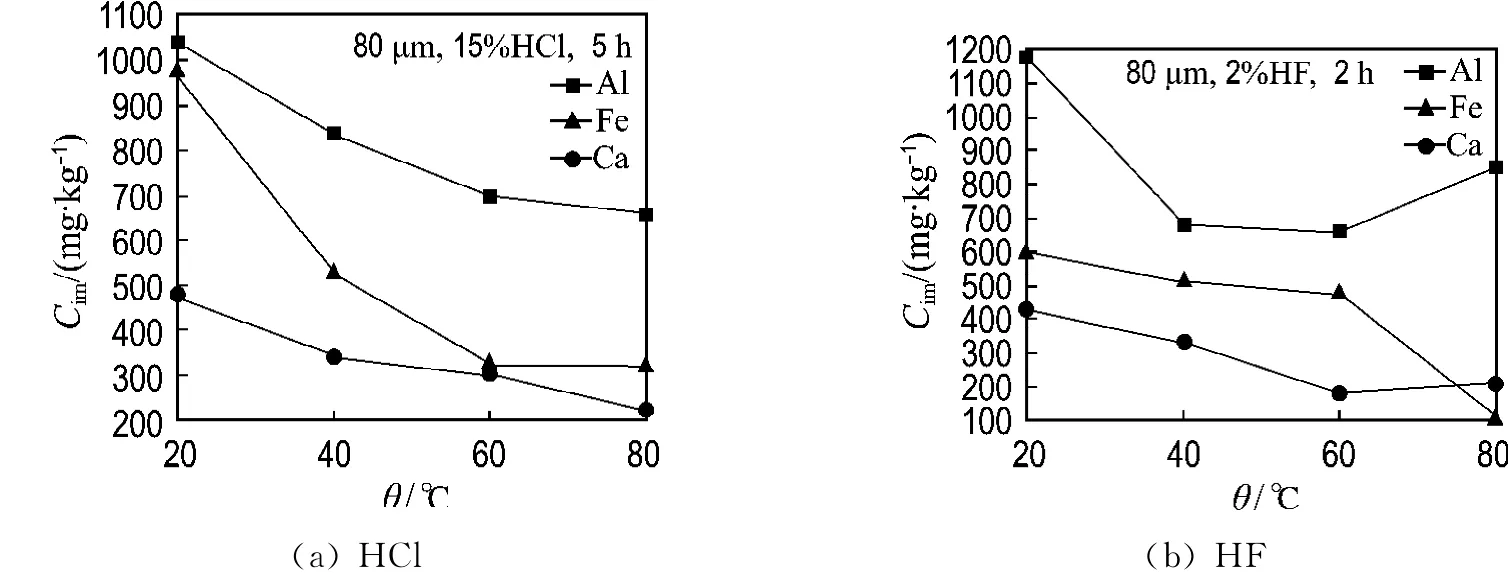
Fig.3 Final impurity content after leaching with hydrochloric acid and hydrofluoric acid at different temperatures

Fig.4 Final impurity content after leaching with hydrochloric acid and hydrofluoric acid at different leaching time

Fig.5 Final impurity content after leaching with hydrochloric acid and hydrofluoric acid at different particle sizes

Fig.6 The concentration of major impurities at different particle sizes
2.2 The final purity of silicon at the optimum processing parameters
From the above analyses it is known that,the optimum process parameters for acid leaching are as follows:15%hydrochloric acid,80℃,10 h and 100μm.The final contents of major impurities after leaching with hydrochloric acid and hydrofluoric acid solutions at the optimum processing parameters are presented in Fig.7.Although the acid leaching parameters are the optimum processing parameters,final total content of major impurities treated with hydrochloric acid is lower than that treated with hydrofluoric acid.The final purity of silicon powders after leaching with hydrochloric acid and hydrofluoric acid solutions at the optimum processing parameters is denoted in Fig.8.The final purity of silicon powders after leaching with hydrochloric acid solution is better than that after leaching with hydrofluoric acid solution and the final purity of silicon powders after leaching with hydrochloric acid and hydrofluoric acid mixture solutions is a little better than that after leaching with hydrochloric acid alone.In the meantime,there is no significant difference between silicon treated in one step with mixture acid,and treated in two steps,first with hydrochloric acid and then with hydrofluoric acid.This result is consistent with the pervious reference[3].When hydrochloric and hydrofluoric acids are used for leaching simultaneously,care must be taken because it might cause explosions under this status.Hydrogen generates during acid leaching process,in addition,because of the presence of silicides(especially calcium and magnesium silicides)in MG-Si,self-igniting silicon hydrides are formed.So adequate safety precaution should be adopted to avoid the above danger and it is better to select hydrochloric acid as the acid leaching solution.
2.3 The acid leaching critical value

Fig.7 Final contents of major impurities after leaching at the optimum processing parameters
Silicon of 99.9%is obtained by leaching with hydrochloric acid alone from MG-Si with iron(0.234%),aluminum(0.271%)and calcium(0.058%)at the optimum processing parameters.On the other hand,silicon of above 99.9%can also be obtained after leaching with hydrochloric acid and hydrofluoric acid.The significant improvement achieved by mixture solutions is due to the Si-Fe and Si-Fe-Ti phases which are not leached by hydrochloric acid.Although the silicon powders of 99.9%can be obtained after the acid leaching,the major metallic impurities in MG-Si can not be fully got rid of only with the acid leaching because there is a critical value in the acid leaching process.

Fig.8 Final purity of silicon powders after leaching at the optimum processing parameters
There are two main reasons as follows:the distribution of impurities in MG-Si is non-uniform and their distribution can be divided into two situations:one is light-colored inclusions which are confined to the grain boundaries and can be clearly defined at grain boundaries;the other one is darkcolored inclusions which are distributed as clutterlike particles in MG-Si.In order to separate these impurities from MG-Si as much as possible in the wet pre-treatment process,MG-Si need to be crushed.During the course of this processing,the impurities exist in MG-Si powders by three forms.The first one is a single particle alone in the MGSi powders;the second one is partly enchased in the silicon and partly naked to the air;the third one is completely coated by silicon with no contact with the outside world.
When the MG-Si powders are processed in the leaching agent,the first form of impurity can quickly react with the leaching agent and go into the solution.Leaching rate can be improved through strengthening stirring,increasing the leaching agent concentration and reaction temperature and so on.The second form of impurity contacts with the outside world only partially,the reaction between the agent and the impurity need to keep from outside to inwards,and this reaction is controlled by a chemical reaction process,and leaching rate can be improved by increasing the reaction temperature,the leaching agent concentration and reducing the original particle radius.The third form of impurity in silicon is coated completely without contact with the outside world.Because silicon does not react with the leaching agent and reaction between the leaching agent and the impurities is difficult,the reaction process is controlled by internal diffusion.Increasing the reaction temperature,reducing the original particle radius and the thickness of solid membrane can improve their leaching rate.When MG-Si powders exist in the wet pre-treatment process,the first form of impurity can quickly react with the leaching agent and thus it can be removed commendably.The second form of impurity can also be removed after a period of reaction time.The third form of impurity coated in the silicon is difficult to contact and react with the leaching agent because silicon is a good barrier coating for impurities[13].
3 Conclusion
(1)The optimum processing parameters for acid leaching are as follows:15%hydrochloric acid,80℃,10 h and 100μm.The removal efficiencies of aluminum,iron and calcium impurities are up to 70.90%,94.82%and 82.69%,respectively.
(2)The formation of insoluble fluorides is a rejection for the impurity removal under hydrofluoric acid and it is dangerous under the mixture of hydrofluoric and hydrochloric acids.In order to avoid the above situation,it is better to select hydrochloric acid as the acid leaching solution.
(3)The content of major impurities increases as the particle sizes of MG-Si powders decrease,because the more friable phases containing impurities concentrate in the finer fraction during sieving.
(4)The major metallic impurities in the silicon can not be fully got rid of only with the acid leaching because there is a critical value in the acid leaching process.Although acid leaching process is only as a pre-treatment step,it is very important and indispensable.
[1]NAUMOV A V.Additional information on the development of solar power and market for silicon raw materials in 2007-2010[J].Journal of Materials Electronic Technology,2007(1):15-20
[2]KANTOS I C,GONALVES A P,SANTOS C S,etal.Purification of metallurgical grade silicon by acid leaching[J].Hydrometallurgy,1990,23(2-3):237-246
[3]KOTVAL P S.Process for the production of refined metallurgical silicon:U.S.Patent 4195067[P].1980-05-25
[4]SCHEI A.A metallurgical route to solar-grade silicon[C]//Proceedings of the Flat-Plate Solar Array Project Workshop on Low-Cost Polysilicon for Terrestrial Photovoltaic Solar-Cell Applications.Las Vegas:California Institute of Technology,1985:279-294
[5]SHARMA I G,MUKHERJEE T K.A study on purification of metallurgical grade silicon by molten salt electrorefining[M].Metallurgical and Materials Transactions B,1986,17(2):395-397
[6]TUCKER N P.Alloys of iron research,Part VII,Preparation of high purity silicon[J].Journal of Iron and Steel Industry,1927,15:412-414
[7]VOOS W.Production of pure silicon:U.S.Patent 2972521[P].1961-02-21
[8]HUNT L P,DOSAJ V D,MCCORMICK J R,etal.Production of solar grade silicon from purified metallurgical silicon[C]//Record of the 12th IEEE Photovoltaic Specialists Conference.New York:IEEE,1976:125-129
[9]DIETL J.Hydrometallurgical purification of metallurgical grade silicon[J].Solar Cells,1983,10:145-154
[10]NORMAN C E,THOMAS R E,ABSI E M.Solar grade silicon substrates by a power to-ribbon process[J].Canadian Journal of Physics,1985,63:859-862
[11]JUNEJA J M,MUKHERJEE T K.A study of the purification of metallurgical grade silicon[J].Hydrometallurgy,1986,16:69-75
[12]ZHANG J,LI T J,MA X D,etal.Optimization of the acid leaching process by using an ultrasonic field for metallurgical grade silicon[J].Journal of Semiconductors,2009,30:3002-1-3002-6
[13]ZHAN L Y.Pre-treatment experimental study of direct preparation solar grade silicon from metallurgical grade silicon[D].Kunming:Kunming University of Science and Technology,2007(in Chinese)
