Thrombospondins and synaptogenesis***★
2012-01-04BinWang,WeitaoGuo,YunHuang
Thrombospondins and synaptogenesis***★
thrombospondin; synapse; synaptogenesis; glial cell; α2δ-1; extracellular matrix; neural progenitor cells; Notch; opioid; regeneration; neural regeneration
Research Highlights
(1) This review summarizes the effects and underlying mechanisms of action of thrombospondin in synaptogenesis.
(2) Thrombospondin regulates synaptogenesis through receptor α2δ-1 and neuroligin 1, and promotes the proliferation and differentiation of neural progenitor cells.
(3) Thrombospondin also participates in synaptic remodeling following injury and in the action of some nervous system drugs.
Abbreviations
TSP, thrombospondin; NPCs, neural progenitor cells; CNS, central nervous system; RGCs, retinal ganglion cells; EGF, epidermal growth factor
lNTRODUCTlON
The occurrence and progression of nervous system injury and related diseases is a process involving changes in synapse number, structure and function. Therefore, the effective regulation of synapse formation and function is important for the treatment of nervous system diseases. Synaptic formation and remodeling involve multiple factors. Recent evidence indicates that astrocyte-secreted thrombospondin (TSP) can regulate synaptogenesis through receptor α2δ-1 and neuroligin 1, and promote the proliferation and differentiation of neural progenitor cells (NPCs). It also plays a role in synaptic remodeling post-injury, and modulates the action of some nervous system drugs[1-5].
Understanding the mechanisms of action of TSP on synaptogenesis should provide insight into the regulation of synaptic plasticity, and help in the development of treatments for nervous system disease and injury. However, it remains poorly understood how TSP modulates synaptogenesis. In the present study, we provide a review of the literature and summarize the effects and mechanisms of action of TSP on synaptogenesis. This should provide a reference for researchers examining the role of TSP in different types of synaptogenesis, including thedownstream mechanisms that mediate these effects, as well as thein vivoregulation and clinical application of this crucial protein.
ROLE OF TSP lN SYNAPTOGENESlS
The synapse is the key structure for signal communication between neurons. Large numbers of synapses relay electrical signals that mediate complex thoughts and actions, and synaptic morphological changes underlie learning and memory. Numerous ligands and cell surface molecules are involved in synapse formation and maturation[6]. However, the molecular mechanisms that regulate synaptogenesis remain poorly understood. Current studies have demonstrated that glial cells are critical for synapse formation and function[7-12]. Christophersonet al[13]reported that TSP secreted from astrocytes is a key regulator of synaptogenesis.
TSPs are large oligomeric extracellular matrix proteins. They bind a variety of membrane receptors, extracellular matrix components and cytokines to mediate interactions between cells and between cells and matrix[14-17]. TSPs play roles in cell attachment and migration, and can alter cell morphology, and inhibit angiopoiesis and tumor growth[18-20]. Astrocyte-secreted TSP distributes in synapses of the central nervous system (CNS) and the neuromuscular junction[21]. As extracellular matrix proteins, TSPs can induce cell adherence and alter signaling, thereby exhibiting the potential to induce synaptogenesis.
The TSP family consists of thrombospondins 1-5 (TSP3 and TSP5 are mainly expressed in bone and muscle, and have little association with synaptogenesis). TSP1 and TSP2, trimeric proteins, have similar structures and functional domains; TSP4, a pentameric protein, has a structure that differs from TSP1/TSP2 (Figure 1).

Figure 1 Domain structure of thrombospondins (TSPs)[1].
These family members have different coding genes. Although TSPs are expressed in the brain, their effects on central nerve cells remain unknown[22].
Christophersonet al[13]found that astrocyte-secreted TSP1 and TSP2 can significantly increase the quantity of synapses in retinal ganglion cells (RGCs) culturedin vitro(Figure 2). In addition, the number of excitatory synapses is significantly reduced in TSP1/TSP2 knockout mice. Similar effects of TSP1 were found on hippocampal neurons culturedin vitro[3]. In addition, TSP1 can promote neuronal migration[23]and axonal overgrowth[24].

Figure 2 Thrombospondin (TSP) promotessynaptogenesis in retinal ganglial cells (RGCs; immunofluorescence, scale bar: 30 μm)[13].
In contrast to other TSPs, TSP4 is only expressed by mature astrocytes (postnatal 17 days)[18]. TSP4 represents the mature TSP isoform in the CNS. It is essential for regulating synaptic formation and plasticity. Interestingly, TSP4 is also found in the neuromuscular junction, indicating a possible role in neuromuscular junction structure[22]. A comparison of TSP gene expression among primates showed that TSP2 and TSP4 levels are relatively higher in humans[25]. It is likely that high TSP expression may promote synaptogenesis in the human brain, resulting in higher cognition in humans.
CHARACTERlSTlCS OF TSP lN SYNAPTOGENESlS
TSP can promote presynaptic and postsynaptic remodeling, but TSP expression is significantly reduced in the CNS of adult rodents, and correspondingly, synapse formation and plasticity is significantly limited[26]. This indicates that there is a specific time window in which TSP promotes synaptogenesis in the CNS,i.e. TSP1 and TSP2 are highly expressed during development, but downregulated after maturation[13,27-28]. This suggests that TSP1 and TSP2 regulate synaptogenesis in a narrow developmental window, and that they are not essential for maintaining synapses in the mature organism. However, high TSP expression is found in the mature newt, goldfish and other organisms with strong neural regeneration capacity[29].
While TSP1 and TSP2-induced synapses have normal ultrastructure and presynaptic membrane function, the postsynaptic membranes lack a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, which are silenced[13,27]. This silencing is common in early development of excitatory synapses in the CNS[30]. Christophersonet al[13]cultured RGCs with astrocytes or astrocyte conditioned medium and found a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor labeling in the postsynaptic membrane. The authors hypothesized that astrocytes produce a signaling molecule that can promote the formation of functional synapses.
MECHANlSM OF TSP REGULATlON OF SYNAPTOGENESlS
After removing astrocytes from the culture system, RGC synapse number was significantly reduced, possibly because TSPs not only promote synaptogenesis, but also maintain synaptic stability[13]. In addition, the presynaptic membrane protein synaptotagmin and the postsynaptic membrane protein postsynaptic density protein 95, as well as synapse-associated protein 102 and Homer protein, were not influenced by TSPs, indicating that TSPs do not regulate the synthesis or degradation of these proteins, but instead function by regulating their transport or cellular localization. Therefore, we assume that TSPs bind synaptic regions to induce synaptogenesis by affecting the localization of cell surface receptors. Many known TSP receptors aggregate at the synapse, including integrin-related protein CD47, various types of integrins and low-density lipoprotein receptor-related protein 1. TSP may activate downstream presynaptic and/or postsynaptic signaling cascades to induce synaptogenesis by binding one or several receptors.
Erogluet al[1]reported that no known TSP receptor could induce synaptogenesis. They found that TSP binds von Willebrand factor repeat sequences, which are present in α2. Therefore, they assumed that α2δ may be the TSP receptor. α2δ-1 overexpression in nerve cells of transgenic mice increased the number of excitatory synapses, and blocking the receptor inhibited synaptic plasticity in the sensory cortex of neonatal mice in response to sensory nerve deprivation by whisker trimming[1]. In addition, gabapentin, an antagonist of α2δ-1, can greatly reduce synaptogenesis in the brain of wild-type mice[1]. All the above findings demonstrate the importance of the TSP-α2δ-1 interaction in synaptogenesis. Further study showed that epidermal growth factor (EGF)-like region of TSP can directly bind α2δ-1 to stimulate synaptogenesis[1], demonstrating that α2δ-1 is the neuronal TSP receptor. α2δ-1 knockout using siRNA showed that α2δ-1 is essential for TSP-induced synaptogenesis[1]. α2δ-4 gene disruption in mice results in the lack of ribbon synapses in photoreceptor cells[31]. α2δ-3 mutation produces a deficiency in synaptic transmission and presynaptic membrane structure inDrosophilaneuromuscular junction[32-33]. These results indicate that α2δ family members are involved in synaptogenesis.
Most neuronal synapses have the α2δ TSP receptor, but this receptor is independent of the activity of voltage-gated calcium channels during synaptogenesis[1]. Then, how does α2δ participate in the regulation of synaptogenesis? Kurshanet al[33]proposed that α2δ may play a role through cytoskeletal rearrangements. However, the mechanism by which the α2δ ligand participates in synaptogenesis remains unclear. With a short intracellular region, δ is not essential for synaptogenesis, so there may be another transmembrane protein which interacts with α2δ and relays signals to cells. However, it is unknown if this proposed interaction mediates the effects observed in previous studies[1,33](Figure 3).
Xuet al[27]cultured hippocampal neurons, and found that TSP1 exhibited effects similar to neurexin in inducing synaptogenesis. Neurexin-induced synapses also lack the AMPA receptor, and the binding intensity with which TSP1 binds neuroligin1 is similar to that for TSP1 binding to the low density lipoprotein receptor[27]. This may imply that they share a common mechanism. In hippocampal neurons cultured with the neuroligin1 extracellular domain and in hippocampal neurons subjected to RNAi-mediated neuroligin1 knockout, the ability of TSP1 to promote synaptogenesis is inhibited[35-36], indicating that neuroligin1 mediates the effects of TSP1 on synaptogenesis. TSP is a polymeric protein; thus, its function in inducing synaptogenesis may involve neuroligin1 aggregation. However, further investigation is required to determine whether neuroligin1 also binds neurexins when binding TSP to induce presynaptic membrane differentiation.
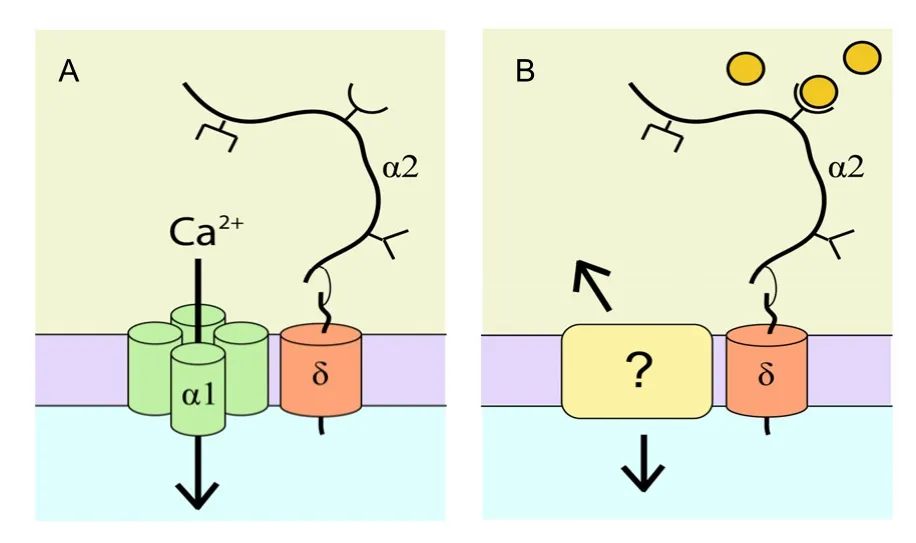
Figure 3 α2δ regulates synaptogenesis through a novel pathway[34].
Astrocytes can induce NPCs to differentiate into neurons by secreting soluble molecules[37-38]. Luet al[3]found that TSP1 deficiency greatly affects the self-renewal capacity of NPCsin vitroandin vitro. As a soluble factor secreted by astrocytes, TSP1 induces NPCs to differentiate into neurons and inhibits their differentiation into oligodendrocytes. TSP1 may influence cell fate through interactions with membrane proteins. Integrins can regulate NPC proliferation through Galectin-1/β1[39], while CD47 promotes nerve development through Cdc42 and Rac[40]. In addition, the Notch1 signaling pathway modulates the proliferation and differentiation of NPCs[41-42]. TSP1 binds Notch1 by complexing with Jagged1[43]and/or Contactin[44]to inhibit NPC differentiation into oligodendrocytes, thereby promoting their differentiation into nerve cells. TSP1 may directly bind EGF-like repeats and/or indirectly regulate nerve maturation through ApoER2 and the low density lipoprotein receptor. Collectively, these observations indicate that TSP1 promotes NPC proliferation and their differentiation into neurons, thereby increasing neuronal number, which benefits synaptogenesis. In addition, TSP can mediate de-adhesion between the cell and matrix in certain states[45], which may promote synaptogenesis. In summary, TSP promotes synaptogenesis by interacting with growth factors, extracellular matrix, adhesion molecules and receptors.
TSP lN CENTRAL NERVOUS SYSTEM DlSEASES
Synaptogenesis defects can result in various nervous system diseases and motor dysfunction. Astrocyte-secreted TSPs are important for nerve cell development, migration and synaptogenesisin vivoandin vitro. Are TSPs also involved in the pathogenesis of epilepsy, stroke or trauma-induced neurological dysfunction? After nervous system injury, TSP expression increases in response to purine signals and mechanical irritation[46]. For example, TSP1 and TSP2 levels are elevated after stroke[47], and α2δ-1 and TSP4 gene expression is upregulated after spinal cord injury[48-49].
Furthermore, increased α2δ-1 expression enhances excitatory synaptic transmission and elevates the neuropathic pain threshold[50-51]. Reduced TSP1 levels are associated with neuropathological manifestations in Down's syndrome patients[52]. These findings demonstrate that TSP1 is associated with the occurrence and progression of nervous system diseases.
Liauwet al[4]studied the role of TSP1/2 in synaptogenesis and synaptic functioning following stroke. They established a model of focal cerebral ischemia in 8 to 12-week-old wild-type and TSP1/2 knockout mice by unilateral occlusion of the middle cerebral and common carotid arteries. TSP1 and TSP2 mRNA and protein expression increased in wild-type mice, mainly in glial fibrillary acidic protein-positive astrocytes. In addition, a comparison of angiopoiesis, synaptic density, axon sprouting, infarction size and functional recovery showed that TSP1/2 knockout mice had impaired recovery compared with wild-type mice. Another study reported that TSP upregulation following stroke contributes to angiopoiesis after ischemia[47].
However, there was no difference in infarction size or blood vessel density between wild-type and TSP1/2-knockout mice, indicating that angiopoiesis is not influenced by TSPs, although synaptic density and axon sprouting are significantly reduced in TSP1/2-knockout mice[4]. In adult human brain, TSP1 and TSP2 levels are very low, but reactive astrocytes and microglia express these proteins[47,53]. Scars mainly comprised glial cells form in lesions after brain tissue injury, increasing TSP1 and TSP2 levels, which may induce epilepsy.
TSP PARTlClPATES lN THE ACTlON OF NERVOUS SYSTEM DRUGS
TSP is not only involved in the pathogenesis of nervous system diseases; it also holds promise for their treatment. Studies show that excitatory synapse formation underlies neuropathic pain and epileptic pathophysiology. Activated astrocytes play important roles in the treatment of epilepsy and spinal cord peripheral nerve injury- induced neuropathic pain[54]. Astrocyte-secreted TSPs may be involved in the repair of synapses after CNS injury. The TSP receptor α2δ-1 is a high-affinity receptor for two anti-epileptic drugs, gabapentin and pregabalin[55]. α2δ-1 gene knockout mice cannot bind gabapentin or pregabalin, indicating the treatment effects of these drugs are mediated through α2δ-1[56]. Gabapentin and pregabalin do not influence single signal dynamics of calcium channels and exhibit only minimal effects on synaptic transmission[57]. Then, what are the mechanisms of action of these drugs? Erogluet al[1]found that gabapentin binding to α2δ-1 restricts the conformation of the von willebrand factor-A domain in the receptor, thereby inhibiting the TSP-α2δ-1 interaction, and interfering with synaptogenesis. This may be the mechanism of action of gabapentin and pregabalin.
Opioids can modulate the functioning of spinal cord synapses and dendrites[58-59]. Ikedaet al[5]studied the influence of opioids on neurons and glial cellsin vivoandin vitro, and found that morphine reduced TSP1 levels in astrocytes, and suppressed synaptogenesis and axonal growth. Extracellular signal-regulated kinase activation can increase TSP1 levels in astrocytes[5]. Therefore, morphine likely reduces TSP1 expression by inhibiting the phosphorylation and activation of extracellular signal-regulated kinase[60]. In addition, morphine may influence transforming growth factor levelsviaextracellular signal-regulated kinase, and transforming growth factor can induce TSP1 expression in various types of cells, including glial cells[61-62]. TSP1 has EGF-like repeats, which can directly or indirectly inhibit negative feedback of the EGF receptor through a matrix metalloproteinase-dependent mechanism[63], reducing TSP1 expression in astrocytes. The EGF receptor is an important receptor for morphine. Long-term morphine treatment can inhibit synaptogenesis and axonal overgrowth, indicating that opioids may interfere with the ability of TSP1 to promote synaptogenesis and stability. TSPs are involved in the mechanism of action of nervous system drugs. This indicates that the mechanism of action of TSPs shares similarities with neuroregulatory mechanisms. They regulate the nervous system through a number of common pathways, possibly involving those affecting synaptic function. Studies of the underlying mechanisms may provide important insight into the functioning of neuroactive compounds.
DlSCUSSlON
Astrocyte-secreted TSPs can promote synaptogenesisin vivoandin vitro. An understanding of the underlying mechanisms may provide a basis for the modulation of synapse formation, structure and function, and advance the clinical treatment of nervous system diseases. There are some issues to be resolved. First, TSP1 and TSP2 levels change with time in the CNS; thus, it is critical to determine whether exogenous TSP1 or TSP2 can restore the synaptogenetic capacity of the mature nervous system or enhance synaptogenesis after nerve injury. In addition, methods for regulating TSP secretion by astrocytes are still lacking. Third, the signaling components downstream of TSPs, as well as related receptors and binding ligands, requires clarification. And it remains uncertain whether the effects of TSPs are only limited to glutamatergic synapses or can be extended to inhibitory GABAergic synapses.
Moreover, further studies are needed to determine how different TSPs regulate synaptogenesis (the effects of TSP subtypes are different because of their mechanisms of action or distribution) in specific brain regions. The intracellular molecular signals and mechanisms by which TSP-α2δ-1 regulates CNS synaptogenesis require further investigation. α2δ-1 is highly expressed in skeletal muscle, myocardium and bone, in addition to the CNS[64], so it is also necessary to explore whether TSP-α2δ-1 participates in the functioning of those tissues. Finally, studies should focus on the effects of TSP deficiency and overexpression on nervous system function, as related to disease and treatment. Future studies should focus on thein vivorole of TSPs to confirm their role in synaptogenesis, complex behaviors and neural circuitry for the treatment of neurological disease and injury.
Astrocyte-secreted TSPs are key regulators of synaptogenesis, acting through α2δ-1 and neuroligin 1, and promote the proliferation and differentiation of neural progenitor cells. TSPs also participate in synaptic remodeling following injury and in the action of some nervous system drugs.
Funding:This study was supported by the Natural Science Foundation of Guangdong Province, No. S2011010004096; the Medical Scientific Research Foundation of Guangdong Province, No. A2010431; A2009477.
Author contributions:Bin Wang conceived and designed the study, retrieved and sorted articles, and wrote the review. All authors screened the articles. Weitao Guo was in charge of funds, designed the study, and revised the manuscript.
Conflicts of interest:None declared.
[1] Eroglu C, Allen NJ, Susman MW, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139(2):380-392.
[2] Blake SM, Strasser V, Andrade N, et al. Thrombospondin-1 binds to ApoER2 and VLDL receptor and functions inpostnatal neuronal migration. EMBO J. 2008;27(22):3069-3080.
[3] Lu Z, Kipnis J. Thrombospondin 1-a key astrocyte-derived neurogenic factor. FASEB J. 2010;24(6):1925-1943.
[4] Liauw J, Hoang S, Choi M, et al. Thrombospondins 1 and 2 are necessary for synaptic plasticity and functional recovery after stroke. J Cereb Blood Flow Metab. 2008; 28(10):1722-1732.
[5] Ikeda H, Miyatake M, Koshikawa N, et al. Morphine modulation of thrombospondin levels in astrocytes and its implications for neurite outgrowth and synapse formation. J Biol Chem. 2010;285(49):38415-38427.
[6] Fox MA, Umemori H. Seeking long-term relationship:axon and target communicate to organize synaptic differentiation. J Neurochem. 2006;97(5):1215-1231.
[7] Ullian EM, Sapperstein SK, Christopherson KS, et al. Control of synapse number by glia. Science. 2001; 291(5504):657-661.
[8] Hughes EG, Elmariah SB, Balice-Gordon RJ. Astrocyte secreted proteins selectively increase hippocampal GABAergic axon length, branching, and synaptogenesis. Mol Cell Neurosci. 2010;43(1):136-145.
[9] Rousse I, St-Amour A, Darabid H, et al. Synapse-glia interactions are governed by synaptic and intrinsic glial properties. Neuroscience. 2010;167(3):621-632.
[10] Eulenburg V, Gomeza J. Neurotransmitter transporters expressed in glial cells as regulators of synapse function. Brain Res Rev. 2010;63(1-2):103-112.
[11] Jones EV, Bernardinelli Y, Tse YC, et al. Astrocytes control glutamate receptor levels at developing synapses through SPARC-beta-integrin interactions. J Neurosci. 2011;31(11):4154-4165.
[12] Suqiura Y, Lin W. Neuron-glia interactions: the roles of Schwann cells in neuromuscular synapse formation and function. Biosci Rep. 2011;31(5):295-302.
[13] Christopherson KS, Ullian EM, Stokes CC, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120(3): 421-433.
[14] Alford AI, Terkhorn SP, Reddy AB, et al. Thrombospondin-2 regulates matrix mineralization in MC3T3-E1 pre-osteoblasts. Bone. 2010;46(2):464-471.
[15] Bornstein P. Thrombospondins as matricellular modulators of cell function. J Clin Invest. 2001;107(8):929-934.
[16] Meng H, Zhang X, Lee SJ, et al. Low density lipoprotein receptor-related protein-1 (LRP1) regulates thrombospondin-2 (TSP2) enhancement of Notch3 signaling. J Biol Chem. 2010;285(30):23047-23055.
[17] Yan Q, Murphy-Ullrich JE, Song Y. Molecular and structural insight for the role of key residues of thrombospondin-1 and calreticulin in thrombospondin-1-calreticulin binding. Biochemistry. 2011;50(4):566-573.
[18] Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol. 2004;36(6):1115-1125.
[19] Giehl K, Graness A, Goppelt-Struebe M. The small GTPase Rac-1 is aregulator of mesangial cell morphology and thrombospondin-1 expression. Am J Physiol Renal Physiol. 2008;294(2):F407-413.
[20] Isenberg JS, Martin-Manso G, Maxhimer JB, et al. Regulation of nitric oxide signalling by thrombospondin 1:implications for anti-angiogenic therapies. Nat Rev Cancer. 2009;9(3):182-194.
[21] Arber S, Caroni P. Thrombospondin-4, an extracellular matrix protein expressed in the developing and adult nervous system promotes neurite outgrowth. J Cell Biol. 1995;131(4):1083-1094.
[22] Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2(11):791-805.
[23] Iruela-Arispe ML, Liska DJ, Sage EH, et al. Differential expression of thrombospondin 1, 2, and 3 during murine development. Dev Dyn. 1993;197(1):40-56.
[24] Yu K, Ge J, Summers JB, et al. TSP-1 secreted by bone marrow stromal cells contributes to retinal ganglion cell neurite outgrowth and survival. PLoS One. 2008;3(6):e2470.
[25] Cáceres M, Suwyn C, Maddox M, et al. Increased cortical expression of two synaptogenic thrombospondins in human brain evolution. Cereb Cortex. 2007;17(10):2312-2321.
[26] Hoffman JR, Dixit VM, O’Shea KS. Expression of thrombospondin in the adult nervous system. J Comp Neurol. 1994;340(1):126-139.
[27] Xu J, Xiao N, Xia J. Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nat Neurosci. 2010;13(1):22-24.
[28] Cahoy JD, Emery B, Kaushal A, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28(1):264-278.
[29] Hoffman JR, O’Shea KS. Thrombospondin expression in nerve regeneration I. Comparison of sciatic nerve crush, transection, and long-term denervation. Brain Res Bull. 1999;48(4):413-420.
[30] Isaac JT. Postsynaptic silent synapses: evidence and mechanisms. Neuropharmacology. 2003;45(4):450-460.
[31] Wycisk KA, Budde B, Feil S, et al. Structural and functional abnormalities of retinal ribbon synapses due to Cacna2d4 mutation. Invest Ophthalmol Vis Sci. 2006; 47(8):3523-3530.
[32] Dickman DK, Kurshan PT, Schwarz TL. Mutations in a Drosophila alpha2delta voltage-gated calcium channel subunit reveal a crucial synaptic function. J Neurosci. 2008;28(1):31-38.
[33] Kurshan PT, Oztan A, Schwarz TL. Presynaptic alpha2delta-3 is required for synaptic morphogenesis independent of its Ca2+-channel functions. Nat Neurosci. 2009;12(11):1415-1423.
[34] Procko C, Shaham S. Synaptogenesis: new roles for an old player. Curr Biol. 2009;19(24):1114-1115.
[35] Wittenmayer N, Körber C, Liu H, et al. Postsynaptic Neuroligin1 regulates presynaptic maturation. Proc Natl Acad Sci U S A. 2009;106(32):13564-13569.
[36] Comoletti D, Flynn R, Jennings LL, et al. Characterization of the interaction of a recombinant soluble neuroligin-1 with neurexin-1beta. J Biol Chem. 2003;278(50):50497-50505.
[37] Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417(6884):39-44.
[38] Oh J, Recknor JB, Recknor JC, et al. Soluble factors from neocortical astrocytes enhance neuronal differentiation of neural progenitor cells from adult rat hippocampus on micropatterned polymer substrates. J Biomed Mater Res A. 2009;91(2):575-585.
[39] Sakaguchi M, Imaizumi Y, Shingo T, et al. Regulation of adult neural progenitor cells by Galectin-1/beta1 Integrin interaction. J Neurochem. 2010;113(6):1516-1524.
[40] Murata T, Ohnishi H, Okazawa H, et al. CD47 promotes neuronal development through Src- and FRG/Vav2-mediated activation of Rac and Cdc42. J Neurosci. 2006;26(48):12397-12407.
[41] Breunig JJ, Silbereis J, Vaccarino FM, et al. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci U S A. 2007;104(51):20558-20563.
[42] Kaltezioti V, Kouroupi G, Oikonomaki M, et al. Prox1 regulates the notch1-mediated inhibition of neurogenesis. PLoS Biol. 2010;8(12):e1000565.
[43] Zhang Y, Argaw AT, Gurfein BT, et al. Notch1 signaling plays a role in regulating precursor differentiation during CNS remyelination. Proc Natl Acad Sci U S A. 2009; 106(45):19162-19167.
[44] Hu QD, Ang BT, Karsak M, et al. F3/contactin acts as a functional ligand for Notch during oligodendrocyte maturation. Cell. 2003;115(2):163-175.
[45] Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest. 2001;107(7):785-790.
[46] Tran MD, Neary JT. Purinergic signaling induces thrombospondin-1 expression in astrocytes. Proc Natl Acad Sci U S A. 2006;103(24):9321-9326.
[47] Lin TN, Kim GM, Chen JJ, et al. Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke. 2003;34(1): 177-186.
[48] Valder CR, Liu JJ, Song YH, et al. Coupling gene chip analyses and rat genetic variances in identifying potential target genes that may contribute to neuropathic allodynia development. J Neurochem. 2003;87(3):560-573.
[49] Wang H, Sun H, Della Penna, et al. Chronic neuropathic pain is accompanied by global changes in gene expression and shares pathobiology with neurodegenerative diseases. Neuroscience. 2002;114(3):529-546.
[50] Li CY, Song YH, Higuera ES, et al. Spinal dorsal horn calcium channel alpha2delta-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. J Neurosci. 2004;24(39):8494-8499.
[51] Li CY, Zhang XL, Matthews EA, et al. Calcium channel alpha2delta1 subunit mediates spinal hyperexcitability in pain modulation. Pain. 2006;125(1-2):20-34.
[52] Garcia O, Torres M, Helguera P, et al. A role for thrombospondin-1 deficits in astrocyte- mediated spine and synaptic pathology in Down's syndrome. PLoS One. 2010;5(12):e14200
[53] Möller JC, Klein MA, Haas S, et al. Regulation of thrombospondin in the regenerating mouse facial motor nucleus. Glia. 1996;17(2):121-132.
[54] Liu L, Rudin M, Kozlova EN. Glial cell proliferation in the spinal cord after dorsal rhizotomy or sciatic nerve transection in the adult rat. Exp Brain Res. 2000;131(1): 64-73.
[55] Gee NS, Brown JP, Dissanayake VU, et al. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271(10):5768-5776.
[56] Field MJ, Cox PJ, Stott E, et al. Identification of the alpha2-elta-1 subunit of voltage-dependent calcium channels as a molecular target or pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci U S A. 2006;103(46):17537-17542.
[57] Garcia K, Nabhani T, Garcia J. The calcium channel alpha2/delta1 subunit is involved in extracellular signaling. J Physiol. 2008;586(3):727-738.
[58] Tsai NP, Tsui YC, Pintar JE, et al. Kappa opioid receptor contributes to EGF-stimulated neurite extension in development. Proc Natl Acad Sci U S A. 2010;107(7):3216-3221.
[59] Liao D, Lin H, Law PY, et al. Mu-opioid receptors modulate the stability of dendritic spines. Proc Natl Acad Sci U S A. 2005;102(5):1725-1730.
[60] Joo CK, Kim HS, Park JY, et al. Ligand releaseindependent transactivation of epidermal growth factor receptor by transforming growth factor-beta involves multiple signaling pathways. Oncogene. 2008;27(5):614-628.
[61] Fitchev PP, Wcislak SM, Lee C, et al. Thrombospondin-1 regulates the normal prostate in vivo through angiogenesis and TGF-beta activation. Lab Invest. 2010;90(7):1078-1090.
[62] Nakao T, Kurita N, Komatsu M, et al. Expression of thrombospondin-1 and Ski are prognostic factors in advanced gastric cancer. Int J Clin Oncol. 2011;16(2):145-152.
[63] Liu A, Mosher DF, Murphy-Ullrich JE, et al. The counteradhesive proteins, thrombospondin 1 and SPARC/osteonectin, open the tyrosine phosphorylation-responsive paracellular pathway in pulmonary vascular endothelia. Microvasc Res. 2009; 77(1):13-20.
[64] Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13(3):298-307.
Bin Wang, Weitao Guo, Yun Huang
Department of Orthopedics, Affiliated Hospital of Guangdong Medical College, Zhanjiang 524001, Guangdong Province, China
Here, we review research on the mechanisms underlying the ability of thrombospondin to promote synaptogenesis and examine its role in central nervous system diseases and drug actions. Thrombospondin secreted by glial cells plays a critical role in synaptogenesis and maintains synapse stability. Thrombospondin regulates synaptogenesis through receptor α2δ-1 and neuroligin 1, and promotes the proliferation and differentiation of neural progenitor cells. It also participates in synaptic remodeling following injury and in the action of some nervous system drugs.
Weitao Guo, M.D., Chief physician, Department of Orthopedics, Affiliated Hospital of Guangdong Medical College, Zhanjiang 524001, Guangdong Province, China guoweitao2000@sina.com
2012-02-01
2012-05-03
We thank Guangdong Medical College and Affiliated Hospital of Guangdong Medical College for technical support; and the staff of the Library of Guangdong Medical College for their help.
Cite this article as:Neural Regen Res. 2012;7(22):1737-1743.
Bin Wang★, Studying for master’s degree, Attending physician, Department of Orthopedics, Affiliated Hospital of Guangdong Medical College, Zhanjiang 524001, Guangdong
Province, China
(N20111126006/WLM)
Wang B, Guo WT, Huang Y. Thrombospondins and synaptogenesis. Neural Regen Res. 2012;7(22): 1737-1743.
www.crter.cn
www.nrronline.org
10.3969/j.issn.1673-5374. 2012.22.009
(Edited by Guo GQ, Li RX/Su LL/Song LP)
杂志排行
中国神经再生研究(英文版)的其它文章
- Early hyperbaric oxygen therapy inhibits aquaporin 4 and adrenocorticotropic hormone expression in the pituitary gland of rabbits with blast-induced craniocerebral injury**★
- Transgene expression and differentiation of baculovirus-transduced adipose-derived stem cells from dystrophin-utrophin double knock-out mouse********☆
- Stem cell transplantation for treating Duchenne muscular dystrophy A Web of Science-based literature analysis*
- Changes in expression and secretion patterns of fibroblast growth factor 8 and Sonic Hedgehog signaling pathway molecules during murine neural stem/progenitor cell differentiation in vitro***☆
- Precision radiotherapy for brain tumors A 10-year bibliometric analysis☆
- ldentification of α7 nicotinic acetylcholine receptor on hippocampal astrocytes cultured in vitro and its role on inflammatory mediator secretion***★
