Early hyperbaric oxygen therapy inhibits aquaporin 4 and adrenocorticotropic hormone expression in the pituitary gland of rabbits with blast-induced craniocerebral injury**★
2012-01-04JianHuoJiachuanLiuJinbiaoWangYongmingZhangChunlinWangYanyanYangWenjiangSunShaonianXu
Jian Huo, Jiachuan Liu, Jinbiao Wang, Yongming Zhang, Chunlin Wang, Yanyan Yang, Wenjiang Sun, Shaonian Xu
Department of Neurosurgery, the 105 Hospital of Chinese PLA, Hefei 230032, Anhui Province, China
Early hyperbaric oxygen therapy inhibits aquaporin 4 and adrenocorticotropic hormone expression in the pituitary gland of rabbits with blast-induced craniocerebral injury**★
Jian Huo, Jiachuan Liu, Jinbiao Wang, Yongming Zhang, Chunlin Wang, Yanyan Yang, Wenjiang Sun, Shaonian Xu
Department of Neurosurgery, the 105 Hospital of Chinese PLA, Hefei 230032, Anhui Province, China
In the present study, rabbits were treated with hyperbaric oxygen for 1 hour after detonator-blastinduced craniocerebral injury. Immunohistochemistry showed significantly reduced aquaporin 4 expression and adrenocorticotropic hormone expression in the pituitary gland of rabbits with craniocerebral injury. Aquaporin 4 expression was positively correlated with adrenocorticotropic hormone expression. These findings indicate that early hyperbaric oxygen therapy may suppress adrenocorticotropic hormone secretion by inhibiting aquaporin 4 expression.
hyperbaric oxygen; blast-induced injury; craniocerebral injury; aquaporin 4; pituitary gland; adrenocorticotropic hormone
Research Highlights
Early hyperbaric oxygen suppresses adrenocorticotropic hormone secretion in the pituitary gland by inhibiting aquaporin 4 expression
lNTRODUCTlON
Stress reactions occurring after craniocerebral injury can cause neuroendocrine disorders, destabilization of the internal environment, and even death[1]. Stress reactions occurring after injury are characterized by hypothalamic-pituitaryadrenal axis activation, as well as changes in related hormones, neurotransmitters and cytokines[2]. Damage to the hypothalamicpituitary-adrenal axis occurs within several hours after craniocerebral injury, function, resulting in excess adrenocorticotropic hormone secretion in the acute stage of injury[3]. In patients with mild or moderate craniocerebral injury, adrenocorticotropic hormone levels were associated with trauma severity and prognosis, while no correlation was found in patients with severe injury[4].
Another study revealed that aquaporin 4 in pituitary gland cells may be involved in hormone secretion following cerebral edema[5], but evidence to support this hypothesis is currently lacking. Blast-induced traumatic brain injury can influence neuroendocrine function[6-7]. In the present study, we, for the first time, used a rabbit model of blast-induced craniocerebral injury to detect aquaporin and adrenocorticotropic hormone expression in the pituitary gland following 1 hour of hyperbaric oxygen treatment. Our objective was to investigate the correlations between these factors and the curative effects of early hyperbaric oxygen on hypothalamicpituitary-adrenal axis dysfunction caused byblast injury.
RESULTS
Quantitative analysis of experimental animals
A total of 150 New Zealand rabbits were randomly assigned to the hyperbaric oxygen (n= 70), untreated blast injury (n= 70) and untreated control (n= 10) groups. Blast-induced craniocerebral injury was induced in the hyperbaric oxygen and untreated blast injury groups. The hyperbaric oxygen group received hyperbaric oxygen for 1 hour after blast injury, while the other groups received air at normal air pressure. Rabbits with blast-induced apnea received respiratory tract nursing. Their breath became deep, slow, and gradually accelerated. They regained consciousness 3-5 hours later, but they were depressed with a poor appetite. Nine rabbits developed epilepsy and three suffered limb palsy (not excluded from the study); five of these rabbits died because of starvation at 3 days after blast injury. These rabbits were replaced. The rabbits with blast injury survived for more than 7 days, and 150 were included in the final analysis. Ten rabbits from each the hyperbaric oxygen and untreated blast injury groups were assessed at 1, 6, 12, 24 and 72 hours, and at 7 and 14 days after blast injury.
Early hyperbaric oxygen inhibited aquaporin expression in the pituitary gland after blast-induced craniocerebral injury
Immunohistochemistry was used to determine aquaporin expression in the adenohypophysis, intermediate lobe and neurohypophyseal cells. Aquaporin was highly expressed in the region surrounding the sinusoidal capillary. Aquaporin expression in the pituitary gland increased over time after blast injury compared with that in the control group, reaching a peak level at 72 hours after injury (P< 0.05). By contrast, aquaporin expression in the pituitary gland decreased after early hyperbaric oxygen treatment, with statistically significant differences from 6 hours after injury compared with the control group (P< 0.05), but then gradually increased to the normal level by 14 days (P> 0.05; Figure 1, Table 1, supplementary Figure 1 online).
Early hyperbaric oxygen inhibited adrenocorticotropic hormone expression in the pituitary gland after blast-induced craniocerebral injury
Immunohistochemistry revealed that adrenocorticotropic hormone was mainly expressed in the cytoplasm, and some positive particles were found in the cytoplasm around the nuclei. Adrenocorticotropic hormone expression in the pituitary gland increased with time after injury compared with the control group, reaching a peak at 72 hours after injury (P< 0.05), while hyperbaric oxygen treatment reduced adrenocorticotropic hormone expression in the pituitary gland (P< 0.05; Figure 2, Table 2, supplementary Figure 2 online). Pearson correlation analysis showed that aquaporin expression was positively correlated with adrenocorticotropic hormone expression (r= 0.959,P< 0.001; Figure 3).
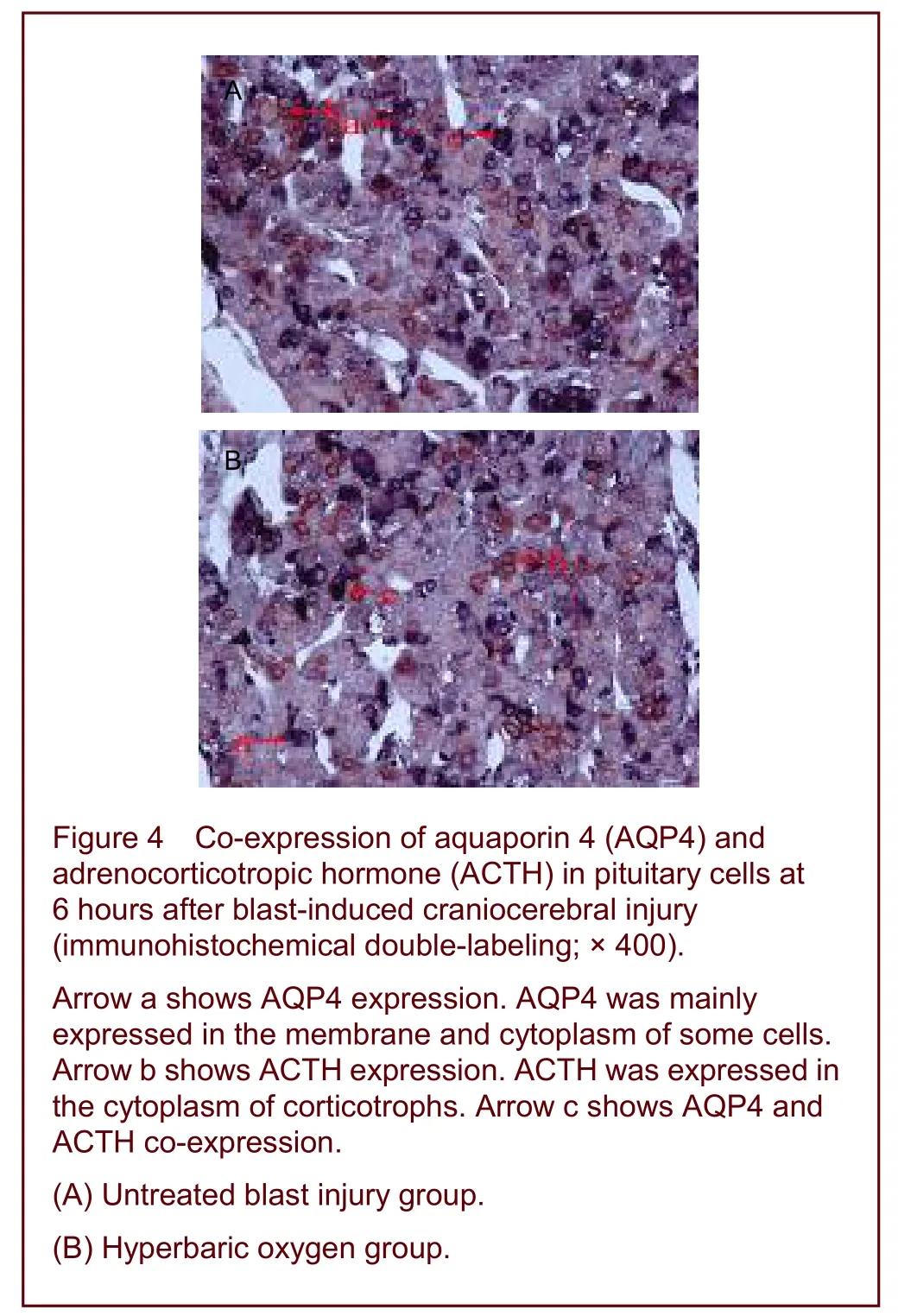
Aquaporin and adrenocorticotropic hormone were co-expressed in pituitary cells after early hyperbaric oxygen therapy
Immunohistochemical double-labeling studies showed that aquaporin was expressed in the membrane and cytoplasm of acidophilic, basophilic, chromophobe and follicular cells in the adenohypophysis, while adrenocorticotropic hormone was expressed in the cytoplasm of basophilic cells.


Table 1 Relative aquaporin 4 expression in the pituitary gland after blast-induced craniocerebral injury (mean absorbance; immunohistochemistry)
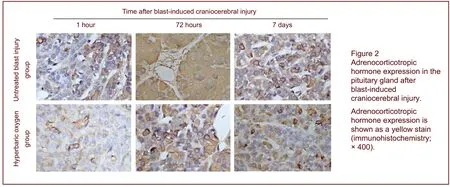

Table 2 Relative adrenocorticotropic hormone expression in the pituitary gland after blast-induced craniocerebral injury (mean absorbance value; immunohistochemistry)
A number of cells co-expressing aquaporin and adrenocorticotropic hormone were observed in the adenohypophysis of the untreated blast injury group, mixed with aquaporin-positive cells and adrenocorticotropic hormone-positive cells. The number of double-labeled cells decreased significantly after hyperbaric oxygen therapy compared with that in the untreated blast injury group at 72 hours after injury (Figure 4).
DlSCUSSlON
Aquaporins are cell membrane transport proteins responsible for water transport. Aquaporin is extensively expressed in the brain, where it plays an important role in water transport and balance[8]. In the present study, aquaporin was expressed on the membrane of adrenal pituicytes, consistent with previous results[5]. The pituitary gland lacks a blood-brain barrier, and its endothelial cells on the venous side of the capillaries are window-shaped, with abundant perivascular space around the vessels[9]. In the present study, aquaporin was extensively expressed in the region surrounding the sinusoidal capillary, indicating that aquaporin may play a physiologically important metabolic role within the pituitary gland and other brain tissues. Aquaporin protein expression changed dynamically in the pituitary gland of rabbits after blast injury; its expression gradually increased over time, reaching a peak at 72 hours after injury, consistent with previous results[10].

Aquaporin expression is highly correlated with the structure and function of the blood-brain barrier[11].
Studies have shown that aquaporin expression is positively correlated with the severity of cerebral edema in mice with water intoxication[12-15].
Manleyet al[16]proposed that aquaporin acts as an osmoreceptor or receptor to generate rapid and sensitive increases in cell capacity by enhancing osmotic pressure. Therefore, there is a dynamic interaction between blood-brain barrier injury-induced osmotic pressure changes, aquaporin and cerebral edema[17-20]. Nielsenet al[21-22]reported that aquaporin participates in pituitary vasopressin secretion and regulation. Studies have also shown that aquaporin in endocrine cells may be involved in the synthesis,transport and regulation of hormones[5,23]. In the present study, the changes in adrenocorticotropic hormone expression were similar to those of aquaporin in the untreated blast injury group, and correlation analysis showed a positive correlation between adrenocorticotropic hormone and aquaporin. These results indicate that aquaporin participates in adrenocorticotropic hormone secretion and regulation. Blast-induced craniocerebral injury in rabbits affects blood-brain barrier permeability, which enhances aquaporin expression in the pituitary gland, resulting in regional osmotic pressure changes and adrenocorticotropic hormone release. However, the precise molecular mechanism requires investigation. Hyperbaric oxygen can balance cerebral blood flow, improve injury-induced brain tissue hypoxia, restore cell membrane permeability, accelerate clearance of accumulated toxic substance and facilitate the repair of injured tissue[24-26]. Results of the present study showed that hyperbaric oxygen therapy did not significantly alter aquaporin expression in the pituitary gland of rabbits at 1 hour after injury, but did reduce adrenocorticotropic hormone expression. It is likely that hyperbaric oxygen therapy suppresses adrenocorticotropic hormone release induced by pituitary gland ischemia/hypoxia. At 72 hours after blast-induced craniocerebral injury, the co-expression of aquaporin and adrenocorticotropic hormone was significantly reduced in the hyperbaric oxygen-treated group compared with the untreated blast injury group. We believe that hyperbaric oxygen improved the partial pressure of oxygen within the pituitary gland, relieved tissue hypoxia and the abnormal distribution of intracellular and extracellular ions, inhibited aquaporin expression, and maintained regional osmotic pressure surrounding adrenocorticotropic hormone cells, thereby reducing adrenocorticotropic hormone secretion.
In summary, early hyperbaric oxygen therapy can inhibit aquaporin expression in anterior pituitary cells, thereby reducing adrenocorticotropic hormone secretion.
MATERlALS AND METHODS
Design
A randomized, controlled animal study.
Time and setting
The animal experiments were performed at the Animal Experimental Center, Daping Hospital of Third Military Medical University, China between February and October 2009. Immunohistochemical studies were performed in the Laboratory of Immunohistochemistry, Anhui Medical University, China, between February and July 2010.
Materials
A total of 150 healthy, adult New Zealand rabbits, males and females, weighing 2.0-2.5 kg, were provided by the Animal Experimental Center, Daping Hospital (license no. SYXK (army) 2007-017). Rabbits were housed at 26 ± 1.5°C with a humidity of 60%. All animal procedures were performed in accordance with theGuidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[27].
Methods
Establishment of blast-induced craniocerebral injury
Rabbits were anesthetized by an intravenous injection of 3% pentobarbital sodium (1 mg/kg)viathe ear, and placed under a self-made explosion frame. A thick cotton blanket was prepared and used to protect the neck and chest against blast or burn injury. A paper detonator (Chongqing 845 Factory, Chongqing, China), equivalent to 600 mg trinitrotoluene, was fixed 6.5 cm above the rabbit’s brain, 1.5 cm to the median, 2 cm anterior to the line of both ears, to induce an explosion. The scalp was sutured after the blast. The patency of the respiratory tract of animals was maintained and cardiopulmonary resuscitation was used if necessary[28-29](supplementary Figure 3 online).
Early hyperbaric oxygen therapy
A transparent oxygen animal experimental chamber was purchased from Shanghai Decompressor Factory (Shanghai, China). Rabbits received hyperbaric oxygen therapy for 1 hour after blast injury, and were exposed to pure oxygen at 2 atmospheres (equivalent to 2.03 MPa). The rabbits received pure oxygen for 5 minutes, followed by a period of increasing pressure for 30 minutes, stable pressure for 1 hour and a period of reducing pressure for 30 minutes. This treatment was performed twice daily, once in the morning and once in the afternoon, with an interval of 8 hours between treatments. Rabbits in the other groups were exposed to air at normal pressure.
Preparation of pituitary gland paraffin sections
Ten rabbits from each group were selected at 1, 6, 12, and 24 hours, and at 7 and 14 days after injury. Rabbits were anesthetized by an intravenous injection of 3% pentobarbital sodium (1 mg/kg). The heart was perfused with 4% paraformaldehyde, and the rabbits were sacrificed. Pituitary gland tissues were harvested (supplementary Figure 4 online)[30], fixed in 4% paraformaldehyde, dehydrated, immersed in paraffin, embedded, and sectioned into three serial sections (5 μm thick). Hyperbaric oxygen therapy was performed 1 hour after inducing blast injury, and sections were prepared immediately after completing hyperbaric oxygen therapy.
Immunohistochemistry for aquaporin and adrenocorticotropic hormone expression in the pituitary gland
The sections were dewaxed, hydrated, washed with PBS three times for 3 minutes each, immersed in 3% H2O2and left at room temperature for 10 minutes. The sections were then washed three times with PBS for 3 minutes each, followed by antigen retrieval in a microwave. The sections were washed with PBS, mixed with goat serum blocking solution at room temperature for 30 minutes, and incubated with 100 μL of rabbit anti-aquaporin or rabbit anti-adrenocorticotropic hormone polyclonal antibodies (1:200; Boster, Wuhan, China) overnight at 4°C. The sections were warmed to room temperature for 10 minutes, washed with PBS three times for 5 minutes each, and then incubated with 100 μL of goat anti-rabbit IgG (1:100; Boster) per section at 37°C for 30 minutes. After washing the sections with PBS three times for 5 minutes each, they were treated with 100 μL of streptavidin-biotin complex solution (Boster) per section, washed with PBS three times for 5 minutes each, and colorized with diaminobenzidine for 5-10 minutes. Sections were then washed with tap water, counterstained with hematoxylin for 1 minute, differentiated with hydrochloric acid and ethanol for about 10 seconds, washed with tap water for 10-15 minutes, dehydrated, cleared, mounted, dried, and observed under a light microscope (Nikon80i; Nikon, Tokyo, Japan). The mean absorbance value in randomly selected fields of view (magnification, × 400) was calculated using the JEDA 801D morphologic image analysis system (Molecular Devices, Sunnyvale, CA, USA) and MetaMorph software (Molecular Devices)[31].
Immunohistochemical double-labeling of aquaporin and adrenocorticotropic hormone
The sections were dewaxed, hydrated, mixed with 3% H2O2and left at room temperature for 10 minutes, followed by antigen retrieval under a microwave (twice at high temperature for 5 minutes, with 5 minutes between each heating). The sections were blocked with goat serum at room temperature for 30 minutes and incubated with 100 μL of rabbit anti-aquaporin polyclonal antibody (1:50) overnight at 4°C. The sections were warmed to room temperature for 10 minutes and incubated with 100 μL of peroxidase-labeled goat anti-rabbit IgG (1:100; Boster) per section at 37°C for 30 minutes. The sections were then washed with PBS three times for 5 minutes each, treated with 100 μL of streptavidin-biotin complex solution (Boster) per section, colorized with blue diaminobenzidine for 10 minutes, washed with distilled water, blocked with goat serum for 30 minutes, and treated with 100 μL of rabbit anti-adrenocorticotropic hormone polyclonal antibody (1:50) overnight at 4°C. The sections were warmed to room temperature for 10 minutes and incubated with 100 μL of peroxidase- labeled goat anti-rabbit IgG (1:100; Boster) per section at 37°C for 30 minutes. The sections were then washed with PBS three times for 5 minutes each, treated with 100 μL of streptavidin-biotin complex solution (Boster) per section, colorized with red diaminobenzidine for 10 minutes, washed with distilled water three times for 5 minutes each, dehydrated, cleared, glycerol-mounted, and observed under a light microscope (magnification, × 400).
Statistical analysis
Data were analyzed using SPSS software version 17.0 (SPSS, Chicago, IL, USA) and are expressed as mean ± SD. Differences between each group at different times were compared using one-way analysis of variance, and paired comparisons were performed using the Student-Newman-Keuls method. Values ofP< 0.05 were considered statistically significant.
Funding:This study was supported by the Eleventh-Five Major Subjects of Nanjing Military Area Command, No. 06Z19; and the Military Medical Science and Technology Innovation Foundation in 2009, No. 09Z009.
Author contributions:Jian Huo, Yanyan Yang, Shaonian Xu and Wenjiang Sun collected and interpreted the experimental data. Jian Huo, Jiachuan Liu, Chunlin Wang, Yongming Zhang and Jinbiao Wang conceived and designed the study. Jian Huo conducted data analysis, wrote the manuscript, and provided technical and statistical support. Jiachuan Liu was in charge of the funds, revised the manuscript and guided the study.
Conflicts of interest:None declared.
Ethical approval: This study was approved by the Animal Ethics Committee of the Third Military Medical University, China.
Supplementary information:Supplementary data associated with this article can be found in the online version, by visiting www.nrronline.org.
[1] Baker AJ, Park E, Hare GM, et al. Effects of resuscitation fluid on neurologic physiology after cerebral trauma and hemorrhage. J Trauma. 2008;64(2):348-357.
[2] Schneider HJ, Stalla GK, Buchfelder M. Expert meeting:hypopituitarism after traumatic brain injury and subarachnoid haemorrhage. Acta Neurochir (Wien). 2006; 148(4):449-456.
[3] Gong DS, Kong YL, Shao NY, et al. Changes of blood anterior pituitary hormone and thyroxine after craniocerebral trauma in patients. Zhonghua Chuangshang Zazhi. 2000;16(1):32-34.
[4] Tanriverdi F, Senyurek H, Unluhizarci K, et al. High risk of hypopituitarism after traumatic brain injury: a prospective investigation of anterior pituitary function in the acute phase and 12 months after trauma. J Clin Endocrinol Metab. 2006;91(6):2105-2111.
[5] Peng XH, Sun SQ. Localization of AQP4 in rat brain. Jiepou Xuebao. 2004;35(2):132-136.
[6] Svetlov SI, Prima V, Kirk DR, et al. Morphologic and biochemical characterization of brain injury in a model of controlled blast overpressure exposure. J Trauma. 2010; 69(4):795-804.
[7] Cheng J, Gu J, Ma Y, et al. Development of a rat model for studying blast-induced traumatic brain injury. J Neurol Sci. 2010;294(1-2):23-28.
[8] Tani K, Mitsuma T, Hiroaki Y, et al. Mechanism of aquaporin-4’s fast and highly selective water conduction and proton exclusion. J Mol Biol. 2009;389(4):694-706.
[9] Sun SQ, Hashimoto PH. Venous microvasculature of the pineal body and choroid plexus in the rat. J Electron Microsc (Tokyo). 1991;40(1):29-33.
[10] Sun WJ, Liu JC, Zhang YM, et al. Expression of AQP-4 and efficacy of hyperbaric oxygen therapy after blast-related brain injury in rabbits. Zhongguo Weiqinxi Shenjing Waike Zazhi. 2011;16(3):136-138.
[11] Papadopoulos MC, Saadoun S, Binder DK, et al. Molecular mechanisms of brain tumor edema. Neuroscience. 2004;129(4):1011-1020.
[12] Euser AG, Bullinger L, Cipolla MJ. Magnesium sulphate treatment decreases blood-brain barrier permeability during acute hypertension in pregnant rats. Exp Physiol. 2008;93(2):254-261.
[13] Yang B, Zador Z, Verkman AS. Glial cell aquaporin-4 overexpression in transgenic mice accelerates cytotoxic brain swelling. J Biol Chem. 2008;283(22):15280-15286.
[14] Vajda Z, Pedersen M, Füchtbauer EM, et al. Delayed onset of brain edema and mislocalization of aquaporin-4 in dystrophin-null transgenic mice. Proc Natl Acad Sci U S A. 2002;99(20):13131-13136.
[15] Tait MJ, Saadoun S, Bell BA, et al. Increased brain edema in aqp4-null mice in an experimental model of subarachnoid hemorrhage. Neuroscience. 2010;167(1):60-67.
[16] Manley GT, Fujimura M, Ma T, et al. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6(2):159-163.
[17] Tomassoni D, Bramanti V, Amenta F. Expression of aquaporins 1 and 4 in the brain of spontaneously hypertensive rats. Brain Res. 2010;1325:155-163.
[18] Sun MC, Honey CR, Berk C, et al. Regulation of aquaporin-4 in a traumatic brain injury model in rats. J Neurosurg. 2003;98(3):565-569.
[19] Kleindienst A, Fazzina G, Amorini AM, et al. Modulation of AQP4 expression by the protein kinase C activator, phorbol myristate acetate, decreases ischemia-induced brain edema. Acta Neurochir Suppl. 2006;96:393-397.
[20] Qing WG, Dong YQ, Ping TQ, et al. Brain edema after intracerebral hemorrhage in rats: the role of iron overload and aquaporin 4. J Neurosurg. 2009;110(3):462-468.
[21] Nielsen S, Nagelhus EA, Amiry-Moghaddam M, et al. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17(1):171-180.
[22] Venero JL, Vizuete ML, Ilundáin AA, et al. Detailed localization of aquaporin-4 messenger RNA in the CNS:preferential expression in periventricular organs. Neuroscience. 1999;94(1):239-250.
[23] Yan JH, Sun SQ. Expression of aquaporin 4 in adult rat pituitary gland. Jiepou Xue Zazhi. 2005;28(3):264-266.
[24] Palzur E, Zaaroor M, Vlodavsky E, et al. Neuroprotective effect of hyperbaric oxygen therapy in brain injury is mediated by preservation of mitochondrial membrane properties. Brain Res. 2008;1221:126-133.
[25] Zhu ZA, Chen X, Zhang H, et al. Effect of hyperbaric oxygenation therapy on apoptosis after cerebral contusion in rats. Zhongguo Linchuang Shenjing Waike Zazhi. 2004; 9(5):367-370.
[26] Buras JA, Holt D, Orlow D, et al. Hyperbaric oxygen protects from sepsis mortality via an interleukin-10-dependent mechanism. Crit Care Med. 2006;34(10):2624-2629.
[27] The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
[28] Zhang YM, Liu JC, Yang YY, et al. Establishment of blast-related brain injury model in rabbits. Zhongguo Weiqinxi Shenjing Waike Zazhi. 2010;15(2):74-76.
[29] Wightman JM, Gladish SL. Explosions and blast injuries. Ann Emerg Med. 2001;37(6):664-678.
[30] Wei SC, Gong ZD. Experimental research on efficacies of GnRH-A immunocastration in rabbits. Xumu Shouyi Xuebao. 2009;40(2):191-196.
[31] Tong H, Chen GH, Liu RY, et al. Age-related learning and memory impairments in adult-onset hypothyroidism in Kunming mice. Physiol Behav. 2007;91(2-3):290-298.
Cite this article as:Neural Regen Res. 2012;7(22):1729-1735.
Jian Huo★, Master, Department of Neurosurgery, the 105 Hospital of Chinese PLA, Hefei 230032, Anhui Province, China
Jiachuan Liu, Master, Chief physician, Master’s supervisor, Department of Neurosurgery, the 105 Hospital of Chinese PLA, Hefei 230032, Anhui Province, China ljc571017@sina.com
2012-01-06
2012-05-26
(N20111027002/YJ)
Huo J, Liu JC, Wang JB, Zhang YM, Wang CL, Yang YY, Sun WJ, Xu SN. Early hyperbaric oxygen therapy inhibits aquaporin 4 and adrenocorticotropic hormone expression in the pituitary gland of rabbits with blast-induced craniocerebral injury. Neural Regen Res. 2012;7(22):1729-1735.
www.crter.cn
www.nrronline.org
10.3969/j.issn.1673-5374. 2012.22.008
We wish to thank the Experiment Center of the Third Military Medical University of Chinese PLA for establishing the animal model, and the Basic Teaching and Research Division, Anhui Medical University in China for technical support with sample detection. We also thank the staff at the Department of Neurosurgery, the 105 Hospital of Chinese PLA for their help with designing the study and writing the manuscript.
(Edited by Ding XH, Yang XF/Su LL/Song LP)
杂志排行
中国神经再生研究(英文版)的其它文章
- Transgene expression and differentiation of baculovirus-transduced adipose-derived stem cells from dystrophin-utrophin double knock-out mouse********☆
- Thrombospondins and synaptogenesis***★
- Stem cell transplantation for treating Duchenne muscular dystrophy A Web of Science-based literature analysis*
- Changes in expression and secretion patterns of fibroblast growth factor 8 and Sonic Hedgehog signaling pathway molecules during murine neural stem/progenitor cell differentiation in vitro***☆
- Precision radiotherapy for brain tumors A 10-year bibliometric analysis☆
- ldentification of α7 nicotinic acetylcholine receptor on hippocampal astrocytes cultured in vitro and its role on inflammatory mediator secretion***★
