双核铜配合物的结构和SOD活性及电化学性质
2011-06-12栾锋平向爱华李庆祥
栾锋平,向爱华,李庆祥
(武汉工程大学化工与制药学院,绿色化工过程省部共建教育部重点实验室,湖北 武汉 430074)
0 引 言

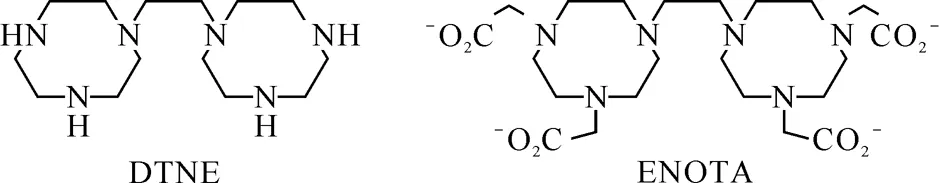
图1 配体DTNE和ENOTA的结构式
1 实验部分
1.1 原料与仪器
所用试剂和溶剂除核黄素、蛋氨酸、氯化硝基四氮唑蓝(NBT)为BR级外,其余均为分析纯.所用水为二次蒸馏水,配体1,2-二(1,4,7-三氮环壬烷)乙烷氢溴酸盐(DTNE·6HBr)根据文献报道的方法合成[15].元素分析在240C型元素分析仪上完成;红外光谱用KBr压片后在Nicolet 5DX FT-IR型红外光谱仪上记录;X-射线衍射数据搜集在德国Bruker SMART-APEX CCD单晶衍射仪上进行;循环伏安在273-型电化学仪上测定型电化学仪上测定.
1.2 配合物的合成[16]
配体1,2-二(1,4,7-三氮环壬烷)乙烷氢溴酸盐(DTNE·6HBr)200 mg(0.26 mmol)溶于5 mL水中,加入100 mg(1.05 mmol)氯乙酸的水溶液(3 mL),用0.1 mol·L-1的NaOH水溶液调pH至11,80 ℃下加热60 h,期间补充NaOH水溶液维持pH=11.溶液冷却后,加入192 mg(0.52 mmol)Cu(ClO4)2·6H2O的水溶液(2 mL),再加入25 mL无水乙醇,室温搅拌3 h后过滤,滤液室温挥发,析出适合X射线结构分析的蓝色晶体.收率:47 mg(25%).IR(KBr)ν:3 464,1 625,1 350 691 cm-1.Anal.calcd for C22H46Cu2N6O13:C 36.21,H 6.35,N 11.52;found C 36.39,H 6.42,N 11.25.
1.3 配合物晶体结构的测定
选取尺寸为0.21 mm×0.20 mm×0.19 mm的化合物的蓝色单晶,数据搜集在SMART-CCD面探衍射仪上进行,用SMART和SAINT程序进行数据还原和晶胞精修,晶体结构用直接法解出,所有非氢原子都用全矩阵最小二乘法对F2各向异性修正(Bruker Shelxtl)[17].氢原子通过理论加氢加上.有关晶体学数据见表1.
CCDC:777328

表1 配合物的晶体学数据
1.4 SOD活性的测定

1.5 电化学研究
循环伏安用273-型电化学分析仪测定.支持电解质是0.1 mol·dm-3的NaClO4的水溶液,测定前溶液中通入高纯氮气15 min以除去其中氧.用三电极系统在氮气气氛中测定,其中,玻碳电极作工作电极,Ag/AgCl电极作参比电极,铂丝作对电极.测定溶液浓度为1.0×10-3mol·dm-3,温度为(25±0.1) ℃.半波电位E1/2用(Epa+Epc)/2近似计算.
2 结果与讨论
2.1 晶体结构
配合物晶体结构见图2,部分键长、键角见表2,从晶体结构图2可知,两个Cu(II)离子分别位于畸变四方锥配位环境的中心,来自三氮环的两个氮原子和来自两个羧酸悬臂的氧原子构成四方锥的底面,桥头氮原子占据了四方锥的顶角.Cu(1)和Cu(2)分别偏离四方锥底面0.025 04和0.023 55 nm,两个底面的标准偏差分别为0.008 83和0.008 93 nm.Cu(1)与四方锥顶点原子之间的距离(Cu(1)-N(3)键长)为0.022 01(4) nm,长于与四方锥底面的四个原子之间的距离0.019 23(4)~0.019 98(5) nm.Cu(2)与四方锥顶点原子(N(4))之间的距离0.022 37(5) nm也长于与四方锥底面四个原子之间的距离0.019 17(4)~0.020 04(5) nm.两个铜原子之间的距离为0.747 nm.该配合物中,两个中心铜离子的配位环境与天然Cu2Zn2SOD的活性中心Cu(II)的配位环境较接近,在天然Cu2Zn2SOD中,活性中心Cu(II)与来自四个组氨酸的咪唑氮原子和一个氧原子的配位,形成一个畸变的四方锥构型[14].
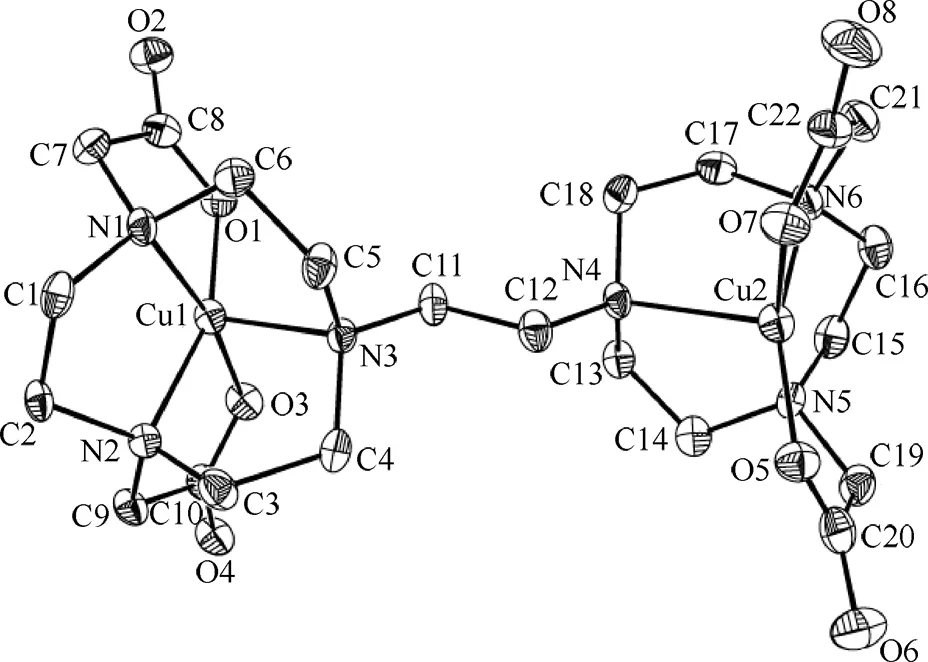
图的晶体结构
2.2 SOD活性
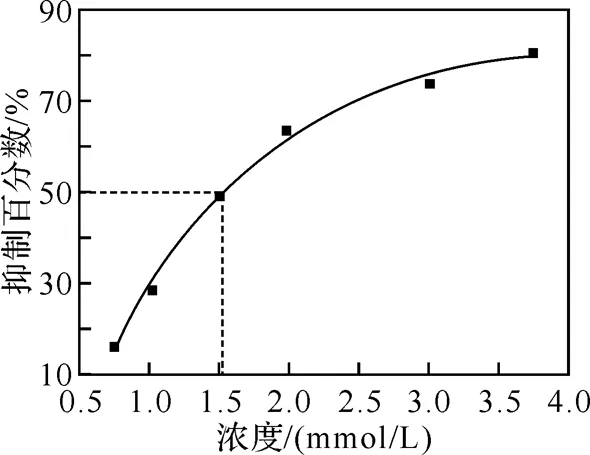
图的SOD活性

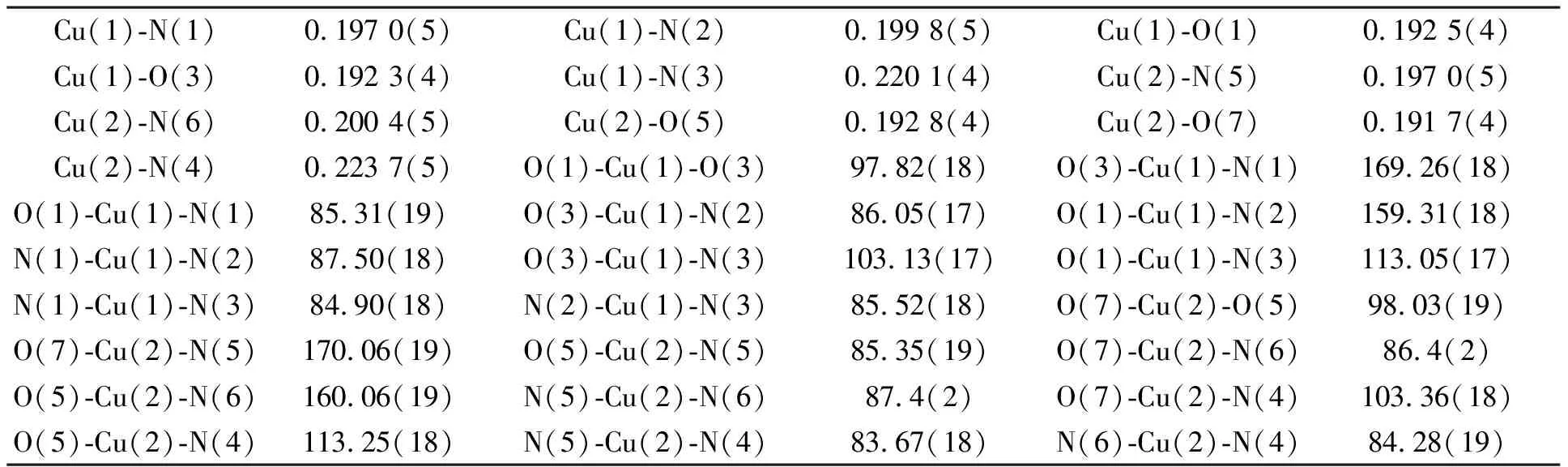
Cu(1)-N(1)0.197 0(5)Cu(1)-N(2)0.199 8(5)Cu(1)-O(1)0.192 5(4)Cu(1)-O(3)0.192 3(4)Cu(1)-N(3)0.220 1(4)Cu(2)-N(5)0.197 0(5)Cu(2)-N(6)0.200 4(5)Cu(2)-O(5)0.192 8(4)Cu(2)-O(7)0.191 7(4)Cu(2)-N(4)0.223 7(5)O(1)-Cu(1)-O(3)97.82(18)O(3)-Cu(1)-N(1)169.26(18)O(1)-Cu(1)-N(1)85.31(19)O(3)-Cu(1)-N(2)86.05(17)O(1)-Cu(1)-N(2)159.31(18)N(1)-Cu(1)-N(2)87.50(18)O(3)-Cu(1)-N(3)103.13(17)O(1)-Cu(1)-N(3)113.05(17)N(1)-Cu(1)-N(3)84.90(18)N(2)-Cu(1)-N(3)85.52(18)O(7)-Cu(2)-O(5)98.03(19)O(7)-Cu(2)-N(5)170.06(19)O(5)-Cu(2)-N(5)85.35(19)O(7)-Cu(2)-N(6)86.4(2)O(5)-Cu(2)-N(6)160.06(19)N(5)-Cu(2)-N(6)87.4(2)O(7)-Cu(2)-N(4)103.36(18)O(5)-Cu(2)-N(4)113.25(18)N(5)-Cu(2)-N(4)83.67(18)N(6)-Cu(2)-N(4)84.28(19)
2.3 配合物的电化学性质
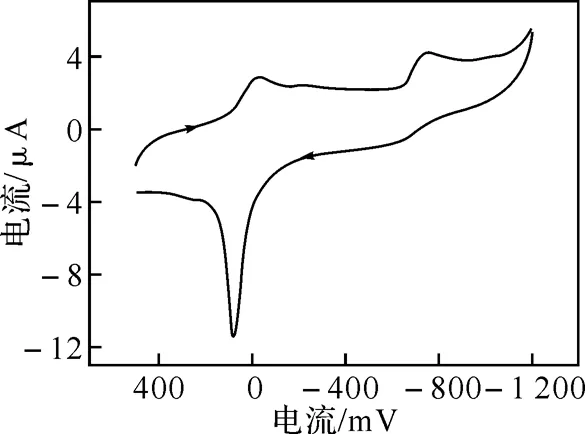
图在水溶液中的循环伏安图
参考文献:
[1] McCord J M,Fridovich I.Superoxide dismutase.An enzymic function for erythrocuprein (hemocuprein) [J].J Biol Chem,1969,244:6049-6055.
[2] Jiang W,Shen T,Han Y C,et al. Divalent-metal-dependent nucleolytic activity of Cu,Zn superoxide dismutase [J].J Bio Inorg Chem,2006,11:835-848.
[3] Scarpellini M,Wu A J,Kampf J W,et al.Corroborative Models of the Cobalt(II) Inhibited Fe/Mn Superoxide Dismutases [J].Inorg Chem,2005,44:5001-5010.
[4] Grove L E,Hallman J K,Emerson J P,et al.Synthesis, X-Ray Crystallographic Characterization,and Electronic Structure Studies of a Di-Azide Iron(III) Complex:Implications for the Azide Adducts of Iron(III) Superoxide Dismutase[J].Inorg Chem,2008,47:5762-5774.
[5] 汪立耀.超氧化物歧化酶的制备及其临床应用[J].武汉化工学院学报,2003(4):16-18.
[6] Valentine J S,Freitas D M.Copper-Zinc Superoxide Dismutase.A Unique Biological "Ligand" for Bioinorganic Studies [J].J Chem Educ,1985,62:990-997.
[7] Zhang Z,Geng Z R,Kan X W,et al.Iron(III), nickel(II) and cadmium(II) complexes of triazamacrocyclic ligand with pendant nitrile groups 1,4,7-tris(cyanomethyl)-1,4,7-triazacyclononane: Synthesis, structural characteristics and artificial nuclease activity[J].Inorg Chim Acta,2010,363:1805-1812.
[8] Wainwright K P.Synthetic and structural aspects of the chemistry of saturated polyaza macrocyclic ligands bearing pendant coordinating groups attached to nitrogen[J].Coord Chem Rev,1997,166:35-90.
[9] Grove L E,Hallman J K,Emerson J P,et al.Synthesis, X-Ray Crystallographic Characterization,and Electronic Structure Studies of a Di-Azide Iron(III) Complex: Implications for the Azide Adducts of Iron(III) Superoxide Dismutase [J].Inorg Chem,2008,47:5762-5774.
[10] Mewis R E,Archibald S.Biomedical applications of macrocyclic ligand complexes[J].Coord Chem Rev,2010,254:1686-1712.
[11] Li Q X,Luo Q H,Li Y Z,et al.A study on the mimics of Cu-Zn superoxide dismutase with high activity and stability: two copper(II) complexes of 1,4,7-triazacyclononane with benzimidazole groups [J].Dalton Trans,2004:2329-2335.
[12] Li Q X,Wang X F,Cai L,et al.Crystal structure,superoxide dismutase activity and electrochemical property of complex[Cu(dtne)]·(ClO4)2·CH3CH2OH[J].Inorg Chem Commun,2009,12:145-147.
[13] Li Q X,Luo Q H,Li Y Z,et al.Shen M C.Studies on Manganese(II) Complexes of N-Benzimidazole-Functionalized 1,4,7-Triazacyclononane:Crystal Structures,Properties and Combined Superoxide Dismutase and Catalase Functions[J].Eur J Inorg Chem,2004,22:4447-4456.
[14] Tainer J A,Getzoff E D,Beem K M,et al.Determination and analysis of the 2 A-structure of copper,zinc superoxide dismutase[J].J Mol Biol,1982,160:181-217.
[15] Sessler J L,Sibert J W,Lynch V.Model studies related to hemerythrin.Synthesis and characterization of a bridged tetranuclear iron(III) complex[J].Inorg Chem,1990,29:4143-4146.
[16] Fry F H,Graham B,Spiccia L,et al.Binuclear copper complexes of bis(1,4,7-triazacyclonon-1-yl) ligandsincorporating acetate pendant arms[J].J Chem Soc,Dalton Trans,1997:827-832.
[17] Sheldrick G M.SHELXTL V5.1 Software Reference Manual[M].Madison:Bruker AXS,Inc,1997.
[18] Luo Q H,Lu Q,Dai A B,et al.A study on the structure and properties of a new model compound of Cu(II)-Zn(II)-superoxide dismutase[J].J Inorg Biochem,1993,51:655-662.
[19] Sawyer D T,Valentine J S.How super is superoxide?[J].Acc Chem Res,1981,14(12):393-400.
