Relationship between blood levels and clinical efficacy of two different formulations of venlafaxine in female patients with depression
2011-12-11BaohuaSONGZhenguoFANWeiminSHENMincaiQIANHaizhiCHENHongDAIShikaiWANG
Baohua SONG, Zhenguo FAN, Weimin SHEN, Mincai QIAN, Haizhi CHEN, Hong DAI, Shikai WANG*
· Research Article ·
Relationship between blood levels and clinical efficacy of two different formulations of venlafaxine in female patients with depression
Baohua SONG, Zhenguo FAN, Weimin SHEN, Mincai QIAN, Haizhi CHEN, Hong DAI, Shikai WANG*
Background:Different formulations of the same medication can have distinct dissociation profiles and release rates resulting in different onset times and durations of action; these differences may in fluence the clinical efficacy and adverse reactions of the medication.Objective:Assess the relationship between the plasma concentrations and the clinical efficacy and adverse reaction pro file of two formulations of venlafaxine (VL) in female patients with major depression.Methods:Sixty female patients hospitalized for depression were randomly assigned to use EFFEXOR XR®, the extendedrelease formulation of venlafaxine (hereafter, the XR group), or Bolexin®, the immediate-release formulation of venlafaxine(hereafter, the IR Group), for six weeks. The mean (SD) total daily dose in the XR group was 168 (32) mg/d administered in one oral dose in the morning and that in the IR group was 160 (67) mg/day administered in three oral doses over the day. At the end of the first, second, fourth and sixth week of treatment the plasma concentrations of VL and its active metabolite, O-desmethylvenlafaxine (ODV), were determined using high-performance liquid chromatography and the clinical efficacy and adverse reactions were accessed using the Hamilton Depression Rating Scale (HAMD) and the Treatment Emergent Symptoms Scale (TESS). A reduction in the baseline HAMD score of 50% or greater was deemed remission.Results:No serious adverse reactions were observed. There were no significant differences in the mean HAMD scores between the two groups at any of the time points evaluated. Mean HAMD scores after six weeks of treatment were significantly lower in both treatment groups; the overall remission rate was 65.6% in the XR group and 69.2% in the IR group (χ2=0.77,p=0.380). Changes in the HAMD scores from baseline were only weakly correlated with changes in the plasma concentrations of ODV (rs=-0.10,p=0.470) and VL (rs=-0.11,p=0.403).Conclusion:Both the extended-release and immediate-release formulations of VL were efficacious and well tolerated in female patients with depression. Plasma concentrations of VL and ODV are not closely related to clinical efficacy so they are not suitable as markers for the clinical effectiveness of treatment with venlafaxine.
Depression; Venlafaxine; O-desmethylvenlafaxine; High-performance liquid chromatography
1 Introduction
Studies in China and elsewhere[1-5]have demonstrated the effectiveness of venlafaxine (VL) —the first approved serotonin-norepinephrine reuptake inhibitor—in the treatment of depression. In clinical practice VL is now widely used as a first-line antidepressant agent. However, only a few studies have assessed the relationship of the plasma concentration of VL to its clinical efficacy[6-8], and those studies have had mixed results. Further work in this area of therapeutic drug monitoring is needed to achieve the goal of individualized drug regimens that maximize efficacy and minimize adverse reactions.
The main metabolite of VL in its two currently available formulations (extended-release and immediate-release) is O-desmethylvenlafaxine (ODV),which has a pharmacological activity of 0.20-3.33 times that of its parent drug[9]. Currently the most widely used method of determining plasma concentrations of VL is high-performance liquid chromatography (HPLC),but few studies have simultaneously assessed plasma levels of VL and ODV[10,11]. The pharmacokinetic activity of VL appears to be similar in men and women[12],but hormonal fluctuation in females could potentiallyinfluence the relationship of clinical symptoms and plasma levels of the medication. To help clarify these issues the current study compares the clinical efficacy and side effect pro file of the two formulations of VL in hospitalized females with depression and determines the correlation between clinical effectiveness and the plasma concentrations of VL and ODV.
2 Subjects and Methods
2. 1 Subjects
As shown in Figure 1, potential subjects in the study were females who met diagnostic criteria for a depressive episode (as listed in the third edition of the Chinese Classification of Mental Disorders and the 10th edition of the International Classification of Diseases), were hospitalized on the psychiatric ward of our hospital from September 2006 to May 2008,and who had a score of 18 or higher on the 17-item Hamilton Depression Rating Scale (HAMD) at the time of admission. Potential subjects were excluded if they were not willing to participate; if the depressive symptoms were secondary to using a psychoactive substance; if there was another co-morbid mental disorder or substance abuse; if the individual was currently taking antidepressant medication; if the individual was suicidal, pregnant or lactating; or if there was a serious physical or neurological illness or significant abnormalities on physical exam, routine blood tests, electrocardiogram, or liver and renal function tests.
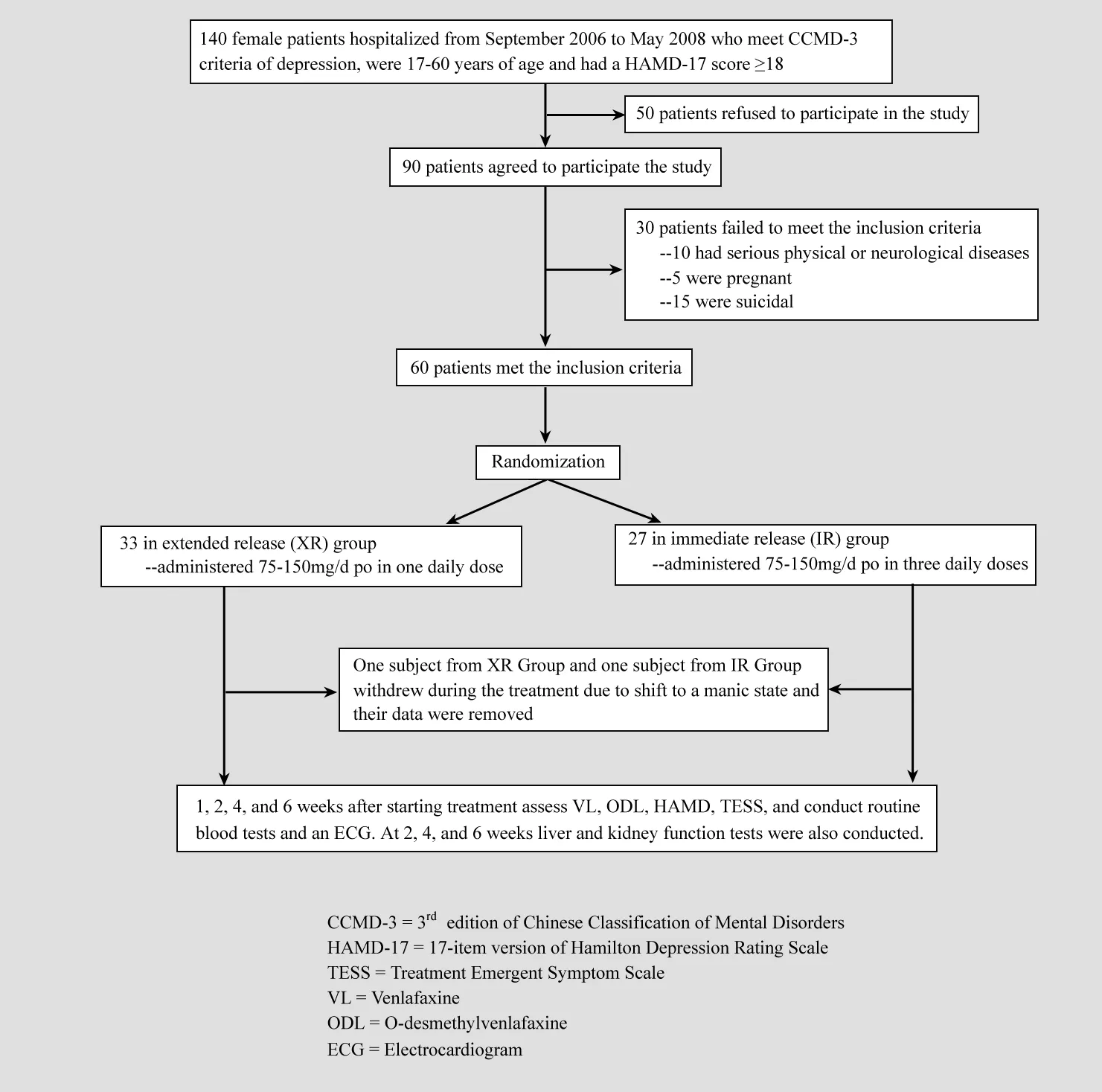
Figure 1. Flowchart for the study
Sixty enrolled subjects were randomly assigned to treatment with extended-release venlafaxine (XR group,n=33) or immediate-release venlafaxine (IR group,n=27). One subject from the XR Group and one from the IR Group withdrew from the study because they converted into a manic episode during the treatment.The data for these two subjects were deemed invalid and not included in the statistical analysis of the results.The mean (SD) and range of the age and duration of illness for the 32 subjects in the XR group were 37.4(11.4) years (18-60 years) and 6.4 (9.6) years (17 days-28 years), respectively; the corresponding values in the 26 patients in the IR groups were 35.1 (13.1) years(17-60 years) and 5.9 (10.2) years (15 days-34 years),respectively. The differences in age and duration of illness between the groups were not statistically significant.
This study was approved by the ethics committee of the Third People’s Hospital of Huzhou. Written informed consent was obtained from each included subject or her guardian.
2. 2 Methods
2. 2. 1 Dosing regimen
Subjects in the XR Group were administrated one daily oral dose of extended-release venlafaxine capsules (EFEXOR XR® manufactured by Wyeth-Ayerst International Inc, Drug Import Registration Certificate No. X20000237 for 75mg capsules and X20000256 for 150mg capsules). Subjects in IR Group were administrated three daily oral doses of immediaterelease VL capsules (Bolexin® manufactured by Chengdu Great Southwest Pharmaceutical Inc., Lot No. 060905).All patients were started at 75mg/d and this dosage was increased to 150 mg/d after 3-5 days based on the response of the individual patient. This dosage was then maintained for six weeks. No concomitant antidepressant agent or antipsychotic medication was used but low-dose benzodiazepines could be administered if the subject had severe insomnia.
2. 2. 2 Assessment of efficacy and adverse reactions
The HAMD was assessed at baseline and at the end of the first, second, fourth and sixth weeks of treatment by the ten treating psychiatrists participating in the study. These psychiatrists had good inter-rater reliability:interclass correlation coefficient for total HAMD score=0.90. Remission of symptoms was de fined as a reduction in the baseline HAMD score of 50% or greater. Adverse reactions were evaluated at the end of the first, second, fourth and sixth weeks of treatment using the Treatment Emergent Symptom Scale (TESS).Routine blood tests were conducted weekly and blood tests of liver and kidney functioning were conducted biweekly over the six weeks of treatment.
2. 2. 3 Determination of plasma levels of VL and ODV
At 6 AM in the morning (at least 12 hours after the last dose) on the last day of the first, second, fourth and sixth weeks of treatment five ml of cubital venous blood was collected. The blood was centrifuged and the serum was stored at -20℃. Serum concentrations of VL and ODV were determined with the Agilent 1100 HPLC system which includes the HPLC, in-line degasser, autosampler, column oven and a UV detector. This HPLC method has a high sensitivity for VL (with a quantitative limit of detection of 6.0 μg·L-1and a limit of detection of 2.4 ng) and has been shown to be quite stable[13]. Data acquisition and processing were conducted using the Agilent Chem Station[14].
2. 3 Statistical methods
The SPSS Version 11.5 software package was used to analyze the data. The HAMD scores and VL and ODL concentrations at the different time-points were compared between the two groups with repeated measures analysis of variance. Serum values of VL and ODL are not normally distributed so the analysis of variance used a normal transformation of the ranks of the original values[15]. The relationship of VL and ODV plasma concentrations to clinical efficacy was evaluated by correlating the mean efficacy in weeks 1, 2, 4 and 6(the mean % change in the HAMD score from baseline)with the mean plasma concentration at those four time points using spearman ranked correlation coefficients.
3 Results
With the exception of the two subjects who dropped out due to conversion to mania, all other subjects completed the full six weeks of treatment.
3. 1 Efficacy comparison
As shown in Table 1 after six weeks of treatment the mean HAMD total scores were significantly lower in both treatment groups. Table 2 shows that the efficacy(% change in HAMD score from baseline) for both groups was good. At the end of the six weeks 65.6% of patients in the XR group had met criteria for remission(>50% drop in baseline HAMD score) and 69.2% in the IR group met criteria for remission (χ2=0.77, p=0.380).However, the repeated measures ANOVA identified no differences between the groups in either absolute HAMD scores or in the efficacy measures.
3. 2 Blood concentration of VL and ODV
The median (range) serum levels of VL and ODV over the six weeks of the study in the XR group were 95(14-624) μg·L-1and 172 (34-336) μg·L-1, respectively; the corresponding values in the IR group were 75 (15-498)μg·L-1and 166 (23-385) μg·L-1, respectively. As shown in Table 2 the plasma levels of both VL and ODV in the two groups varied significantly over time, but there was no significant difference between the two groups at any of the time periods.
3. 3 Correlation between drug concentration and clinical efficacy
The correlation of change in HAMD scores with the plasma levels of VL (combining week 1 to week 6 values for both groups) was weak and non-significant (rs=0. 11,p=0.403), and the correlation of the change in HAMD scores with plasma levels of OVD was also weak and non-significant (rs=-0. 10, p=0.470). This correlation was also weak and non-significant for the two formulations of venlafaxine: in the XR group the correlation of clinical efficacy with VL plasma levels was rs=-0.02 (p=0.933)and with OVD plasma levels was rs=-0.11, p=0.550;the corresponding values in the IR group were rs=-0.22(p=0.273) and rs=-0. 08 (p=0.706), respectively.
3. 4 Comparison of adverse reactions
As stated above, two patients (one in each group)converted to mania during the treatment period and were, thus, removed from the study. None of the remaining patients experienced severe adversereactions that required removal from the study. The most common adverse reactions in both groups were nausea and anorexia (8 cases in the XR group and 6 in the IR group), which primarily occurred in the early stages of the treatment. Less common adverse effects were dizziness (4 cases in the XR group and 3 cases in the IR group), headache (3 cases in the XR group and 2 cases in the IR group), and insomnia (2 cases in the XR group and 2 case in the IR group). These side effects were mild and well tolerated. One subject in the IR group had abnormal liver function tests that resolved with symptomatic treatment. No other abnormalities were identified in the routine blood tests or in the electrocardiographic examinations. The number and severity of adverse effects was not significantly different between the two groups.
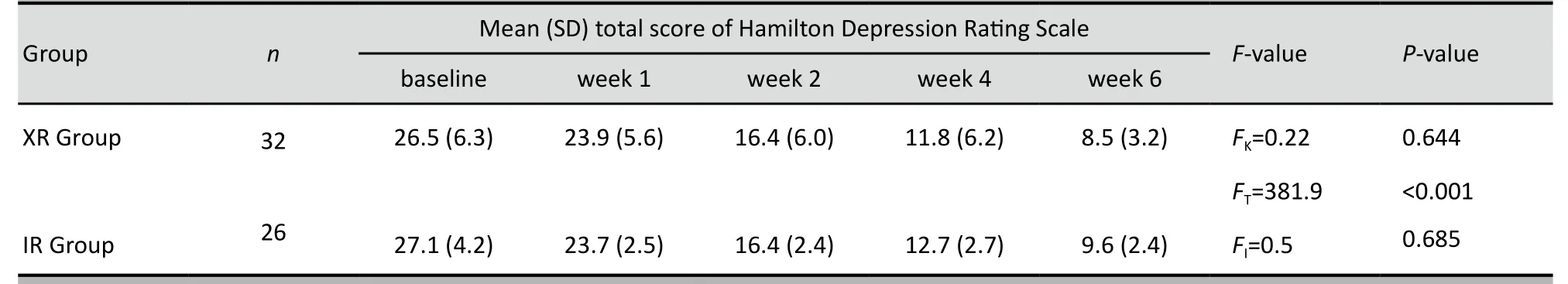
Table 1. Mean (SD) total HAMD score at different time points in depressed women treated with extended-release (XR)and immediate-release (IR) formulations of venlafaxine

Table 2. Mean (SD) percent reduction in baseline HAMD score at different time points in depressed womentreated with extended-release (XR) and immediate-release (IR) formulations of venlafaxine
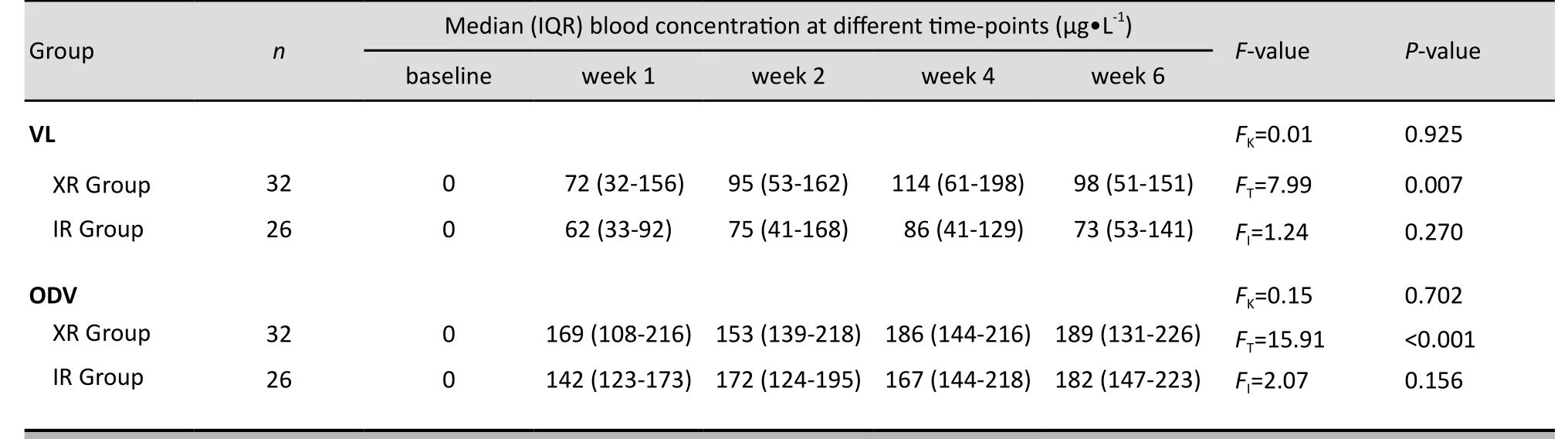
Table 3. Comparison of blood concentrations of venlafaxine (VL) and O-desmethylvenlafxine (ODV) in female depressed patients taking extended-release (XR) and immediate-release (IR) formulations of venlafaxine
Eight subjects from the XR group received adjunctive treatment for insomnia ( five with alprazolam and three with nitrazepam) and eight subjects from the IR group received adjunctive treatment for insomnia (six with alprazolam and two with nitrazepam). The dosages of these adjunctive drugs were not significantly different between the two groups.
4 Discussion
4. 1 Main findings
Confirming previous reports[3-5]we found that the extended-release and immediate-release formulations of venlafaxine were safe, tolerable and similarly effective in the treatment of depression.
Previous studies[16]found that VL and its main active metabolite ODV both show linear pharmacokinetic characteristics in the dosage range of 75mg to 450 mg per day. The peak plasma level is lower and occurs later (at 5.5 hours) in the XR formulation than in the IR formulation (peak at 2-3 hours), so the plasma concentration curve is flatter for the XT formulation of the medication. Other studies[17]report high variability among patients for steady-state plasma concentrations of VL and ODV, with coefficients of variation of 77% and 33%, respectively; in the current study the coefficient of variation for VL was 45% and that for ODV was 28%.Given the large individual differences in plasma levels, it is widely believed that there is no relationship between the efficacy of treatment of depression with venlafaxine and the plasma concentrations of VL and ODV[1,2]so there is serious doubt about the utility of monitoring VL and OVD levels. Our study also found no significant relationship between clinical efficacy and the plasma levels of VL and ODV, contradicting the findings of a previous study in China[13].
4. 2 Limitations
This study has four main limitations. The study was limited to hospitalized depressed patients who were female, so it is unclear if these results would be relevant for less severely ill individuals (who do not need hospitalization) or for males. The follow-up period of six weeks was longer than most studies, but this was still not long enough to assess the relationship of serum levels to efficacy during maintenance treatment of depression. The failure to find differences in rates of adverse reactions between the groups may have been related to the relatively small number of subjects in the study (i.e., Type II errors). Individuals who assessed the clinical outcome (i.e., HAMD) knew which formulation of venlafaxine the patient was using so this lack of blinded assessment may have resulted in some biases.
4. 3 Implications
This study from China confirms findings from other countries that find serum levels of VL and ODV too variable to be suitable for use as markers of the therapeutic efficacy of venlafaxine. Replication studies with larger samples, with other types of depressed patients, with longer follow-up periods, and with alternative methods of assessing plasma levels of venlafaxine and its key metabolites[18-21]are needed to con firm this growing consensus.
Conflict of Interest
The authors report no conflict of interest.
Funding
This study was supported by the Third People’s Hospital of Huzhou, it did not received funds from any pharmaceutical company.
1. Kuang WH, Li J, Huang Y. The relationship between the plasma concentration and the clinical efficacies in the depression treated with venlafaxine. West China Journal of Pharmaceutical Sciences, 2003, 18(3):179-181. (in Chinese)
2. Li J, Duan CM. The relationship between the oral dose/plasma concentration and the clinical efficacies in the depression treated with venlafaxine. Chinese Journal of Nervous And Mental Diseases, 1999, 25(2):95-99. (in Chinese)
3. Fan ZG. Efficacy of Venlafaxine for outpatients with depression.Chinese Journal of Clinical Pharmacy, 2001, 10(6), 386-387. (in Chinese)
4. Yuan YG, Zhang SN. Comparison between different dosages of venlafaxine extended-release formulation in the treatment of depression. Journal of Clinical Psychiatry, 2003, 13(4):236-237.
5. Huang YJ, Qing XX, Liu L, Qing HP, Liu Y, Jin KH, et al. Efficacy and adverse effect of venlafaxine XR in treatment of depression.Chinese Mental Health Journal, 2006, 20(5):342. (in Chinese)
6. Khan A, Upton GV, Rudolph RL, Entsuah R, Leventer SM. The use of venlafaxine in the treatment of major depression and major depression associated with anxiety: a dose-response study.Venlafaxine Investigator Study Group. J Clin Psychopharmacol,1998, 18(1):19-25.
7. Charlier C, Pinto E, Ansseau M, Plomteux G. Venlafaxine: the relationship between dose, plasma concentration and clinical response in depressive patients. J Psychopharm acol, 2002,16(4):369-372.
8. Gex-Fabry M, Balant-Gorgia AE, Balant LP, Rudaz S, Veuthey JL, Bertschy G. Time course of clinical response to venlafaxine:relevance of plasma level and clearlity. Eur J Clin Pharmacol, 2004, 59(12):883-891.
9. Su FL, Li HD. Advance in pharmacokinetics of antidepressants:venlafaxine. Chinese J of Clinical Pharmacology, 2007, 23(3):223-226. (in Chinese)
10. Matoga M, Pehourcq F, Titier K, Dumora F, Jarry C. Rapid high-performance liquid chromatographic measurement of venlafaxine and O-desmethylvenlafaxine in human plasma.Application to management of acute intoxications. J Chromatogr B Biomed Sci Appl. 2001;760(2):213-218.
11. Nichols AI, Focht K, Jiang Q, Preskorn SH, Kane CP.Pharmacokinetics of venlafaxine extended release 75 mg and desvenlafaxine 50 mg in healthy CYP2D6 extensive and poor metabolizers:a randomized, open-label, two-period, parallelgroup, crossover study. Clin Drug Investig, 2011, 31(3):155-167.
12. Klamerus KJ, Parker VD, Rudolph RL. Effects of age and gender on venlafaxine and O-desmethylvenlafaxine pharmacokinetics.Pharmacother, 1996, 16(5):915-923.
13. Wang ZG, Zhu XS, Ma RT. Simultaneous determination of venlafaxine and O-desmethylvenlafaxine in human serum by RP-HPLC. Chinese Pharmaceutical Journal, 2011, 46(1): 54-57. (in Chinese)
14. Tolstikov VV, Fiehn O. Analysis of highly polar compounds of plant origin:combination of hydrophilic interaction chromatography and electrospray ion trap mass spectrometry. Anal Biochem,2002, 301(2):298-307.
15. Conover WJ, Iman RL. Rank transformations as a bridge between parametric and nonparametric statistics. American Statistician,1981, 35 (3):124-129.
16. Shen YC. Psychiatry. 5th Ed. Beijing:People’s Health Press,2010:898-900. (in Chinese)
17. Charlier C, Pinto E, Ansseau M, Plomteux G. Venlafaxine:the relationship between dose plasma concentration and clinical response in depressive patients. J Psychopharmacol, 2002,16(4):369-372.
18. Gex-Fabry M, Rudaz S, Balant-Gorgia AE, Brachet A, Veuthey JL, Balant LP, et al. Steady-state concentration of venlafaxine enantiomers:model-based analysis of between-patient variability. Eur J Clin Pharmacol, 2002, 58(5):323-331.
19. Reis M, Lundmark J, Bjork H, Bengtsson F. Therapeutic drug monitoring of racemic venlafaxine and its main metabolites in an everyday clinical setting. Ther Drug Monit, 2002, 24(4):545-553.
20. Liu W, Wang F, Li HD. HPLC-MS/ESI determination of venlafaxine and O-desmethylvenlafaxine enantiomers serum concentration.Chinese Journal of Pharmaceutical Analysis, 2008(2):211-215. (in Chinese)
21. Liu W, Cai HL. HPLC-MS/ESI determination of venlafaxine and its three metabolites in human plasma. Chinese Journal of Pharmaceutical Analysis, 2008, 28(3):367-371. (in Chinese)
不同剂型文拉法辛治疗女性抑郁症患者的血药浓度与临床疗效的关系
宋宝华 范振国 沈卫民 钱敏才 陈海支 戴 红 王世锴
浙江省湖州市第三人民医院精神科313000
王世锴,电子信箱:tiantian9825@yeah.net
背景不同剂型的药物在体内的解离和释放速度存在差异,因此其起效和作用时间也有所不同,并可能会进一步影响其疗效和不良反应。
目的探讨不同剂型的文拉法辛治疗女性抑郁症患者的血药浓度与药物临床疗效和不良反应间的关系。
方法将60例女性抑郁症住院患者以单纯随机的方式分别编入文拉法辛缓释剂组(怡诺思组)及文拉法辛速释剂组(博乐欣组),进行为期6周的对照研究。怡诺思组患者每日晨起一次顿服药物,日平均剂量(标准差)为168(32)mg;博乐欣组患者每日分3次服用药物,日平均剂量(标准差)为160(67)mg。在研究第1、2、4、6周末以高效液相色谱法测定文拉法辛及其活性代谢产物氧去甲基文拉法辛浓度,以汉密尔顿抑郁量表(Hamilton Depression Rating Scale,HAMD)和治疗时出现的症状量表(Treatment Emergent Symptoms Scale,TESS)评定临床疗效和药物不良反应,HAMD评分减分率≥50%为有效。
结果两组均未见有严重的药物不良反应。在各研究时点两组HAMD评分组间差异均无统计学意义。治疗6周后两组HAMD评分均明显下降;缓释剂组的有效率为65.6%,速释剂组的有效率为69.2%(χ2=0.77,p=0.380)。治疗前后HAMD评分的改变与氧去甲基文拉法辛(rs=-0.10,p=0.470)及文拉法辛(rs=-0.11,p=0.403)血药浓度呈较弱的负相关。
结论两种剂型的文拉法辛对女性抑郁症患者均显示了良好的临床疗效和耐受性,但文拉法辛与氧去甲基文拉法辛血药浓度未与临床疗效密切相关,所以此两项指标不适合用来判定文拉法辛的临床疗效。
抑郁症 文拉法辛 氧去甲基文拉法辛 高效液相色谱法
10.3969/j.issn.1002-0829.2011.06.003
Department of Psychiatry, Third People’s Hospital of Huzhou, Huzhou 313000, China
*Correspondence: tiantian9825@yeah.net
(received: 2011-05-31; accepted: 2011-11-02)
猜你喜欢
杂志排行
上海精神医学的其它文章
- Mental health literacy in Changsha, China
- Eight-week follow-up of depressive and anxiety symptoms in patients with chronic hepatitis B, patients with hepatitis B cirrhosis and normal control subjects
- Cost of treating medical conditions in psychiatric inpatients in Zhejiang, China
- 苯丙胺类兴奋剂所致精神障碍的临床诊治问题
- Biostatistics in Psychiatry (6)Estimating treatment effects in observational studies
- Management of Behavioral and Psychological Symptoms of Dementia (BPSD)—no easy solution
