Alkylation of p-Cresol with tert-Butanol Catalyzed by NovelMultiple-SO3H Functioned Ionic Liquid*
2011-05-15BAOShaohua鲍少华QUANNannan权南南ZHANGJing张敬andYANGJianguo杨建国
BAO Shaohua (鲍少华), QUAN Nannan (权南南), ZHANG Jing (张敬) and YANG Jianguo (杨建国)
Alkylation of-Cresol with-Butanol Catalyzed by NovelMultiple-SO3H Functioned Ionic Liquid*
BAO Shaohua (鲍少华), QUAN Nannan (权南南), ZHANG Jing (张敬) and YANG Jianguo (杨建国)**
Shanghai Key Laboratory of Green Chemistry and Chemical Process, Department of Chemistry, East China Normal University, Shanghai 200062, China
The alkylation of-cresol with-butanol (TBA) to 2--butyl--cresol (TBC) catalyzed by a novel multiple-SO3H functioned ionic liquid (IL1) was investigated. Meanwhile, the catalytic activity of this novel ionic liquid was compared with other four traditional ionic liquids. The results showed that IL1 has superior catalytic activity to other four traditional ionic liquids with the conversion of 85.3% and selectivity of 95.2%. Also, the reaction conditions were investigated to obtain the optimum conditions. Operational simplicity, small amount of usage, high activity, reusability and selectivity are the key features of this methodology.
ionic liquid,-cresol, 2--butyl--cresol, alkylation
1 INTRODUCTION
The alkylation of phenol (as well as its derivatives) with different alcohols is an important reaction, because of its great significance in the production of a variety of fine chemicals. Among these reactions, the alkylation of-cresol with-butanol (TBA) gives 2--butyl--cresol (TBC), 2,6-di--butyl--cresol (2,6-DTBC), and-alkylated products (TBPE), which are widely used in the manufacture of phenolic resins, antioxidants [1] and polymerization inhibitors [2]. Catalysts used for alkylation of aromatics include Lewis acids [3], Brønsted acids [4], montmorillonite [5], cation-exchange resins [6], mesoporous materials [7], zeolites [8, 9], molecular sieves [10], and supercritical or near-critical water [11]. Most above methods suffer from at least one or more disadvantages, though some ones have convenient protocols to provide high yield. The traditional liquid acid catalysts may cause equipment corrosion and environmental pollution, and solid acids are deactivated rapidly from coke formation at high reaction temperature. Although cation-exchange resins shows good catalytic activity and thermal stability, but their fouling is a major problem. Recently, ionic liquids, known as environmentally benign catalysts, have been investigated extensively. Shen. [12] reported that 1-butyl-3-methyl-imidazolium hexafluorophosphate ([bmim]PF6) can catalyze the alkylation of phenol with TBA with high phenol conversion and selectivity to 2,4-di--butylphenol. Gui. [13] reported that functionalized ionic liquid 1-(4-sulfonicacid) butyl-3-methylimidazolium hydrogen sulfate ([Mim(CH2)4SO3H] [HSO4]) can also be used to catalyze the reaction with high phenol conversion and selectivity to 2,4-di--butylphenol. The above ionic liquids show high activity, stability and reusability. However, the main disadvantages of those ionic liquids are their relatively large dosage. To the best of our knowledge, the alkylation of-cresol with-butanol using multiple-SO3H functioned ionic liquid as catalyst has not been reported yet. In this paper, we reported a novel multiple-SO3H functioned ionic liquid (Fig. 1) that is capable of catalyzing alkylation of-cresol to-butanol with the advantages of easy separation, low dosage, excellent reusability and high activity. Meanwhile, a possible reaction mechanism was discussed.
2 EXPERIMENTAL
All organic reagents were commercial products with high purity available (>98%) and used for the reaction without further purification. Imidazole, 1,4-butylenedibromide, sodiumhydroxide, dimethyl sulfoxide,-cresol,-butyl alcohol were purchased from Shanghai Chemicals Co. Cyclohexane was supplied by Jiangsu Qiang Sheng Chemical Co.
2.1 Synthesis of ionic liquids
2.1.1
Imidazole (25 mmol), sodiumhydroxide (25 mmol) and dimethyl sulfoxide (6 ml) were mixed and stirred magnetically at 60 °C until imidazole was dissolved. Subsequently, 1,4-butylenedibromide (6 ml) was added dropwise into the mixture at 60 °C and stirred continuously for 2 h. After that, the mixture was dumped into water, and settled for a few hours to form white solid product. Then the obtained white solid was mixed with quadruplemolar 1,4-butane sulfonate (20 ml) under stirring at room temperature for 72 h. After solidification of the mass, the product (zwitterions) was washed with ethyl ether three times and then dried under vacuum (393 K, 1.33 Pa). The white solid zwitterions was obtained in good yield (>95%).

Figure 1 The synthetic route of the novel ionic liquid (IL1)
Figure 2 Structure of IL2 through IL5
2.1.2
For comparison, IL2-5 were also synthesized respectively according to the literature [14-17]. The structure of these ionic liquids can be seen in Fig. 2.
2.2 Synthesis of 2-tert-butyl-p-cresol
The liquid-phase alkylation reaction of-cresol with TBA using the obtained ionic liquids as catalysts was performed in a 100 ml glass reactor, fitted with a reflux condenser and a magnetic stirrer. A typical procedure was as follows: 5 mmol-cresol, 5 mmol TBA, 2.5 mmol IL1 and 3 ml cyclohexane were added into the flask respectively. The reaction was carried out at the desired temperature. On completion, the mixture was cooled to room temperature. The products were in the upper phase and analyzed by gas chromatograph (GC). The ionic liquid in the lower phase was collected and recycled.
2.3 Analysis
An Agilent 6890 (Agilent Co., USA) gas chromatograph equipped with a flame ionization detector (FID) was employed for the analysis. The column was programmed with an initial temperature of 50 °C, going to 250 °C at the rate of 20 °C·min-1, and making an isotherm at 250 °C for 20 min. The qualitative analysis of product was completed by the system of GC-MS. The concentrations of reactant and product were calculated according to the chromatograph peak areas given by software Chemstations.
3 RESULTS AND DISCUSSION
Alkylation of-cresol with TBA is a typical acid-catalyzed reaction giving a mixture of the isomers of-butylated phenols in terms of alkyls and the position of alkyl substituents, which are all possible products. The reaction scheme is shown in Fig. 3. Both C- and O-alkylated products can be formed depending on the reaction conditions such as the reaction temperature and the amount of TBA,., which agree with the results reported elsewhere [18].
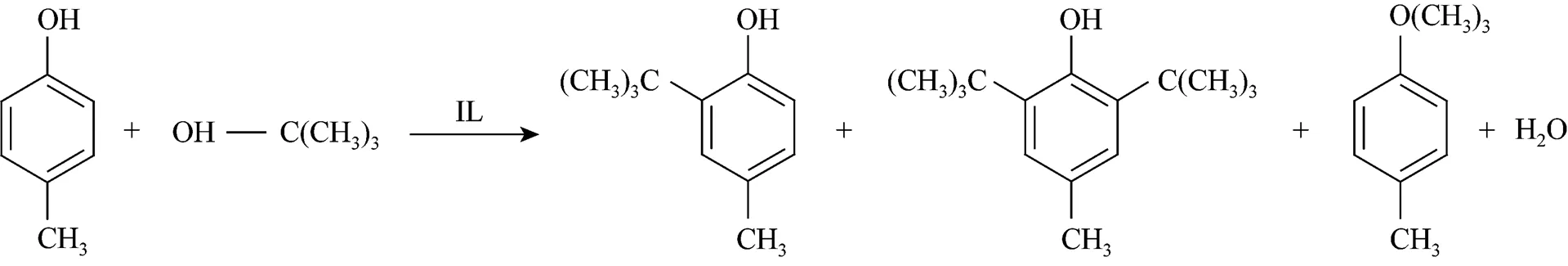
Figure 3 Reaction scheme for alkylation of-cresol with TBA
3.1 Effect of reaction time on tert-butylation of p-cresol
Firstly, the effect of reaction time on the yield of 2-TBC was investigated (Fig. 4). It can be seen that the conversion and selectivity increased rapidly with the time and reached the equilibrium level after 7 h with the conversion of-cresol of 85% and the selectivity of TBC to 94%. After that, the selectivity to TBPEdecreased quickly, maybe because TBPE is transformedto TBC through a rearrangement reaction [16]. Therefore, 7 h was chosen as the optimal reaction time.
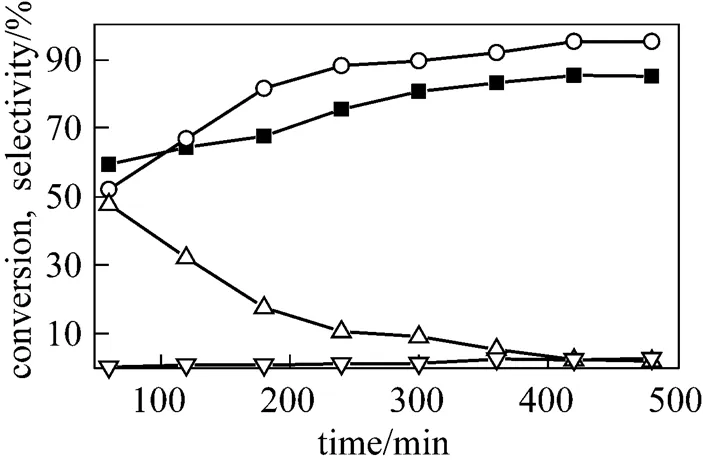
Figure 4 Effect of the reaction time on-butylation of-cresol
■ conversion of-cresol;○ selectivity to TBC;△ selectivity to TBPE;▽ selectivity to 2,6-DTBC
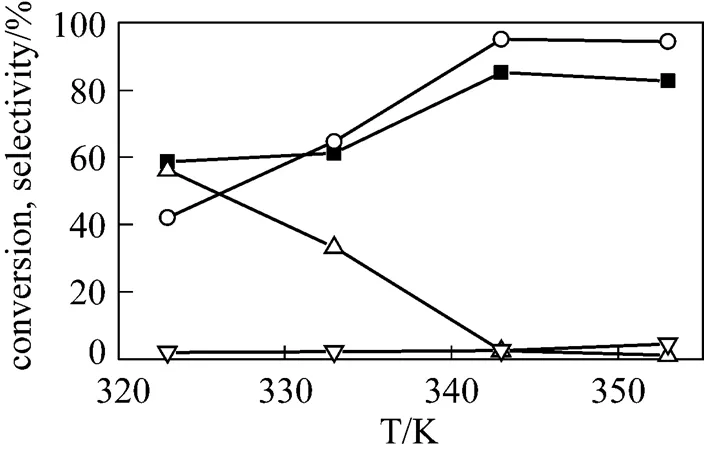
Figure 5 Effect of the reaction temperature on the- butylation of-cresol
■ conversion of-cresol;○ selectivity to TBC;△ selectivity to TBPE;▽ selectivity to 2,6-DTBC
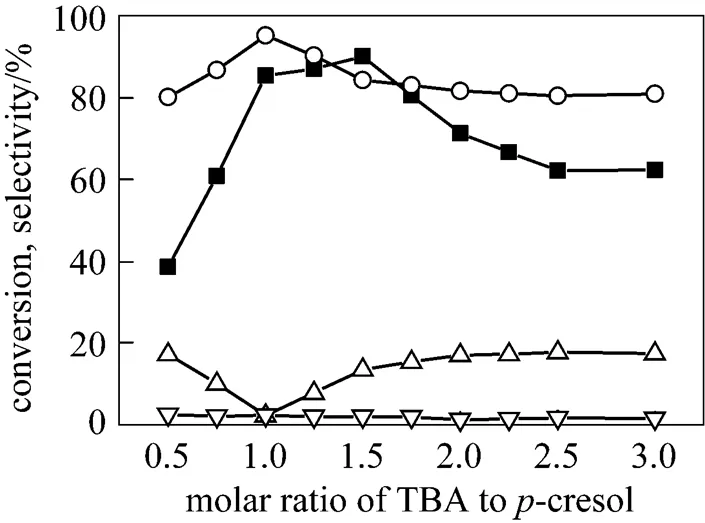
Figure 6 Effect of the reactant ratio on the-butylation of-cresol
■ conversion of-cresol;○ selectivity to TBC;△ selectivity to TBPE;▽ selectivity to 2,6-DTBC
3.2 Effect of reaction temperature on tert-butylation of p-cresol
The influence of the reaction temperature on-butylation of-cresol was also investigated in the range of 323–353 K and the results were shown in Fig. 5. With the increase of temperature, the conversion of-cresol and the selectivity to TBC increased and reached the climax value rapidly. At the same time, the selectivity to TBPE decreased greatly and then reached an equilibrium level at 343 K, while the selectivity to 2,6-DTBC was almost unchanged. Therefore, it was concluded that the increasing reaction temperature does not lead to the further C-alkylation. The possible reason may be due to the dehydration of TBA at higher reaction temperature or the dealkylation of the alkylated products at higher reaction temperature [19]. So the optimal reaction temperature was inferred to be 343 K.
3.3 Effect of the reactant ratio on the tert-butylation of p-cresol
The influence of the molar ratio on the reaction was shown in Fig. 6. It was observed that as the amount of TBA increased, the conversion of-cresol and selectivity to TBC initially reached maxima and then decreased, while the selectivity to TBPE increased gradually and then reached a steady state. From the above phenomenon, we can deduce that the weak acidic media favored O-alkylation product as well as strong acidic media favored C-alkylation product. The reason is perhaps that excess TBA results in the decrease of the catalyst concentration in the reaction system due to water formed in the dehydration of TBA. At the same time, a large amount of the by-products, such as the oligomers of isobutene and O-alkylated products are also formed due to excess TBA [20]. Accordingly, the optimal molar ratio of reactants is determined at 1︰1 (-cresol to TBA).
3.4 Effect of the amount of ionic liquids on the tert-butylation of p-cresol
A series of reactions with different molar ratio of IL1 to-cresol were carried out to establish the effect of the amount of ionic liquid on the tiled reaction. From the Fig. 7, we can see as the molar ratio of IL1 to-cresol increased, both the conversion of-cresol and the selectivity to TBC increased and reached a stabilized level. At the same time, the selectivity to TBPE and 2,6-DTBC decreased and increased respectively. This is attributed to the proportional increase in the amount of IL1 leading to the increase of acidity in the reaction system, further affecting the distribution of products [21]. Considering both conversion and selectivity, the optimum molar ratio of IL1 to-cresol is 0.4︰1.
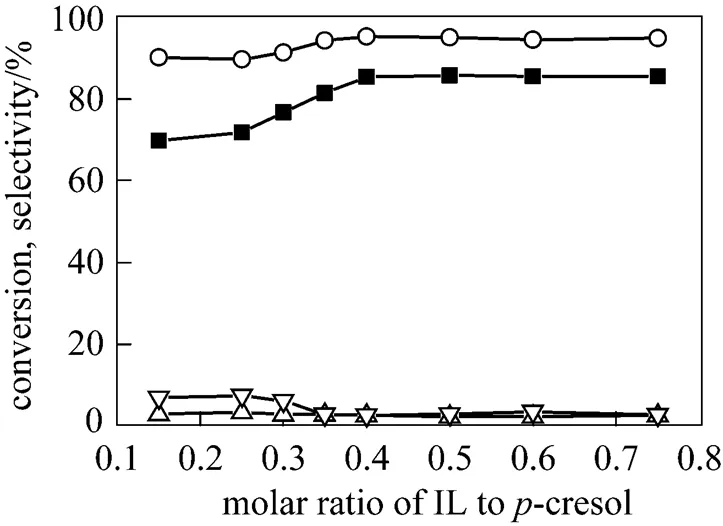
Figure 7 Effect of the amount of ionic liquids on the-butylation of-cresol
■ conversion of-cresol;○ selectivity to TBC;△ selectivity to TBPE;▽ selectivity to 2,6-DTBC
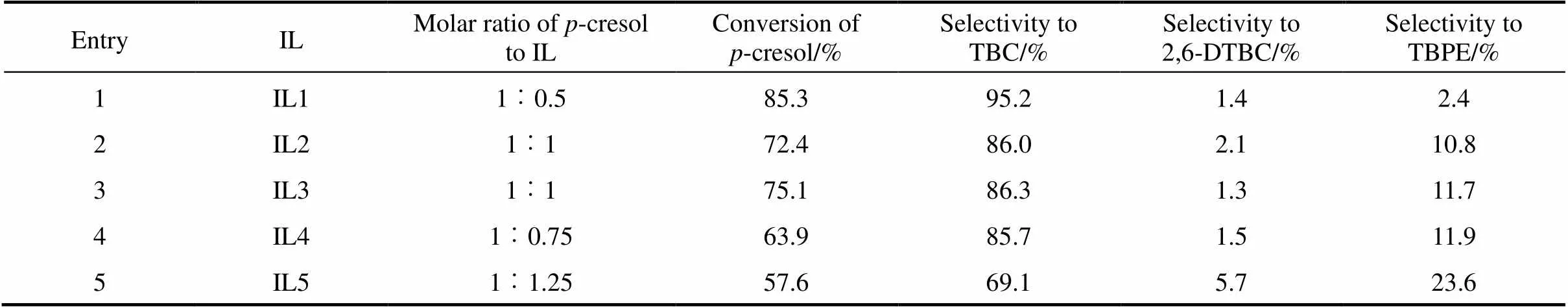
Table 1 Alkylation of p-cresol with TBA in different ionic liquids①
Compared with the traditional ionic liquids in Ref. [13], both the molar usage and quality usage of this novel ionic liquid are less than those traditional ionic liquids. And when considering the synthetic cost, the novel ionic liquid is almost the same as the traditional ionic liquids.
3.5 Effect of different ionic liquids on the tert- butylation of p-cresol
The novel ionic liquid (IL1) was compared with the imidazole ionic liquids and pyridinium ionic liquids which have different cations and anions respectively (Fig. 2). The influence of different ionic liquids on the reaction was depicted in Table 1. From Table 1, it was clearly that when all of the ionic liquids were under their optimization reaction conditions, the yield of TBC over IL1 was the highest, which exhibited that the novel ionic liquid (IL1) has much higher activity than others. At the same time, it could be seen that the main products of this reaction are TBC, 2,6-DTBC, TBPE and a small quantity of other products are also produced. It also could be seen that the conversion from IL4 (Entry 4) was obvious better than that from IL5 (Entry 5), indicating that catalytic properties of the ionic liquids are controlled predominantly by the anion, and the conversion and product selectivity were mostly dependent on the acidity. The conversion from IL3 (Entry 3) was slight better than that from IL2 (Entry 2), which indicated that the length of the side chain of the cation has slight impact on the catalytic performance. Owing to the advantages of easy separation, low dosage, reusability and high activity, the novel ionic liquid is a promising alternative for the traditional acids in the butylation of-cresol.
3.6 Recycle of ionic liquid
The reusability is one of the most important features of the novel catalyst for its applications. After reaction, the used ionic liquid was extracted by ether and vacuumized for 4 h at 373 K for the next cycle. The recovery of IL is 95% on the average. The used ionic liquid was assessed by1H NMR spectroscopy and no traces of products and reactant were detected. The results of the catalyst recycling experiment are shown in Table 2. It could be seen that the novel ionic liquid could be recycled for at least five times without any apparent loss of activity.
Table 2 Recycling of the IL1 in the alkylation of p-cresol with TBA①
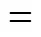
3.7 Discussion on mechanism
Alkylation is a typical electrophilic substitution reaction, and the key step may be the electrophilic reaction of the-butyl cation ([t-C4H9]+) with the C atom of the aromatic ring or the O atom of the hydroxyl group of-cresol. According to the reaction results, it can be concluded that TBC is a stable product in the-butylation of-cresol using ionic liquids as the catalyst, although a lot of O-alkylated product (TBPE) is formed at the beginning of the reaction. To help the mechanism study, we use the ab initio calculations based on quantum mechanics, and the results are in agreement with Liu[21, 22]. We conclude that the electronegativity of the active sites of the reactant, the structural stabilities of the intermediate products, or the final products may have a strong effect on the reaction routes [22].


4 CONCLUSIONS
In conclusion, the alkylation of-cresol with TBA catalyzed by the novel mutipule-SO3H ionic liquid (IL1) was successful. Under the optimum reaction conditions, the conversion of-cresol and the selectivity to TBC were 85.3% and 95.2% respectively. IL1 could be recycled for at least five times without any apparent loss of the conversion and selectivity. Moreover, compared with the reported results, this process possesses more advantages, such as low dosage of ionic liquid, low energy consumption due to low reaction temperature, the high activity and reusability of catalyst,. The present study showed that this novel mutipule-SO3H ionic liquid has excellent catalytic activity and can be used as an effective substitute for traditional acids.
1 Krishnan, A.V., Ojha, K., Pradhan, N.C., “Alkylation of phenol with tertiary butyl alcohol over zeolites”,...., 6 (2), 132-137 (2002).
2 Kamalakar, G., Komura, K., Sugi, Y., “The-butylation of phenol in supercritical carbon dioxide over H-Y zeolite”,.., 34 (10), 1446-1447 (2005).
3 Tian, L., Cao, F.H., Fang, D.Y., Guo, S.Z., “Alkylation of toluene with 1,3-pentadiene over [bupy]BF4-AlCl3ionic liquid catalyst”,...., 15 (5), 680-682 (2007).
4 Zhang, T., Zhang, F.Q., Mei, D., Wang, G.F., Wang, J.G., “A comparative study on the alkylation of benzene with propene over HBeta zeolites in different phases”,.., 6 (6), 385-388 (2005).
5 Choudary, B.M., Rao, B.P.C., “Fe3+-montmorillonite: A bifunctional catalyst for one pot Friedel-Crafts alkylation of arenes with alcohols”,.., 3 (8), 363-375 (2002).
6 Herrmann, W.A., Bohm, V.P.W., “Heck reaction catalyzed by phospha- palladacycles in non-aqueous ionic liquids”,..., 572 (1), 141-145 (1999).
7 Sakthivel, A., Badamali, S.K., Selvam, P., “-selective-butylationof phenol over mesoporous H-AlMCM-41”,., 39 (3), 457-463 (2000).
8 Fu, J.Q., Ding, C.H., “Study on alkylation of benzene with propylene over MCM-22 zeolite catalyst byIR”,.., 6 (12), 770-776 (2005).
9 Zhang, K., Zhang, H., Xua, G., Xiang, S., Xu, D., Liu, S., Li, H., “Alkylation of phenol with-butyl alcohol catalyzed by large pore zeolite”,..., 207 (1-2), 183-190 (2001).
10 Kulkarni, S.J., Madhavi, G., Ramachander Rao, A., Krishna Mohan, K.V.V., “Side-chain alkylation of acetophenone with formaldehyde over alkali and alkaline earth metal ion modified basic zeolites”,.., 9 (4), 532-538 (2007).
11 Sato, T., Sekiguchi, G., Adschiri, T., Arai, K., “Non-catalytic and selective alkylation of phenol with propan-2-ol in supercritical water”,.., 1 (17), 1566-1567 (2001).
12 Shen, H.Y., Judeh, Z.M.A., Ching, C.B., “Selective alkylation of phenol with-butyl alcohol catalyzed by [bmim]PF6”,., 44 (5), 981-983 (2003).
13 Gui, J., Ban, H., Cong, X., Zhang, X.T., Hu, Z.D., Sun, Z.L., “Selective alkylation of phenol with-butyl alcohol catalyzed by Brönsted acidic imidazolium salts”,...., 225 (1), 27-31 (2005).
14 Cole, A.C., Jensen, J.L., Ntai, I., Tran, K.L.T., Weaver, K.J., Forbes, D.C., Davis, J.H.Jr., “Novel Brønsted acidic ionic liquids and their use as dual solvent catalysts”,...., 124 (21), 5962-5963 (2002).
15 Gui, J., Cong, X., Liu, D., Zhang, X.T., Hu, Z.D., Sun, Z.L., “Novel Brønsted acidic ionic liquid as efficient and reusable catalyst system for esterification”,.., 5 (9), 473-477 (2004).
16 Liu, X.M, Zhou, J.X, Guo, X.W., Liu, M., Ma, X.L., Song, C.S., Wang, C., “SO3H-functionalized ionic liquids for selective alkylation of-cresol with-butanol”,..., 47 (15), 5298-5303 (2008).
17 Bonhote, P., Dias, A.P., Papageorgiou, N., Kalyanasundaram, K., Graetzel, M., “Hydrophobic, highly conductive ambient-temperature molten salts”,.., 35 (5), 1168-1178 (1996).
18 Devassy, B.M., Shanbhag, G.V., Lefebvre, F., Halligudi, S. B., “Alkylation of-cresol with-butanol catalyzed heteropoly acid supported on zirconia catalys”,..., 210 (1-2), 125-130 (2004).
19 Subramanian, S., Mitra, A., Satyanarayana, C.V.V., Chakrabarty, D.K., “-selective butylation of phenol over silicoaluminophosphate molecular sieve SAPO-11 catalyst”,.., 159 (1-2), 229-240 (1997).
20 DeCastro, C., Sauvage, E., Valkenberg, M.H., Holderich, W. F., “Immobilised ionic liquids as Lewis acid catalysts for the alkylation of aromatic compounds with dodecene”,.., 196 (1), 86-94 (2000).
21 Liu, X.M., Guo, X.W., Zhou, J.X., “SO3H-functionalized ionic liquids for selective alkylation of-cresol with-butanol”,.., 9 (1), 1-7 (2008).
22 Liu, X.M., Zhou, J.X., Guo, X.W., Liu, M., Ma, X.L., Song, C.S., Wang, C., “SO3H-functionalized ionic liquids for selective alkylation of-cresol with-butanol”,...., 47 (15), 5298-5303 (2008).
23 Yadav, G.D., Nirav, S.D., “Alkylation of phenol with methyl--butylether and-butanol over solid acids: Efficacies of clay-based catalysts”,.., 236 (1-2), 129-147 (2002).
24 Yadav, G.D., Pujari, A.A., Joshi, A.V., “Alkylation of-cresol with methyl-butyl ether (MTBE) over a novel solid acid catalyst UDCaT-1”,.., 1 (6), 269-274 (1999).
** To whom correspondence should be addressed. E-mail: jgyang@chem.ecnu.edu.cn
2010-03-04,
2010-11-13.
the National High Technology Research and Development Program of China (2006BAE03B06), Shanghai Leading Academic Discipline Project (B409), and Shanghai International Cooperation of Science and Technology Project (06SR07101).
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Dynamic Simulation and Analysis of Industrial Purified TerephthalicAcid Solvent Dehydration Process*
- Preparation of p-Hydroxybenzaldehyde by Hydrolysis of DiazoniumSalts Using Rotating Packed Bed*
- Liquid-solid Equilibria in Quinary System Na+, K+, Mg2+//Cl-, at 25 °C*
- Pervaporation Separation of Butanol-Water Mixtures UsingPolydimethylsiloxane/Ceramic Composite Membrane*
- Reaction Kinetics of Biodiesel Synthesis from Waste Oil Using a Carbon-based Solid Acid Catalyst
- Enzyme-catalyzed Synthesis of Vitamin E Succinate Using aChemically Modified Novozym-435*
