薄壁中孔碳材料的精细控制合成
2011-12-12朱月香谢有畅
王 羽 隗 罡 蔡 斌 朱月香 谢有畅
(北京大学化学与分子工程学院,分子动态与稳态结构国家重点实验室,北京分子科学国家实验室,北京100871)
1 Introduction
Mesoporous carbon materials have important applications in adsorption of large molecules,1-3as catalyst supports in fuel cells,4-6and as electrode materials in electric double-layer capacitors,7-10due to their large surface area,pore volume,and chemical and mechanical stability.This has motivated many studies on controllable synthesis of such materials to meet the requirements of specific applications.Recently,the template method has attracted much attention and has been proved to be effective,because the porous structure of the carbon replica largely corresponds to that of the template.11-13Thus carbon materials with different texture can be obtained by choosing appropriate templates.
Ordered mesoporous silicas(OMSs),such as MCM-48 and SBA-15,are among the most studied hard templates because of their ordered pore structure,and many well-defined carbon replicas have been obtained from those templates.4,14-18Anodic aluminum oxide(AAO)19-21has also been utilized for preparing tubular mesoporous carbon materials due to its well-aligned pore channels.An important drawback of the OMSs and AAO templates is that their synthesis is time-consuming and requires a special technique which is not easily scaled up for industrial application.
To find a more cost-efficient method,a wide diversity of disordered templates have been used to prepare mesoporous carbon,such as alumina,22silica nanoparticles,1MgO,23Ni(OH)2/ NiO,8CaCO3,24,25TiO2,26and natural clay minerals.27Those works focused on low-cost and commercial available templates,convenient synthesis procedure,or easy removal of the templates.Nevertheless,some carbon materials had very broad pore size distribution(PSD),1and some had quite a few micropores.8,24-26In addition,the relationship between the pore structures of templates and the corresponding carbon materials was not clear.
In order to enhance the controllability during the synthesis of mesoporous carbons which are able to meet the various demands in different application areas,key factors influencing the texture of the carbon materials need to be found out.Recently,a review summarized the control of pore structure in MgO-templated nanoporous carbons.28Its two main conclusions,size of mesopores in the resultant carbons was tunable by selecting MgO precursor and relative volume between mesopores and micropores was controlled by carbon precursor, could be universal in other disordered template systems and may enlighten the controllable synthesis of mesoporous carbons.However,in this review,there was no detailed discussion about the thickness of pore walls and it seemed that in this carbon coating method mesopores in the resulting carbons mainly generated by removing nanoparticles of the templates.
We herein proposed another possible synthesis mechanism, demonstrating that mesopores of the carbons had two sources, one from the removal of the template particles and the other from the original pores of the template.In order to get sufficient evidence to the mechanism,we chose three types of γ-alumina as templates and investigated the pore structures of the carbon materials obtained.Pore volume of the carbon materials calculated based on the mechanism showed good agreements with the experimental data.This mechanism,for the first time, pointed out the importance of template pores in a disorderedtemplate system and the correlation between the texture of the templates and that of the corresponding carbon materials was clearly elucidated.In the meanwhile,a series of carbon materials with narrow pore size distribution(PSD)in small mesopore range(5-15 nm),high mesopore ratio,large surface area,and thin pore walls were obtained,which have competitive mesoporous properties among the reported results.
2 Experimental
2.1 Source of alumina templates
The three types of alumina used are denoted Al2O3-I (SBA150,Engelhard Corp.,America,chemically pure),Al2O3-II(prepared by calcination of pseudoboehmite from Shandong Aluminum Corp.,chemically pure),and Al2O3-III(Shanxi Aluminum Plant,chemically pure).All of the templates were calcined at 800°C for 4 h before use.
2.2 Synthesis of carbon-covered alumina(CCA)
CCA samples were prepared with sucrose(analytical reagent)as the carbon source,using the previously reported procedure.22The alumina templates were impregnated with aqueous solutions of sucrose.In a typical procedure,Route A,the sucrose:alumina mass ratio was kept below the monolayer dispersion threshold value,29which is 0.27:1 for the alumina template with surface area of 100 m2·g-1.Hence the sucrose:alumina mass ratios in alumina systems I,II,and III were 0.30:1, 0.40:1,and 0.40:1,respectively.After drying at 90°C,the precursors were calcined at 800°C under N2purge gas(flow rate of 50 mL·min-1)for 1 h.The as-prepared black powder was denoted as CCA-x-1,where x represents the type(I,II,or III)of alumina.
The impregnation and carbonization steps were repeated one to three times,to obtain CCA-x-2,CCA-x-3,and CCA-x-4 with higher carbon content,where the numbers(2-4)indicate the times of impregnation/carbonization steps.For example, sample CCA-I-4 means the CCA using Al2O3-I as template, with four impregnation/carbonization steps.
To test the effect of the sucrose dosage,a special CCA control sample following Route B,CCA-I-1-Q,was prepared using a sucrose:alumina mass ratio of 1.2:1(Q stands for quadruple of sucrose mass ratio in this sample,compared with that in sample CCA-I-1),which greatly exceeded the monolayer dispersion threshold of sucrose.
2.3 Synthesis of mesoporous carbon materials
The CCA samples were immersed overnight in 24%hydrofluoric acid(HF,guaranteed reagent)solution at room temperature to dissolve the alumina templates.The carbon samples,obtained as insoluble fractions,were washed several times with distilled water and absolute alcohol,then dried in air at 110°C. The resulting carbons were denoted as C-CCA.
2.4 Characterization
Nitrogen sorption isotherms were obtained with a Micromeritics ASAP 2010 volumetric adsorption system(America)at liquid-nitrogen temperature(77 K).All samples were degassed at 200°C prior to the measurements.Specific surface area was determined using the Brunauer-Emmett-Teller(BET)method based on adsorption data in the relative pressure(p/p0)range of 0.05 to 0.2.PSD was evaluated by the Barrett-Joyner-Halenda (BJH)method from the desorption branch of the isotherm,except when the most probable pore size was around 4 nm,in which case the PSD curve was calculated from the adsorption branch,to avoid the tension strength effect(TSE).30The total pore volume(Vtotal)was estimated from the single point adsorption at p/p0of 0.995.The micro pore volume(Vmicro)was obtained from the t-plot method.
The carbon contents of the CCA precursors were measured using a thermogravimetric analyzer(SDT Q600,TA Instruments,America)from room temperature to 800°C at a heating rate of 10°C·min-1and an air flow rate of 100 mL·min-1; α-Al2O3was used as the reference.
Transmission electron microscopy(TEM)images were recorded on a Hitachi H-9000NAR high-resolution microscope (Japan)at an acceleration voltage of 100 kV.
X-ray diffraction(XRD)was performed with a Rigaku D/ MAX-200 powder diffractometer(Japan)using Ni-filtered Cu Kαradiation at 40 kV and 100 mA.
Raman spectroscopy was carried out with a confocal microprobe Raman system(Horiba Jobin Yvon LabRAM HR800, France)with excitation wavelength 632.8 nm from an internal He-Ne laser.
3 Results and discussion
3.1 Texture of alumina templates
After calcination,all three types of alumina used in the preparation of the carbon materials were still γ-alumina,according to the XRD results(Figures not shown),with the BET surface areas of 127,176,and 167 m2·g-1and total pore volumes of 0.49,0.40,and 0.38 cm3·g-1,respectively.Fig.1 shows the nitrogen sorption isotherms and PSD of the alumina templates. Although the pores in these alumina templates were disordered,all of the aluminas had relatively narrow PSD in the mesopore range.The most probable pore sizes of Al2O3-II and Al2O3-III were very similar at about 6-7 nm,while that of Al2O3-I was about 12 nm,with little overlap of its PSD with the PSDs of the other two types of alumina.Thus texture correlation of the as-prepared carbon samples and their templates could be obtained by comparing the results for the alumina templates with different PSDs.
3.2 Effect of synthesis parameters on carbon texture
3.2.1 Effect of the completeness and firmness of the carbon framework in the precursor CCA on resultant carbon texture
Fig.2 shows nitrogen sorption isotherms and PSDs of carbon samples prepared using the different aluminas as templates. For all three alumina systems,carbon samples C-CCA-x-3 and C-CCA-x-4 showed relatively narrow PSD curves,whose shape and position were similar to those of the alumina templates,except for 0.3-3.0 nm broadening or a few nm shift of the most probable pore size.Thus these carbon materials duplicated most of the texture of the corresponding alumina templates after the alumina was removed.As shown in Table 1,all of the C-CCA-x-3 and C-CCA-x-4 samples had BET surface areas larger than 1000 m2·g-1and the micropore ratio in those carbon samples was≤1%.Table 2 lists some mesoporous carbons prepared with other disordered templates reported in literature.Apparently,carbons in this work(C-CCA-I-4 and CCCA-II-3)not only had few micro pores,but also presented the largest pore volume among the carbons with similar pore size.
However,the PSDs of samples C-CCA-I-1 and C-CCA-I-2 derived from the desorption branch were artificially narrow,unlike the PSD derived from the adsorption branch,most probably due to the TSE.30To obtain more realistic texture information,the PSD curves derived from the adsorption branch were adopted for C-CCA-I-1 and C-CCA-I-2.The PSD curve of CCCA-I-2 was fairly broad and centered at about 6 nm,while the pore size of C-CCA-I-1 was even smaller.Similar results were also obtained for the Al2O3-II and Al2O3-III systems.This should be attributed to the lower carbon contents of their precursor CCAs,which directly affects the toughness of the carbon layer.

Fig.1 Nitrogen sorption isotherms(A)and pore size distributions(PSDs)(B)of alumina templates derived by the BJH method from the desorption branches of the isotherms

Fig.2 Nitrogen sorption isotherms(I-IIIA)and PSDs(I-III B)of C-CCAsamples prepared usingAl2O3I-III as templatesThe PSD curves were derived by the BJH method from the desorption branches of the isotherms except for C-CCA-I-1(a)and C-CCA-I-2(a),which were derived from the adsorption branches to avoid the tension strength effect(TSE).30The vertical dotted lines in Figs.I-III B denote the most probable pore sizes of the alumina templates.
As reported previously,22,31carbon in CCA is uniformly distributed on an alumina surface as graphene flakes.The number of graphene layers in the CCAsamples listed in Table 1 was deduced from the carbon content(wC)measured by thermogravimetric analysis and the estimated carbon content(wg,g·g-1)of CCA with one intact graphene layer,which was calculated according to formula(1).

The data in Table 1 indicate that the carbon in CCA-I-1 is too low to form an intact graphene layer and the nominal graphene layer in CCA-I-2 is only 1.1.Even if the graphene layer is highly graphitized,the disordered porous structure of alumina makes it difficult to form strong junctions between every two graphene flakes.Thus,the carbon framework in CCA-I-1 and CCA-I-2 tended to collapse without the support of the alumina template.
3.2.2 Effect of sucrose impregnation-carbonization patterns on resultant carbon texture
Increasing the sucrose dosage in a single-pass impregnation-carbonization procedure could possibly give precursor CCAs with higher carbon content than those attainable with the optimized multipass pattern.However,our experimental results show that neglecting the monolayer-dispersion threshold of sucrose,carbon materials prepared with the simplified procedure had inferior mesoporous properties to those of the carbon materials prepared by the optimized multipass procedure(Table 1 and Fig.3).For the sample C-CCA-I-1-Q prepared by impregnation with sucrose:alumina mass ratio of 1.2:1,the shape of the PSD curve was deformed and the peak shifted to much lower pore size,compared with that of C-CCA-I-4.The pore volume of C-CCA-I-1-Q was only about 75%of that of CCCA-I-4,while its micropore ratio was four times as that of CCCA-I-4.

Table 1 Texture properties of resultant carbons and carbon contents(wC)of the corresponding CCAs

Table 2 Texture of mesoporous carbons prepared by different disordered templates

Fig.3 Nitrogen sorption isotherms(A)and PSDs(B)of C-CCA-I samples prepared by different impregnation-carbonization patterns

Fig.4 TEM images of carbon samples(A)C-CCA-I-4,(B)C-CCA-II-4,(C)C-CCA-III-4
3.3 TEM,XRD and Raman characterization of typical carbon samples
The TEM images shown in Fig.4 illustrate the nanostructures of the resultant carbon materials.The materials from all three alumina templates have disordered porous structure produced by stacking of small carbon flakes.The pores in C-CCA-I-4 are larger than those in the other two carbon samples,in accordance with the difference in template pore size. The thin pore walls can be seen at the edges of the carbon materials.The XRD patterns in Fig.5 show two broad peaks with 2θ values about 24.0°and 43.7°,which are indexed to the (002)and(100)/(101)reflections,respectively,of graphite.The weak diffraction intensity indicates low crystallinity of the samples,and the broader d002(2θ value lower than 26.6°)indicates disordered stacking of the graphene flakes,in accordance with the TEM results.The ID/IGvalue from Raman spectra is also used for characterizing the ordering of the carbon structure. The broad and intense D peak of the carbon samples in Fig.6 provides further confirmation of the disordered carbon structure.
3.4 Mechanism and verification calculation
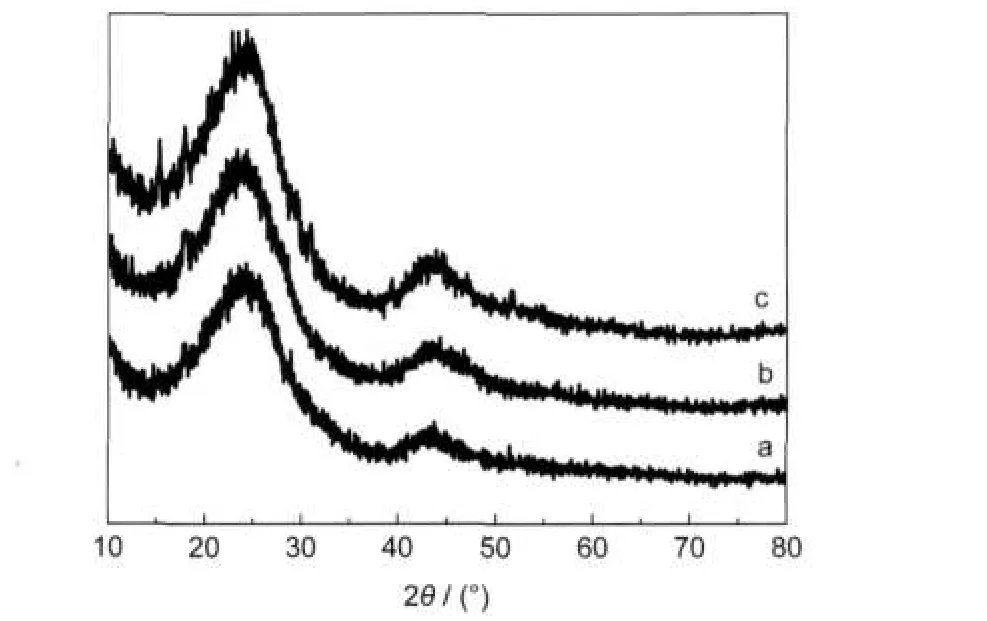
Fig.5 XRD patterns of(a)C-CCA-I-4,(b)C-CCA-II-4,and (c)C-CCA-III-4
From these results,a typical mechanism of this synthesis can be proposed as follows with Al2O3-I system for example (Fig.7).In each impregnation step,when the amount of sucrose is below the monolayer-dispersion threshold sucrose spontaneously disperses on the alumina surface as a monolayer.During in situ pyrolysis,due to the interaction between the sucrose molecules and the alumina surface,sucrose is converted to graphene flakes that uniformly cover the alumina surface without aggregating.After repeating the impregnation and carbonization procedure,a complete carbon framework with 1-2 graphene layers thick forms.Finally,the optimized carbon material is obtained by removing the alumina template.

Fig.6 Raman spectra of(a)C-CCA-I-4,(b)C-CCA-II-4,and (c)C-CCA-III-4
The completeness and firmness of the carbon framework is a key factor that affects the mesoporous properties of the carbon materials thus obtained.When the carbon framework is complete and sufficiently strong,it almost duplicates the pore structure of the alumina template and has good mesoporous properties,after removal of alumina.When the carbon framework is far from complete(e.g.,C-CCA-I-1 in Section 3.2.1)or not sufficiently strong(e.g.,C-CCA-I-2),it collapses without the support of alumina,and the resulting carbon material does not copy the texture of the template.
The reason for the increased proportion of micropores in sample C-CCA-I-1-Q in Section 3.2.2 can also be understood from Fig.7.When the amount of sucrose exceeds the monolayerdispersion threshold in a single impregnation step,crystalline sucrose forms and leads to the formation of not only a thin carbon layer but also microporous particulate nano-carbon,after pyrolysis.Those crumbs of carbon block some of the alumina pores and cause the shift of the PSD curve as well as the decrease in the total pore volume of the final carbon materials.
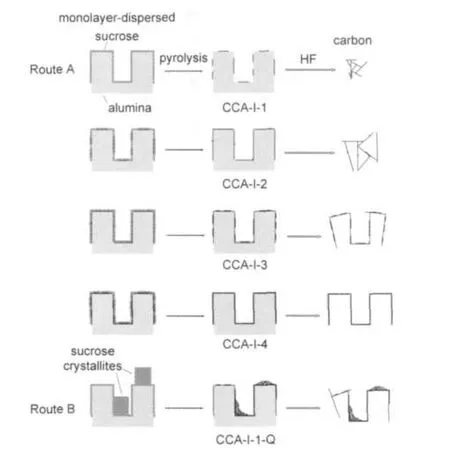
Fig.7 Schematic representation of the synthesis mechanism with Al2O3-I system for instance
To test the proposed mechanism,the predicted pore volume after removal of the alumina template was calculated as follows.Assuming that the carbon framework with a thickness of 1-2 graphene layers does not change its texture after removing the alumina template,as shown in Fig.7,the pore volume in the final carbon material is composed of two parts:the original pores in the precursor CCA(VCCA)and new pores generated by removing alumina(VAl2O3).Thus,the predicted pore volume of the carbon material,Vcalc,can be calculated by formula(2),in which wCis the carbon content in CCA andργ-Al2O3is the density of γ-Al2O3(3.67 g·cm-3).

The calculated results are listed in Table 3.For most of the carbon samples prepared under optimized synthesis conditions (C-CCA-I-4,C-CCA-II-3,C-CCA-II-4,C-CCA-III-3,and CCCA-III-4),the difference between the calculated and measured pore volume was less than 7%.The good match of Vcalcand Vmeasin these samples establishes the feasibility of the proposed synthesis mechanism,and also implies that the carbon materials obtained have very thin walls with thickness of 1-2 graphene layers.

Table 3 Comparison of calculated and measured pore volumes of carbon samples
In the case of C-CCA-I-3 and C-CCA-II-3,for which the calculated number of graphene layers is 1.4,the difference between Vmeasand Vcalcfor C-CCA-I-3(-25%)is much larger than for C-CCA-II-3(-4%).This indicates that at similar carbon content and average thickness of carbon layer,the framework of carbon samples prepared by alumina with larger pores can more easily shrink than in the case of the framework from alumina with smaller pores.Nevertheless,sample C-CCA-I-4 with higher carbon content and thicker graphene layer(1.7 layers)duplicated the alumina pore structure quite well.The difference between the calculated and measured pore volumes was only 3%in that case.
The difference for C-CCA-I-1-Q was only 4%,indicating that the carbon framework of this sample did not collapse much after removing the alumina template,probably because of its high carbon content,even though the particulate nanocarbon generated from crystalline sucrose blocked some of the pores and caused a shift of the PSD curve.The generation of new pores after removing alumina also leads to the broadening and slight shift of the peaks in the PSD curves.
Comparing the VCCAwithVAl2O3data of the same sample in Table 3,it is worth noting that even covered with a thin layer of carbon,VCCAwas still comparable or larger thanVAl2O3.This result revealed the importance of VCCA,which should not be neglected during the synthesis procedure of mesoporous carbon with large mesopore volume.
4 Conclusions
Mesoporous carbon materials with narrow PSD,large surface area,large pore volume,and high mesopore ratio were synthesized from carbon-covered alumina.A mechanism of this synthesis was proposed by comparing the results of three alumina systems,to elucidate the correlation between the textures of the alumina templates and the resultant carbon materials.Forming a complete and robust carbon layer in the precursor CCA is a crucial step for controlled production of carbon materials with excellent mesoporous properties,and controlling the sucrose:alumina mass ratio in each impregnation step helped to form a uniform carbon layer and minimize the generation of micropores.It is worthy of noting that the resultant mesoporous carbon materials had very thin pore walls with a thickness of only 1-2 graphene layers.The synthesis method presented herein exploits low-cost raw materials and has great potential for scale-up in practical applications.
(1) Han,S.J.;Sohn,K.;Hyeon,T.Chem.Mater.2000,12,3337.
(2)Hartmann,M.;Vinu,A.;Chandrasekar,G.Chem.Mater.2005, 17,829.
(3)Zhuang,X.;Wan,Y.;Feng,C.M.;Shen,Y.;Zhao,D.Y.Chem. Mater.2009,21,706.
(4) Joo,S.H.;Choi,S.J.;Oh,I.;Kwak,J.;Liu,Z.;Terasaki,O.; Ryoo,R.Nature 2001,412,169.
(5) Nam,J.H.;Jang,Y.Y.;Kwon,Y.U.;Nam,J.D.Electrochem. Commun.2004,6,737.
(6) Cui,X.Z.;Shi,J.L.;Zhang,L.X.;Ruan,M.L.;Gao,J.H. Carbon 2009,47,186.
(7) Li,L.X.;Song,H.H.;Chen,X.H.Electrochim.Acta 2006,51, 5715.
(8)Wang,D.W.;Li,F.;Liu,M.;Lu,G.Q.;Cheng,H.M.Angew. Chem.Int.Edit.2008,47,373.
(9) Xia,K.S.;Gao,Q.M.;Jiang,J.H.;Hu,J.Carbon 2008,46, 1718.
(10) Numaoa,S.;Judaia,K.;Nishijoa,J.;Mizuuchib,K.;Nishia,N. Carbon 2009,47,306.
(11) Lu,A.H.;Schüth,F.Adv.Mater.2006,18,1793.
(12) Lee,J.;Kim,J.;Hyeon,T.Adv.Mater.2006,18,2073.
(13) Liang,C.D.;Li,Z.J.;Dai,S.Angew.Chem.Int.Edit.2008,47, 3696.
(14) Ryoo,R.;Joo,S.H.;Jun,S.J.Phys.Chem.B 1999,103,7743.
(15) Lee,J.;Yoon,S.;Oh,S.M.;Shin,C.H.;Hyeon,T.Adv.Mater. 2000,12,359.
(16) Jun,S.;Joo,S.H.;Ryoo,R.;Kruk,M.;Jaroniec,M.;Liu,Z.; Ohsuna,T.;Terasaki,O.J.Am.Chem.Soc.2000,122,10712.
(17) Lu,A.H.;Schmidt,W.;Spliethoff,B.;Schüth,F.Adv.Mater. 2003,15,1602.
(18) Gierszal,K.P.;Jaroniec,M.;Liang,C.D.;Dai,S.Carbon 2007, 45,2171.
(19) Kyotani,T.;Tsai,L.;Tomita,A.Chem.Mater.1995,7,1427.
(20) Parthasarathy,R.V.;Phani,K.L.N.;Martin,C.R.Adv.Mater. 1995,7,896.
(21) Cott,D.J.;Petkov,N.;Morris,M.A.;Platschek,B.;Bein,T.; Holmes,J.D.J.Am.Chem.Soc.2006,128,3920.
(22)Lin,L.;Wang,P.;Wang,S.R.;Zhu,Y.X.;Zhao,B.Y.;Xie,Y. C.Carbon 2006,44,3120.
(23) Inagaki,M.;Kato,M.;Morishita,T.;Morita,K.;Mizuuchi,K. Carbon 2007,45,1121.
(24)Zhao,C.R.;Wang,W.K.;Yu,Z.B.;Zhang,H.;Wang,A.B.; Yang,Y.S.J.Mater.Chem.2010,20,976,
(25)Xu,B.;Peng,L.;Wang,G.Q.;Cao,G.P.;Wu,F.Carbon 2010, 48,2377.
(26) Ng,Y.H.;Ikeda,S.;Harada,T.;Park,S.;Sakata,T.;Mori,H.; Matsumura,M.Chem.Mater.2008,20,1154.
(27) Shi,L.M.;Yao,J.F.;Jiang,J.L.;Zhang,L.X.;Xu,N.P. Microporous Mesoporous Mat.2009,122,294.
(28) Morishita,T.;Tsumura,T.;Toyoda,M.;Przepiórski,J.; Morawski,A.W.;Konno,H.;Inagaki,M.Carbon 2010,48, 2690.
(29)Wang,Y.;Lin,L.;Zhu,B.S.;Zhu,Y.X.;Xie,Y.C.Appl.Surf. Sci.2008,254,6560.
(30) Groen,J.C.;Peffer,L.A.A.;Pérez-Ramírez,J.Microporous Mesoporous Mat.2003,60,1.
(31) Lin,L.;Lin,W.;Zhu,Y.X.;Zhao,B.Y.;Xie,Y.C.;Jia,G.Q.; Li,C.Langmuir 2005,21,5040.
