Clinical Impact of t(14;18) in Diffuse Large B-cell Lymphoma
2011-07-18HongweiZhangNiuliangChengZhenwenChenJinfenWangSuhongLiWeiBai
Hong-wei Zhang, Niu-liang Cheng, Zhen-wen Chen, Jin-fen Wang, Su-hong Li, Wei Bai
1Department of Biochemistry and Molecular Biology, Shanxi Medical University, Taiyuan 030013, China;
2Department of Pathology, Fen Yang College of Shanxi Medical University, Fenyang, China;
3Department of Pathology, Shanxi Province Tumor Hospital, Taiyuan, China
Clinical Impact of t(14;18) in Diffuse Large B-cell Lymphoma
Hong-wei Zhang1,#, Niu-liang Cheng1*, Zhen-wen Chen2, Jin-fen Wang3, Su-hong Li3, Wei Bai3
1Department of Biochemistry and Molecular Biology, Shanxi Medical University, Taiyuan 030013, China;
2Department of Pathology, Fen Yang College of Shanxi Medical University, Fenyang, China;
3Department of Pathology, Shanxi Province Tumor Hospital, Taiyuan, China
Objective:Recent studies have suggested that t(14;18) is present in a significant proportion of diffuse large B-cell lymphomas (DLBCLs). However, the prognostic significance of this translocation and its relationship with BCL-2 protein expression remains controversial. Our study aimed to investigate the predictive power of t(14;18) and BCL-2 protein expression in the prognosis of DLBCLs.
Methods:Biopsy specimens from 106 DLBCLs were analyzed using interphase fluorescence in situ hybridization (FISH). Immunophenotypic analysis of CD20, CD3, CD10, BCL-6, MUM1 and BCL-2 was performed by immunohistochemistry. SPSS 13.0 software was used for statistical analysis.
Results:The t(14;18) was identified in 27 of 106 cases (25.5%). The percentages of tumor cells expressing CD10, BCL-6, MUM1 and BCL-2 were 21.7%, 26.4%, 56.6% and 73.6%, respectively. The presence of this translocation was significantly correlated with the expression of CD10 and immunophenotypic subtype (p<0.001). No association was observed between BCL-2 protein expression and the presence of t(14;18). Multivariate analysis confirmed that both t(14;18) and BCL-2 expression were significantly associated with survival. Moreover, patients with t(14;18) had worse prognosis, compared with those with BCL-2 expression (for overall survival: hazard ratio, 4.235; 95%CI, 2.153-8.329, p<0.001 vs. hazard ration, 2.743; 95%CI, 1.262-5.962, p=0.011).
Conclusions:The t(14;18) is a useful prognostic tool for the evaluation of DLBCL immunophenotype and prognosis. The prognosis of GCB (germinal centre-like B cell) DLBCL patients should be made with the consideration of the presence of this translocation, and the detection of t(14;18) should be included as a routine diagnostic test in these cases.
Chromosome translocation, t(14;18), FISH, Survival analysis, Diffuse large B-cell lymphoma
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is categorized as a distinct lymphoma entity, according to the World Health Organization (WHO) classification[1]. It is the most common subtype of non-Hodgkin lymphomas (NHLs), representing approximately 40% to 50% adult NHL cases. The incidence of NHL is currently increasing. It is now the fifth most frequent cancer worldwide[2-4].
Evidence suggests that DLBCL is not a single disease but rather a heterogeneous group of tumors with various clinical courses, histology and molecular and cytogenetic characteristics. Currently, the International Prognostic Index (IPI) model is most widely used to predict the outcome of DLBCL[5]. However, even patients within identical IPI categories may exhibit striking variability in 11111111111111prognosis, suggesting the heterogeneity of this malignancy. Molecular and cytogenetic studies of NHLs have shown that chromosomal and genetic abnormalities are associated with the biological and clinical features of these diseases and that they could serve as useful prognostic markers.
The t(14;18)(q32;q21) translocation, reported in approximately 10% to 40% DLBCL cases[6-8], is a characteristic feature of follicular lymphoma and is considered the initiating event of lymphomagenesis[9]. The translocation between these two chromosomes juxtaposes theBcl-2locus (located at 18q21) next to the regulatory regions of the IGH (locus at 14q32), which modifies the regulatory region of the proto-oncogeneBcl-2leading to BCL-2 overexpression. BCL-2 is an anti-apoptotic protein whose overexpression opposes mitochondrial apoptotic pathways. The presence of t(14;18) may be important for the pathogenesis of DLBCL with t(14;18). However, the prognostic effect of this translocation and its relationship with BCL-2 protein expression remain controversial[10,11].
In previous reports, polymerase chain reaction (PCR) was often used to detect t(14;18), whereas it has beenreported that FISH is the gold standard in the study of genetic abnormalities. Therefore, the purpose of this study was to determine the incidence of t(14;18) in DLBCL by FISH and its correlation with BCL-2 protein expression and patient prognosis, providing a scientific foundation for the prognosis of DLBCL patients.
MATERIALS AND METHODS
Case Selection
A total of 106 specimens from DLBCL patients treated at the Shan Xi Tumor Hospital in China were examined in this study. The inclusion criterion was a diagnosis of DLBCL between 2000 and 2007. The t(14;18) translocation was detected by FISH. All cases were confirmed by pathologic review and were classified according to the World Health Organization system[1]. Clinical and follow-up information were obtained from corresponding medical records at the Hematology Department of the same hospital. All patients were newly diagnosed and previously untreated; they received CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone) or CHOP-like chemotherapy. Among these patients, nine received rituximab together with combination chemotherapy, and eight received additional radiotherapy. Written informed consent was obtained from all patients, according to the Declaration of Helsinki.
FISH Analysis
Interphase FISH was performed on paraffinembedded tissue sections to detect t(14;18) as previously described[12]. Commercially available dual-color, dual-fusion IGH/BCL-2 probe sets (05J71-001) were used, according to the manufacturer’s instructions (www.Vysis.com). The LSI IGH/BCL-2 dual-color fusion translocation probe is a mixture of a LSI IGH probe labeled with SpectrumGreen and a LSI BCL-2 probe labeled with SpectrumOrange. A translocation was defined by the presence of two yellow fusion signals or adjacent red and green signals on abnormal chromosomes 18 and 14, respectively. At least 200 intact, non-overlapping nuclei were assessed by two pathologists using a Leica TCS SP5 Laser Scanning Confocal Microscope (LSCM). The positive predictive cutoff value used was 3.9% of suspected tumor cells and was determined by examining ten control samples[12].
Immunohistochemistry (IHC)
IHC was performed on archived paraffin-embedded tissue samples by the EnVision method, using the antibodies of CD20 (L26; Maxin Bio, China), CD3 (MAB-0200; Maxin Bio, China), CD10 (clone 56C6; Maxin Bio, China), BCL-6 (GI191E/A8; Santa Cruz, China), MUM1 (clone MUM1p; Santa Cruz, China), and BCL-2 (MAB-0012; Santa Cruz, China). For BCL-6 and MUM1, the immunohistochemical results were considered positive if at least 30% of the tumor cells showed nuclear immunoreactivity. For CD10 and BCL-2, membranous reactivity in more than 30% of cells was considered positive[13]. According to Hans’s method, immunohistochemical detection of CD10, BCL-6 and MUM1 was used to classify DLBCL into GCB and non-GCB groups[13]. The GCB subgroup included all tumors with the CD10+or CD10–/BCL-6+/MUM1–immunophenotype. Other cases were classified into the non-GCB subgroup, including MUM1+tumors, regardless of their BCL-6 status (CD10–/BCL-6+/ MUM1+or CD10–/BCL-6–/MUM1+).
Statistical Analysis
Overall survival (OS) was defined as the time interval between the date of diagnosis and the date of death or the last follow-up. Deaths from other causes during lymphoma remission were censored. Progression-free survival (PFS) was measured as the time from diagnosis until progression, relapse after response, or death from lymphoma. Pearson’sχ2test or Fisher’s exact test was used to determine the differences between the variables examined. The Kaplan-Meier method with the log rank test was performed to estimate the OS rate and to compare survival differences between groups.P-value<0.05 was considered statistically significant, and allPvalues were two-sided. Survival differences between groups were analyzed using the Cox proportional hazard model. All statistical analyses were performed using SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
Clinical Characteristics of Patients
The follow-up period of the 106 patients ranged from 0.5 to 104 months, and their median survival time was 23.4 months. In total, 73 patients were diagnosed with primary nodal presentation, and 33 patients were diagnosed with extranodal presentation, with a median age of 56.5 years (range, 15 to 92). In all, 44 patients presented high ECOG performance status (≥2), and 39 patients presented a high IPI score (≥3). There were 56 cases of Ann Arbor stage I-II, and the remaining cases were at III-IV stage. Elevated levels of serum lactate dehydrogenase (LDH) were observed in 55 patients, ranging from 242 to 1651 IU/L (normal: ≤240 IU/L). All patients, except two cases, received intensive combination chemotherapy after admission, and one patient underwent radiation therapy.
t(14;18) Chromosomal Translocation
The t(14;18) translocation was observed in 25.5% (27 of 106) of our cases with DLBCL (Figures 1A and 1B). The primary sites of t(14;18) were variable: lymph node (n=16), eye (n=1), thyroid gland (n=2), cervical (n=1), gastrointestinal tract (n=4), skin (n=1) and breast (n=2). There was no significant difference in the incidence of t(14;18), according to the primary site. The relationships between t(14;18) and the CD10 immunophenotype were observed. Of 23 CD10-positive cases, 14 (60.9%) were t(14;18)-positive (P<0.001). Of 26 GCB DLBCL cases, 14 (53.8%) were t(14;18)-positive (P<0.001). However, no significant correlation was observed between BCL-2 protein expression and the presence of t(14;18) (Table 1).
Immunophenotypic Findings
Immunohistochemical findings obtained from 106 DLBCL biopsy specimens are listed in Table 1. The neoplastic cells in all cases of B-cell lymphoma were CD20-positive and CD3-negative. For CD10, BCL-6, MUM1 and BCL-2, the positive rates were 21.7% (23/106), 26.4% (28/106), 56.6% (60/106) and 73.6% (78/106), respectively. According to Hans’s method, 26 (24.5%) patients were the GCB type, and 80 (75.5%) patients were the non-GCB type[13]. Furthermore, the expression of BCL-2 protein correlated with immunophenotype. Of 78 patients with positive BCL-2 protein expression, 63 (80.8%) were the non-GCB type, and 15 (19.2%) were the GCB type. There was a significant difference in BCL-2 expression between different immunophenotypes (P<0.05).
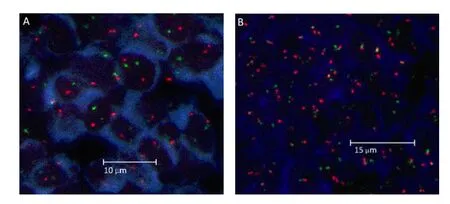
Figure 1.Fluorescence in situ hybridization images obtained using a LSI IGH/BCL-2 dual-color and dual-fusion probe to detect the IGH/BCL-2 translocation. A, Nuclei of tumor cells without the IGH/BCL-2 translocation, showing two orange and two green signals, B, Within tumor cells with the IGH/BCL-2 translocation, one orange and one green with two yellow (orange +green fusion signal) signals were frequently observed in individual nuclei.
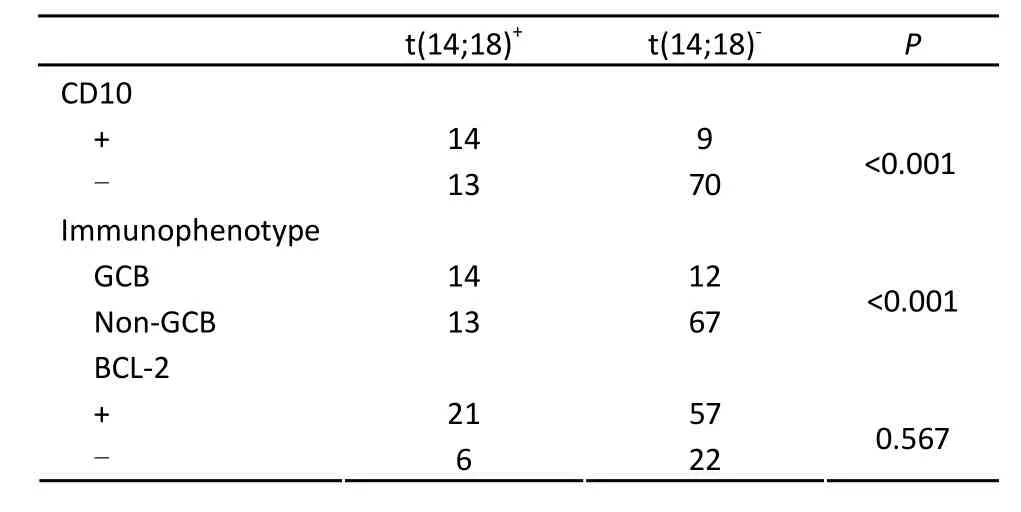
Table 1.The correlation of t(14;18) with immunophenotype in 106 patients with DLBCL
Comparison of OS and PFS between t(14;18)-positive and t(14;18)-negative Groups among the Patients with DLBCL
The prognostic implications of t(14;18) in DLBCL were assessed by univariate analysis with the log rank test (Figure 2). There was a statistically significant difference in OS and PFS between the t(14;18)-positive group (n=27) and the t(14;18)-negative group (n=79) (P<0.001). The median OS and the PFS of the former group were 18.5 and 11.0 months, respectively, and those of the latter group were 40.0 and 35.3 months, respectively, suggesting that the presence of t(14;18) is a strong indicator of poor prognosis of patients with DLBCL. In addition, two patients in the t(14;18)-positive group received rituximab, together with combination chemotherapy. They were diagnosed as the GCB type, and their survival time were 21.3 and 10 months (OS) and 10.3 and 4.5 months (PFS), respectively. However, because the patient number was too small, statistical analysis could not be performed.
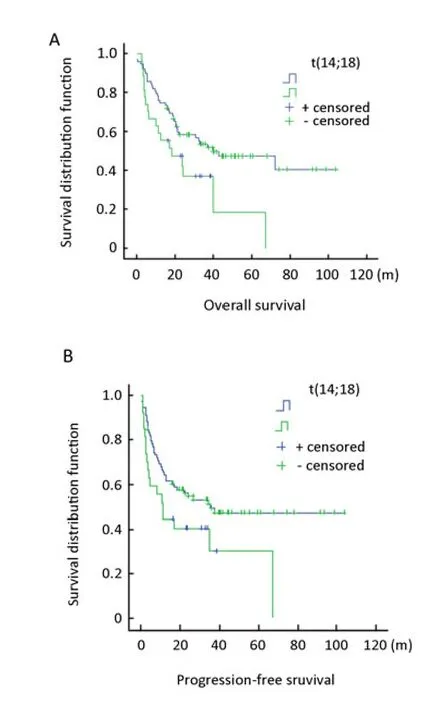
Figure 2.OS and PFS of patients with DLBCL, according to the t(14;18) status. A, Patients with t(14;18) showed poorer overall survival (OS) time than without t(14;18). B, Patients with t(14;18) showed poorer progression free survival (PFS) time than those without t(14;18).
Comparison of OS and PFS According to BCL-2 Protein Expression
一类是确定性函数关系,变量之间的关系可以用函数表示.例如,圆的面积S与半径r之间就是确定性函数关系,可以用S=πr2表示.
The prognostic implications of BCL-2 expression for DLBCL patients were also evaluated with the log rank test. As shown in Figure 3, BCL-2-positive DLBCL patients (n=78), with a median OS of 22.0 months (P=0.005) and the PFS of 16.0 months (P=0.004), showed significantly poorer prognosis than BCL-2-negative patients (n=28), with a median OS of 72.0 months (P= 0.005) and the PFS of 71.4 months (P=0.004).
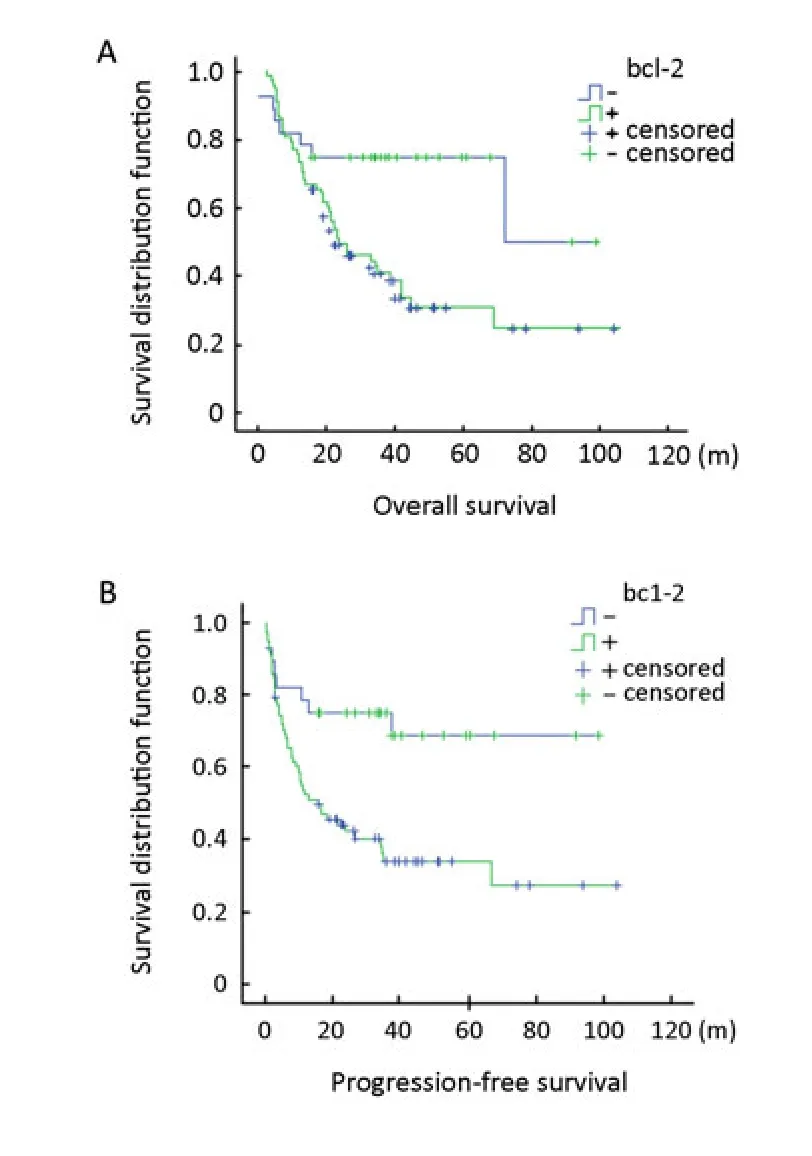
Figure 3.OS and PFS of patients with DLBCL, according to the BCL-2 status. A, Patients with BCL-2-positive tumors showed poorer overall survival (OS) time than those with BCL-2-negative tumors. B, Patients with BCL-2-positive tumors showed poorer progression free survival (PFS) time than those with BCL-2-negative tumors.

Table 2.Multivariate analysis of prognostic factors in DLBCL
Multivariate Analyses
The OS and the PFS were significantly worse in DLBCL patients with the following characteristics: performance status of 2-4, high serum LDH level, Ann Arbor stage III/IV, IPI≥3, the non-GCB phenotype, B symptom, BCL-2 expression and t(14;18). Multivariate analysis in a Cox proportional hazard model confirmed that among the eight prognostic factors examined, performance status of 2-4, BCL-2 expression and t(14;18) have significant predictive power for adverse prognosis in patients with DLBCL. (Table 2)
DISCUSSION
In this study, we examined the presence of t(14;18) in 106 DLBCL cases, detecting its occurrence in 27 cases (25.5%). Previously, Yuko et al. studied the t(14;18) abnormality in DLBCL cases and reported an incidence of 15% (9 of 61 cases)[14]. In studies ofBcl-2gene rearrangement using Southern blot hybridization, rearrangements of theBcl-2-MBRgene were observed in about 16 to 28% cases[15]. Our results are in agreement with the results of these previous studies.
The t(14;18) translocation was associated with GCB DLBCL and CD10 protein expression. Of 26 GCB DLBCL cases, 14 (53.8%) were t(14;18)-positive (P<0.001). Of 23 CD10 positive cases, 14 (60.9%) were t(14;18)-positive (P<0.001). An association between the presence of t(14;18) and CD10 expression[16]has been reported and suggested to represent a FL origin. Some studies have demonstrated that t(14;18) exclusively occurs in cases with a GCB gene expression profile[17,18]. These cases were shown to be CD10-positive by IHC. However, in our study, t(14;18) was also observed in 13 (16.2%) non-GCB DLBCL cases, raising the possibility that an additional mutation or translocation event may have resulted in the loss of expression of one of the GCB-associated antigens and that these cases would have been classified as the GC type without gene expression analysis.
No statistical correlation was found between t(14;18) and BCL-2 protein expression, consistent with the results of some previous reports[17,19]but contrary to the findings of Barrans et al[20]. It is possible that BCL-2 expression is essential in early lymphomagenesis, but additional genetic abnormalities developing during tumor progression may abrogate this requirement. The finding of BCL-2 protein expression in cases without t(14;18) indicates that other mechanisms can lead to BCL-2 expression.
The prognostic significance of t(14;18) in DLBCL is controversial. In the present study, the OS rates of t(14;18)-positive cases and t(14;18)-negative cases were significantly different (p =0.028). The PFS rates of t(14;18)-positive and t(14;18)-negative cases also differed significantly (P=0.044). These findings are similar to those previous reports, showing that t(14;18) negates the beneficial prognostic effect of the presence of the GCB phenotype[11]. However, another study reported that t(14;18) has no significant effects on OS or disease-free survival[14].
In conclusion, t(14;18) is a useful prognostic tool for the evaluation of DLBCL immunophenotype and prognosis. Most GCB DLBCL cases with t(14;18) were associated with adverse prognosis. Therefore, the prognosis of GCB DLBCL patients should be made with the consideration of the presence of t(14;18), and the detection of this translocation should be included as a routine diagnostic test for these cases.
REFERENCES
1. Stein H, Warnke RA, Chan WC, et al. In WHO classification of tumors of haematopoietic and lymphoid tissues: diffuse large B-cell lymphoma, not otherwise specified. 4th ed. IARC: lyon, 2008.
2. Jemal A, siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin 2007; 57: 43-66.
3. Gloeckler Ries LA, Reichman ME, Lewis DR, et al. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist 2003; 8: 541-52.
4. Stewart BW, Kleihues P. In World Cancer Report. Lyon; IARC Press, 2003.
5. Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 2008; 9: 105-16.
6. Gascoyne RD, Adomat SA, Krajewski S, et al. Prognostic significance of bcl-2 protein expression and bcl-2 gene rearrangement in diffuse aggressive non-Hodgkin’s lymphoma. Blood 1997; 90: 244-51.
7. Kramer MHH, Hermans J, Wijburg E, et al. Clinical relevance of BCL-2, BCL-6 and MYC rearrangements in diffuse large B-cell lymphoma. Blood 1998; 92: 3152-62.
8. Tibiletti MG, Martin V, Bernasconi B, et al. BCL2, BCL6, MYC, MALT 1, and BCL10 rearrangements in nodal diffuse large B-cell lymphomas: a multicenter evaluation of a new set of fluorescent in situ hybridization probes and correlation with clinical outcome. Human Pathology 2009; 40: 645-52.
9. Magrath I. Molecular basis of lymphomagenesis. Cancer Res 1992; 52: 5529-40.
10. Biasoli I, Morais JC, Scheliga A, et al. CD10 and Bcl-2 expression combined with the International Prognostic Index can identify subgroups of patients with diffuse large-cell lymphoma with very good or very poor prognoses. Histopathology 2005; 46: 328-33.
11. Yoon SO, Jeon YK, Paik JH, et al. MYC translocation and an increased copy number predict poor prognosis in adult diffuse large B-cell lymphoma (DLBCL), especially in germinal centre-like B cell (GCB) type. Histopathology 2008; 53: 205-17.
12. Ventura RA, Martin-Subero JI, Jones M, et al. FISH analysis for the detection of lymphoma associated chromosomal abnormalities in routine paraffin-embedded tissue. J Mol Diagn 2006; 8: 141-51.
13. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004; 103: 275-82.
14. Yuko Hirose, Yasufumi Masaki, Hiromi Karasawa, et al. Incidence of diffuse Large B-cell lymphoma of germinal center B-cell origin in whole diffuse large B-cell lymphoma: tissue fluorescence in situ hybridization using t(14;18) compared with immunohistochemistry. Int J Hematol 2005; 81: 48-57.
15. Weiss LM, Warnke RA, Sklar J, et al. Molecular analysis of the t(14;18) chromosomal translocation in malignant lymphomas. N Engl J Med 1987; 317: 1185-89.
16. Fang JM, Finn WG, Hussong JW, et al. CD10 antigen expression correlates with the t(14;18)(q32;q21) major breakpoint region in diffuse large B-cell lymphoma. Mod Pathol 1999; 12: 295–300.
17. Huang JZ, Sanger WG, Greiner TC, et al. The t(14;18) defines unique subset of diffuse large B-cell lymphoma with germinal center B-cell expression profile. Blood 2002; 99: 2285-90.
18. Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002; 346: 1937–47.
19. Javeed Iqbal, Sanger WG, Horsman DE, et al. Bcl-2 translocation defines a unique tumor subset within the germinal center B-cell-like diffuse large B-cell lymphoma. American Journal of Pathology 2004; 165: 159-66.
20. Barrans SL, Evans PAS, O ’Connor SJM, et al. The Bcl-2/IgH is associated with germinal center-derived diffuse large B-cell lymphoma and is a strong predictor of outcome. Clin Cancer Res 2003; 9: 2133-39.
10.1007/s11670-011-0160-x
2010-12-14;Accepted2011-03-17
*Corresponding Author
E-mail: chengniuliangty@yaghoo.ocm
#Author’s present address: Department of hematology, Shanxi Province Tumor Hospital, Taiyuan, China
©Chinese Anti- Cancer Association and Springer-Veriag Berlin Heidelberg 2011
猜你喜欢
杂志排行
Chinese Journal of Cancer Research的其它文章
- Effects of an Engineered Anti-HER2 Antibody chA21 on Invasion of Human Ovarian Carcinoma Cell In Vitro
- Reduction of Plasma MicroRNA-21 is Associated with Chemotherapeutic Response in Patients with Non-small Cell Lung Cancer
- The Use of CT Perfusion to Determine Microvessel Density in Lung Cancer: Comparison with FDG-PET and Pathology
- Immediate Versus Delayed Treatment with EGFR Tyrosine Kinase Inhibitors after First-line Therapy in Advanced Non-small-cell Lung Cancer
- DNA Repair Gene Polymorphisms in the Nucleotide Excision Repair Pathway and Lung Cancer Risk: A Meta-analysis
- Over-expression of Metastasis-associated in Colon Cancer-1 (MACC1) Associates with Better Prognosis of Gastric Cancer Patients
