一维异核配合物[KNd(bta)2(H2O)6]·H2O的水热合成,晶体结构和荧光性质
2011-11-09马长燕陈文斌林焦敏郭仕恒周爱菊
马长燕 陈文斌 林焦敏 郭仕恒 周爱菊 董 文
(广州大学化学与化工学院,广州 510006)
一维异核配合物[KNd(bta)2(H2O)6]·H2O的水热合成,晶体结构和荧光性质
马长燕 陈文斌 林焦敏 郭仕恒 周爱菊 董 文*
(广州大学化学与化工学院,广州 510006)
以双四唑胺(H2bta)、NdCl3·6H2O、KOH为原料,用水热法合成了1个一维异核的Nd3+配合物[KNd(bta)2(H2O)6]·H2O。通过X-射线单晶衍射、元素分析、红外光谱对该配合物进行了结构表征。该化合物属于单斜晶系,P21/m空间群。每个Nd3+与2个来自bta2-离子的4个氮原子和5个水分子形成了1个盖帽的四方双锥,通过bta2-和水分子桥联配体把Nd3+和K+连接成一维链状结构,链与链之间通过O-H…N和N-H…O氢键作用形成三维超分子结构。紫外吸收光谱和荧光光谱表征结果显示该配合物的水溶液在292和412 nm表现出双四唑胺的特征吸收峰和荧光发射特征峰。
双四唑胺;异核配合物;晶体结构;荧光性质
0 Introduction
In recent years,the supramolecular complexes based on the multidentate ligand bistetrazolylamine (H2bta)and 3d or 4f metal ions have been received much attention due to their multifunctionality including magnetic and luminescent properties[1-6].The H2bta and its deprotonated anions (Scheme 1)contain nine nitrogen electron-donating atoms,possess conjugated N=N double bonds between two five-numbered tetrazole rings,which enhance their electronic fluidity and molecular rigidity[7].
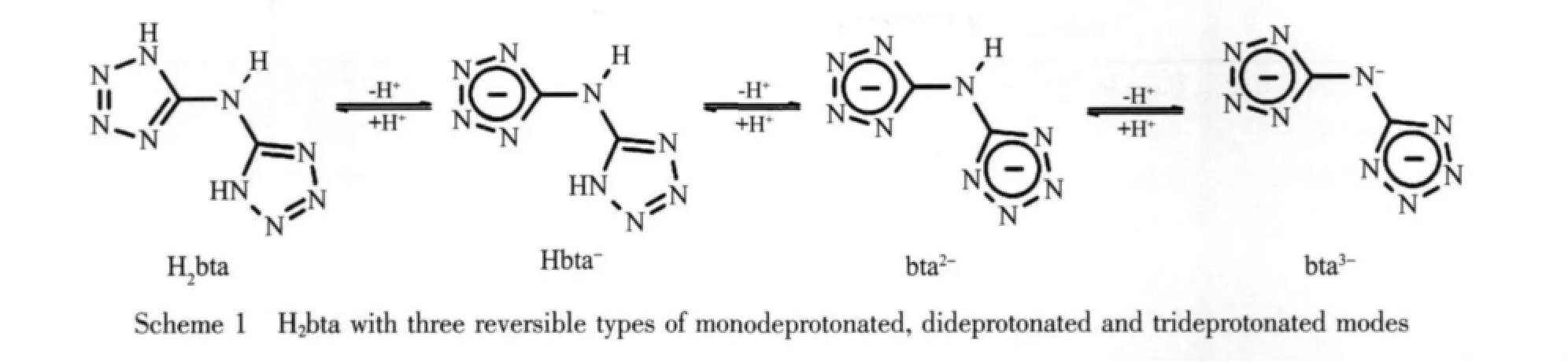
According to the reported studies,the ligand H2bta is especially interesting not only because of its various coordination modes to metal ions,resulting from completely or partially deprotonated sites allowing for the large diversity of topologies,but also because of its abilities to form complementary intermolecular hydrogen bonds such as O-H…N,N-H…O and N…H-N to assemble various supramolecular structures[6-11].Here,we report the synthesis,crystal structure and luminescent property of a novel heteronuclear complex [KNd(bta)2(H2O)6]·H2O(1).
1 Experimental
1.1 Materials and instrumentation
All reagents were purchased from commercial sources without further purification.Deionized water was used for the conventional synthesis.Elemental analyses of C,H and N were carried out using a Perkin-Elmer analyzer model 240.IR (KBr pellets)spectrum was obtained in the 400~4 000 cm-1range using a Bruker Tensor 27 spectrophotometer.The UV spectrum was obtained on a UV-2550 UV-Vis Spectrophotometer.The excitation and emission spectra were recorded on a F-4500 Fluorescence Spectrophotometer.
1.2 Synthesis of 1
A mixture of H2bta (0.031 g,0.2 mmol),NdCl3· 6H2O (0.062 g,0.2 mmol)and 0.05 mol·L-1KOH aqueous solution (20 mL)was heated in a 25 mL Teflon-lined autoclave at 160℃for 3 d,followed by slow cooling (5℃·h-1)to room temperature.The resulting mixture was filtered and washed with 95% methanol,and light purple crystals of 1 were collected and dried in air(50%yield based on H2bta).Elemental anal.calcd.for[KNd(bta)2(H2O)6]·H2O(%):C 7.85,H 2.62,N 41.20;found(%):C 7.68,H 2.80,N 41.01.
1.3 X-ray crystallography
The diffraction data was collected on a Bruker Smart APEX-Ⅱ CCD at296(2)K,usinggraphite monochromatized Mo Kα radiation (λ=0.071 073 nm).Empirical absorption corrections were applied by SADABS program.The structure of complex 1 was solved by direct methods and refined by full-matrix least-squares procedure on F2in SHELXL-97 program.All non-hydrogen atoms were refined anisotropically and hydrogen atoms were placed by the calculated positions and refined isotropically.
The crystal data,details on the data collection and refinement of 1 are summarized in Table 1.The selected bond lengths and angles are presented in Table 2.Table 3 gives the hydrogen bonding lengths and bond angles for 1.
CCDC:777028.

Table 1 Crystallographic data for 1
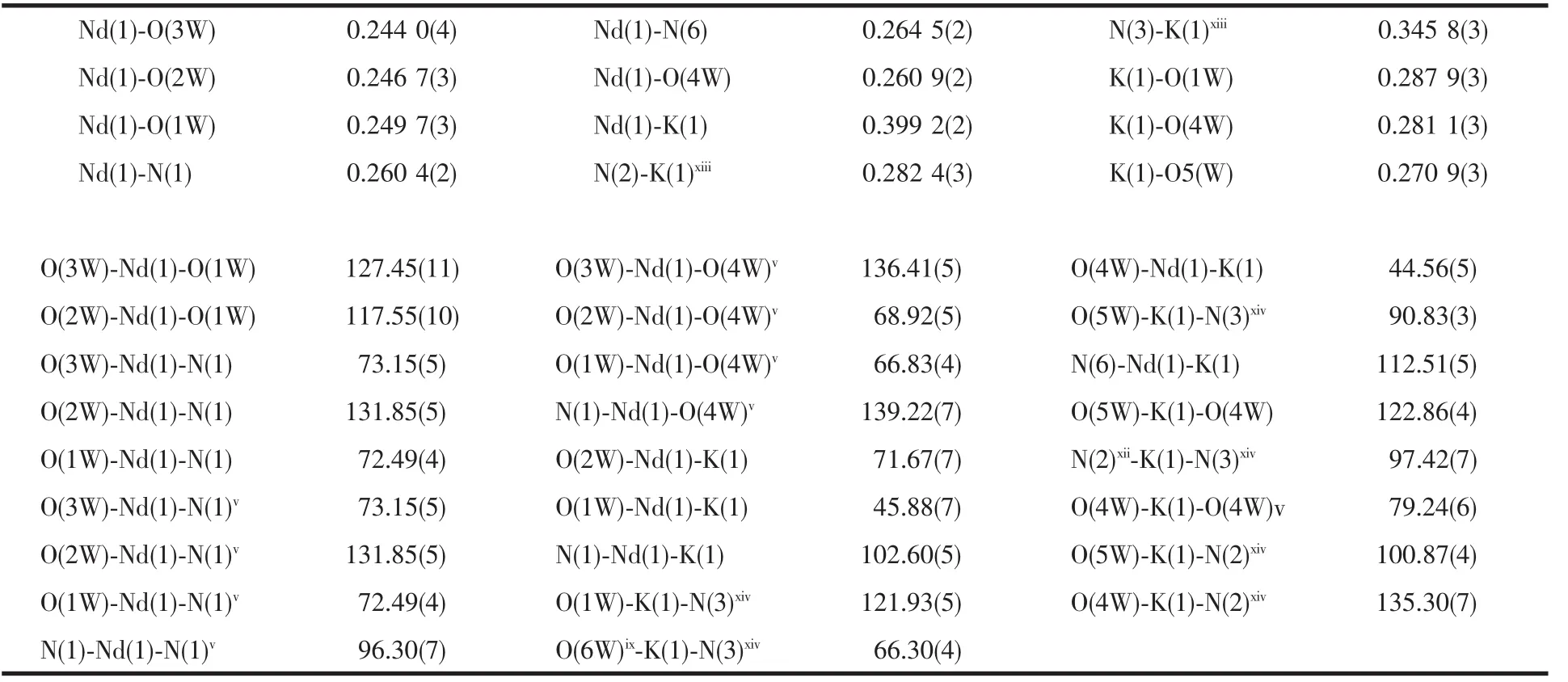
Table 2 Selected bond lengths(nm)and angles(°)for 1

Table 3 Hydrogen bonding lengths and bond angles for 1
2 Results and discussion
2.1 Crystal structure of 1
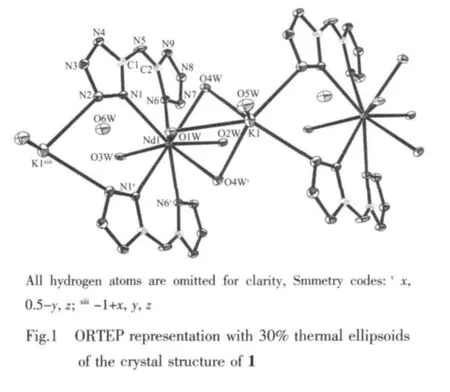
The atomic labeling scheme of 1 is shown in Fig.1. X-ray structural analysis reveals that 1 crystallizes in the monoclinic system,space group P21/m.Each unit of 1 consists of a Nd3+ion,a K+ion,two bta2-ligands,six coordinated water molecules and one solvent water molecule.The Nd(1)coordinatesto four tetrazole nitrogen atoms coming from two units of bta2-ligands and five oxygen atoms of water molecules to form a distorted capped square antiprism.Each H2bta acts as a μ2bridging ligand to link each Nd(1)and K(1).Each K+coordinates to two tetrazole nitrogen atoms coming from two bta2-ligands and four oxygen atoms of water molecules.Nd(1)and K(1)are linked by the bridging O (2),O(3)and O(3v)(Symmetry codes:vx,0.5-y,z) oxygen atoms of coordinated water molecules resulting in a 1D chain.In the crystal structure of 1,the six noncoordination nitrogen atoms of each bta2-anion and all water molecules take part in forming the rich intermolecular hydrogen-bonds(Table 2).There are two different hydrogen-bonds of O-H…N,and N-H…O with the shortest O…N distance of 0.280 6 nm(Fig.2). The extensive range of hydrogen bonds link the complex units and water molecules to form a three dimensional supramolecular structure(Fig.2).

2.2 IR spectrum
The IR spectrum of complex 1 exibites four characterized absorption region:the νN-Hvibration at 3 598 cm-1,the sharpest band split into νC=Nvibration band at 1 658 cm-1and νN=Nvibration band at 1 589 cm-1,the bands of the C-N stretching vibration at 1 037 cm-1,and the γN-Hvibration band at 730 cm-1.The bands at 3336 cm-1indicate the O-H stretching of water.
2.3 Spectroscopic studies
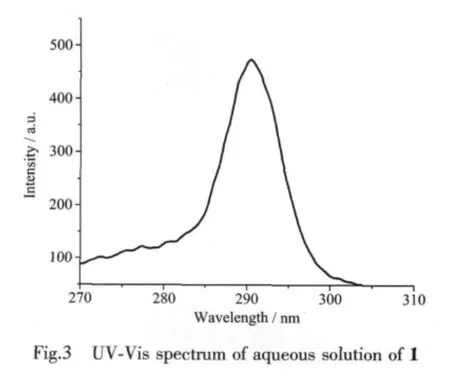
The UV-Vis absorption spectrum of 1 in aqueous solution (10-5mol·L-1)is shown in Fig.3.The aqueous solution of 1 displays the peak position around 290 nm that is attributed to the π-π transitions.
The photoluminescent spectrum of aqueous solution of 1 at room temperature is shown in Fig.4.The excitation peak at 292 nm of aqueous solution of 1 is corresponding with position of UV-Vis absorption peak (Fig.4a).The aqueous solution of 1 exhibits the emission maximum at 412 nm under the UV light excitation at 290 nm (Fig.4b).The luminescence of 1 should be attributed to the π-π transitions of bta2-ligand and the absorption band at 315 nm may be assigned to the intraligand n-π transitions.

[1]Gao E Q,Liu N,Cheng A L,et al.Chem.Commun.,2007: 2470-2472
[2]Jiang T,Zhang X M.Cryst.Growth Des.,2008,8:3077-3083
[3]Lu Y B,Wang M S,Zhou W W,et al.Inorg.Chem.,2008,47 (19):8935-8942
[4]Klapoetke T M,Thomas M,Mayer P,et al.J.Mater.Chem., 2008,18:5248-5258
[5]Guan Y F,Wang D Y,Dong W,et al.Acta Cryst.,2007,E63 (12):m3150/1-m3150/7
[6]GUAN Yang-Fan(管阳凡),ZHOU Ai-Ju(周爱菊),DONG Wen(董文),et al.Chinese J.Struct.Chem.(Jiegou Huaxue), 2009,28:1018-1022
[7]Guan Y F,Zhou A J,Dong W,et al.J.Struct.Chem.,2009, 938:150-155
[8]GUAN Yang-Fan(管阳凡),WANG Dong-Yao(王东耀), DONG Wen(董文),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,62(2):318-3229
[9]Lin J M,Huang B S,Dong W,et al.CrystEngComm,2009,11 (2):329-336
[10]Lin J M,Guan Y F,Dong W,et al.Dalton Trans.,2008,28: 6165-6169
[11]Friedrich M,Mayer P,Weber B,et al.Inorg.Chem.,2005,44 (22):8044-8052
Hydrothermal Synthesis,Crystal Structure and Photoluminescence of a One-Dimensional Heteronuclear Complex[KNd(bta)2(H2O)6]·H2O
MA Chang-Yan CHEN Wen-Bin LIN Jiao-Min GUO Shi-Heng ZHOU Ai-Ju DONG Wen*
(Department of Chemistry,Guangzhou University,Guangzhou 510006,China)
A heteronuclear complex[KNd(bta)2(H2O)6]·H2O(1)has been synthesized and charactered by X-ray diffraction,elemental analysis,IR spectroscopy.The crystal of 1 is of monoclinic,space group P21/m with a=0.7576 (1)nm,b=1.2800(0)nm,c=0.9976(0)nm,β=101.0330(10)°,V=0.9495(1)nm3,D=2.139 g·cm-3,Z=2,F(000)= 602,Goof=1.056,R1=0.0250,wR2=0.0561.Complex 1 shows a new one-dimensional chain structure and further extended into a 3D supramolecular structure through O-H…N and N-H…O intermolecular hydrogen bonding interactions.The aqueous solution of complex 1 shows interesting fluorescent property caused by H2bta ligand at room temperature.CCDC:777028.
bistetrazolylamine;heteronuclear complex;X-ray diffraction;fluorecent property
O614.33+5
A
1001-4861(2011)07-1436-05
2010-12-14。收修改稿日期:2011-03-29。
国家自然科学基金资助项目(No.21041005)。
*通讯联系人。E-mail:dw320@yahoo.com.cn
