Growth and Physiological Features of Cyanobacterium Anabaena sp. Strain PCC 7120 in a Glucose-Mixotrophic Culture*
2011-05-15YUGuoce喻国策SHIDingji施定基CAIZhaoling蔡昭铃CONGWei丛威andOUYANGFan欧阳藩
YU Guoce (喻国策), SHI Dingji (施定基), CAI Zhaoling (蔡昭铃), CONG Wei (丛威) and OUYANG Fan (欧阳藩)
Growth and Physiological Features of Cyanobacteriumsp. Strain PCC 7120 in a Glucose-Mixotrophic Culture*
YU Guoce (喻国策)1,2,**, SHI Dingji (施定基)3, CAI Zhaoling (蔡昭铃)2, CONG Wei (丛威)2and OUYANG Fan (欧阳藩)2
1Institute of Nuclear and New Energy Technology, Tsinghua University, Beijing 100084, China2State Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100080, China3Research Center of Photosynthesis, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China
Mixotrophic growth is one potential mode for mass culture of microalgae and cyanobacteria particularly suitable for the production of high value bioactive compounds and fine chemicals. The typical heterocystous cyanobacteriumsp. PCC 7120 was grown in the presence of exogenous glucose in light. Glucose improved the cell growth evidently, the maximal specific growth rate under mixotrophic condition (0.38 d-1) being 1.6-fold of that of photoautotrophic growth. Mixotrophy caused a variation in cellular pigment composition, increasing the content of chlorophylland decreasing the contents of carotenoid and phycobiliprotein relative to chlorophyll. Fluorescence emission from photosystem II (PSII) relative to photosystem I was enhanced in mixotrophic cells, implying an increased energy distribution in PSII. Glucokinase (EC 2.7.1.2) activity was further induced in the presence of glucose. The mixotrophic culture was scaled up in a 15 L airlift photobioreactor equipped with an inner and an outer light source. A modified Monod model incorporating the specific growth rate and the average light intensity in the reactor was developed to describe cell growth appropriately. The understanding of mixotrophic growth and relevant physiological features ofsp. PCC 7120 would be meaningful for cultivation and exploitation of this important cyanobacterial strain.
cyanobacteria,sp. PCC 7120, growth features, mixotrophic culture, photobioreactor
1 INTRODUCTION
Mixotrophic culture is a potential mode for mass production of microalgae and cyanobacteria by using heterotrophic capability of the photosynthetic microorganisms [1-3]. It is expected that mixotrophic growth can achieve high cell densities and synthesize light-induced products such as photosynthetic pigments, and is especially suitable for the production of high value bioactive compounds, fine chemicals and pharmaceuticals. Some microalgae and cyanobacteria, which were regarded as obligate photoautotrophs, can utilize organic carbon compounds for their growth [4-6], and the potential of growth depends on the genetic background of the specific organism, the nature of the substrate as well as environmental factors. Much work has been conducted on mixotrophic and heterotrophic growth of the green algae[7-10],[11-13],[14, 15], and[16]. These work revealed the characteristics of mixotrophic growth and the advantage of mixotrophic growth over photoautotrophic growth for biomass production as well as the formation of some specific products in some sense.
Though some strains of cyanobacteria can use various sources of fixed carbon [17, 18], studies on mixotrophic and heterotrophic growth of cyanobacteria are limited and little information is available regarding the physiological features of cyanobacterial mixotrophy. The existing work has been focused on mixotrophic culture of[1, 3, 19-21], with few other cyanobacterial strains studied [22, 23]. Mixotrophic growth of filamentous heterocystous cyanobacteria such as, one important category of cyanobacteria, has rarely been reported [24-26]. It was reported that the production of biomass and photosynthetic pigments during mixotrophic growth of.was increased by 1.5- to 2.0-fold compared with that in photoautotrophic culture [1], and a cell density as high as 10.24 g·L-1was achieved in fed-batch mixotrophic culture of.[3]. During mixotrophic culture photosynthetic cells utilize light and organic carbon concurrently as energy sources, and CO2and the organic substrate as carbon sources. The simultaneous involvement of photoautotrophy and heterotrophy may give rise to a relatively more complicated response of cellular physiology. While Marquez. [19] suggested the independence of photosynthetic activity and heterotrophic metabolism of.in mixotrophic culture based on the growth performance in different nutritional modes, it was demonstrated that mixotrophic growth had a significant effect on photosynthetic activity of cyanobacteria [26-28]. A deep understanding of growth characteristics and intrinsic physiological features of cyanobacteria in mixotrophic culture would be constructive for the regulation and optimization of the culture technology.
sp. strain PCC 7120 is a typical filamentous heterocystous cyanobacterium, which has been adopted as a model species for investigation of cyanobacterial physiology and molecular biology [29, 30], and its entire genome sequence has been determined [31].sp. PCC 7120 can be cultured for the production of a variety of fine chemicals and bioactive compounds, such as sulfolipid [32], pigments and exopolysaccharides[33]. Furthermore, sincesp. PCC 7120 is used as a conventional host strain in cyanobacterial genetic engineering, it is very promising to produce high value transgenic proteins through cultivation of this cyanobacterial strain [34, 35]. So far, few studies have been reported on the culture ofsp. PCC 7120 [32, 33], and the potential of mixotrophic growth of the cyanobacterium has rarely been explored to our knowledge.
In our preliminary worksp. PCC 7120 was found to be able to grow on glucose in light and not in darkness, indicating its mixotrophic but not heterotrophic capability. The current work presents some characteristic features of mixotrophic growth ofsp. PCC 7120 with glucose as the sole exogenous carbon substrate. Scale up of mixotrophic culture in a new type airlift photobioreactor and relevant kinetic analysis of mixotrophic growth are also carried out.
2 MATERIALS AND METHODS
2.1 Strain and medium
The cyanobacteriumsp. strain PCC 7120 was obtained from the Research Center of Photosynthesis, Institute of Botany, Chinese Academy of Sciences, Beijing. The basal medium BG-11 [36] was adopted throughout the study, in which sodium chloride was substituted for sodium nitrate unless otherwise indicated and the buffer Tris-HCl (pH 7.5) was used. Glucose was added to give a required initial concentration as an exogenous carbon source in the medium.
2.2 Shake flask culture
sp. PCC 7120 was maintained on plates of BG-11 medium, solidified with mass concentration of 1.5% agar powder. Cells were subcultured in a 50 ml Erlenmeyer flask containing 20 ml BG-11 medium at 30°C, 130 r·min-1and a light intensity of 160mE·m-2·s-1, and then transferred to a 250 ml Erlenmeyer flask containing 130 ml medium. After 5-day cultivation, 20 ml cell suspension was inoculated to 250 ml Erlenmeyer flasks, each containing 130 ml medium with glucose concentration arranged as indicated. The irradiance was provided with five 18 W fluorescent lamps (PHILIPS TLD 18W/54). Continuous illumination with a fixed intensity of 160mE·m-2·s-1was guaranteed throughout all cultures. A sample of 5 ml was taken aseptically at a fixed time every three days. Experiments were performed in duplicate for each case to be examined. Results were expressed as the mean values, and relative standard deviations were less than 10%.
2.3 Photobioreactor culture
The photobioreactor system consisted of a 1.8 L bubble column photobioreactor (BCR, 1.5 L working volume) and a 15 L airlift photobioreactor (ALR, 11 L working volume) (Fig. 1). With a ratio of illuminating area to volume of 33.3 m-1, the BCR was irradiated with four 18 W fluorescent lamps giving the maximum surface light intensity of 624mE·m-2·s-1and the minimum of 110mE·m-2·s-1. The ALR (reactor height 1 m, draft tube height 0.6 m, ratio of the cross-sectional area of the riser to the downcomer 0.75) was characterized by the outer and inner light source, possessing a ratio of illuminating area to volume of 33.2 m-1. Sixteen 30 W fluorescent lamps (PHILIPS TLD 30W/54) were used as the outer light source, providing an average light intensity of 339.1mE·m-2·s-1on the outer surface, and one 30 W fluorescent lamp as the inner light source, giving an average light intensity of 228.5mE·m-2·s-1on the inner surface.
The BCR was inoculated with pure culture grown in shake flasks and operated at 30°C and air aeration of 0.178 vvm. A four-day BCR photoautotrophic culture was pressed into the ALR aseptically and adopted as the inoculum (13%) for the ALR cultivation. The ALR was operated at 30°C and an aeration rate (air containing 3% CO2) of 0.227 vvm. Nitrogenous BG-11 medium was used in photobioreactor cultures and a glucose concentration of 3 g·L-1was used in mixotrophic culture in the ALR. The BCR was sterilized in an autoclave and the ALR by steam in situ prior to cultivation.
2.4 Analytical methods

Samples were centrifuged at 4000 r·min-1for 20 min with the harvested supernatant analyzed for glucose. Glucose was determined by using the Fehling method or a glucose analyzer (YSI MODEL 2700 SELECT, Yellow Springs Instruments, USA).
Light intensity (photon flux density) was measured by using a photosynthetically active radiometer (FGH-1, Factory of Photoelectric Instruments of Beijing Normal University, Beijing, China).
Absorption spectra at room temperature (25°C) were measured by using a dual wavelength double-beam recording spectrophotometer (UV3000, Shimadzu, Japan). Fluorescence emission spectra at 77 K were measured by using a fluorescence spectrophotometer (F-4500, Hitachi, Japan). Two exciting light wavelengths of 436 nm and 580 nm were adopted.

Figure 1 Schematic diagram of the photobioreactor system
Glucokinase (EC 2.7.1.2) activity was measured according to Slein. [38] and Pearce and Carr [39]. Protein concentration was determined by the modified Lowry method [40]. Exponentially growing cells were washed with deionized water, suspended in 0.1 mol·L-1potassium phosphate buffer (pH 7.0) and broken by ultrasonic distintegration in ice bath. The resultant suspension was centrifuged at 9000 r·min-1for 10 min at 2°C yielding the supernatant for enzyme and protein assay. Analysis was done in triplicate, and results were expressed as means of three determinations±standard deviations.
The attenuation of light in cultures was determined as described by Hirata. [41] with an incident light intensity of 234.0mE·m-2·s-1.
2.5 Average light intensity in the airlift photobioreactor
The following assumptions are made for the estimation of the average light intensity in the ALR: (1) the incident light intensity on the reactor surface is uniform; (2) there is no axial distribution of light intensity in the reactor; and (3) the attenuation of light obeys the Lambert-Beer law. Light intensity can thus be taken as a function of cell density and radial position in the photobioreactor.
According to the Lambert-Beer law, the light intensity yielded by one beam from the outer light source at radial positioncan be expressed as



where1is the radius of the tube containing the inner light source.
Similarly, the whole inner light source gives the light intensity at radial position

Thus, the light intensity at radial positionis

The average light intensity in the ALR can be obtained as follows.

The average light intensities at different cell densities can be derived through numerical integration by using the Mathematica software.
3 RESULTS AND DISCUSSION
3.1 Mixotrophic growth on glucose
Mixotrophic growth and photoautotrophic growth ofsp. PCC 7120 are presented in Fig. 2. The presence of exogenous glucose improves the cell growth evidently. The maximal cell density in mixotrophic growth achieves 3.1 g·L-1at a glucose concentration of 18 g·L-1, being 4.5-fold of that under photoautotrophic condition [Fig. 2 (a)]. The specific growth rate increases with glucose concentration until 18 g·L-1, peaking at 0.38 d-1which is 1.6-fold of that of photoautotrophic growth. The chlorophyll contents in mixotrophic cells at different glucose concentrations are generally higher than those in photoautotrophic cells throughout the cultures [Fig. 2 (b)]. Glucose is consumed to a limited extent [Fig. 2 (c)], the specific glucose consumption rate being 0.52 d-1with glucose of 18 g·L-1.

Figure 2 Mixotrophic growth ofsp. PCC 7120 on glucose at a light intensity of 160mE·m-2·s-1
The improvement of mixotrophic growth ofsp. PCC 7120 may be from the contribution of exogenous glucose assimilated as a carbon source and also possibly as an energy source at a relatively low light intensity. It has been reported that fructose is taken up and utilized infor mixotrophic growth [24, 27] and is able to enhance the development and growth ofin mixotrophic culture [25, 26], demonstrating the potential of mixotrophy in.sp. PCC 7120 did not grow on glucose in darkness in our experiments, which implies that the utilization of glucose requires the participation of light, and the weak light due to the shading effect at high cell density may also impede mixotrophic growth. The poor performance in glucose uptake may be related to the nature of photoautotrophy of the cyanobacterium, in which the tricarboxylic acid cycle is incomplete and glucose is metabolized mainlypentose phosphate pathway. The higher chlorophyll content in mixotrophic cells suggests that the positive effect of the exogenous carbon source on the chlorophyll formation may be due to its supply of carbon skeleton molecules for biosynthesis and the enhancement of cellular photosynthetic activity in mixotrophic growth.

Table 1 Relative contents of pigments in photoautotrophicand mixotrophic Anabaena sp. PCC 7120 cells
Note: Data are calculated from Fig. 3. The relative content Carotenoid/Chlorophyllis represented by A495/A680and Phycobiliprotein/Chlorophyllby A625/A680.

Figure 4 Fluorescence emission spectra of photoautotrophic and mixotrophicsp. PCC 7120 cells at 77 K


Figure 3 Absorption spectra of photoautotrophic and mixotrophicsp. PCC 7120 cells at room temperature

3.2 Absorption spectra at room temperature
Absorption spectra ofsp. PCC 7120 cells at room temperature show four peaks (Fig. 3), reflecting the existence of three kinds of pigments in cells, chlorophyll(435 and 680 nm), carotenoid (495 nm) and phycobiliprotein (625 nm). Table 1 gives the relative contents of these pigments represented by the ratios of maximum absorption of carotenoid and phycobiliprotein to that of chlorophyll. It is seen that the relative contents of carotenoid and phycobiliprotein in mixotrophic cells are lower than those in cells growing photoautotrophically. While mixotrophy is advantageous for chlorophyll synthesis, it decreases the formation of photosynthetic accessory pigments relative to chlorophyll. This is interesting and may be related to the physiological regulation of pigment synthesis exerted by photosynthesis and glucose metabolism simultaneously in mixotrophic growth.
3.3 Fluorescence emission spectra at 77 K
Fluorescence emission spectra at 77 K reflect the flow and relative distribution of light energy between the two photosystems, the photosystem I (PSI) and the photosystem II (PSII), in cyanobacterial cells. The exciting light of 436 nm, which is absorbed by chlorophyll, causes two fluorescence peaks at 690 nm and 725 nm emitted by PSII and PSI, respectively [Fig. 4 (a)]. The exciting light of 580 nm, which is absorbed by phycobiliprotein, produces four fluorescence peaks at 645 nm, 660 nm, 690 nm and 725 nm emitted by phycoerythrin, phycocyanin, PSII and PSI, respectively [Fig. 4 (b)]. Table 2 shows the relative intensity of fluorescence emitted by photoautotrophic and mixotrophic cells represented by the ratios of the relevant peak values. It is clear that the relative fluorescence emission intensity of PSII to PSI is higher, and the relative intensities of phycoerythrin and phycocyanin to PSI are lower in mixotrophically growing cells than in photoautotrophically growing cells. In mixotrophic cells, PSI may be reduced by receiving electrons from glucose oxidation through some common electron carriers shared by respiratory and photosynthetic electron chains [42], and may not acquire all its energy from light. The light energy transport from PSII to PSI may be interfered in some way and the energy distributed in PSII relatively accumulates, increasing the emission of fluorescence from PSII. In the meantime, the greater amount of energy kept in PSII may imply an increased efficiency of photosynthetic activity. The lower fluorescence intensities of phycoerythrin and phycocyanin are consistent with the relatively reduced synthesis of phycobiliprotein in cells growing mixotrophically.

Table 3 Specific activity of glucokinase in Anabaena sp. PCC 7120 cells grown under photoautotrophic and mixotrophic conditions

Table 2 Relative intensity of fluorescence emitted by photoautotrophic and mixotrophic Anabaena sp. PCC 7120 cells when excited by 436 nm and 580 nm light
Note: Data are calculated from Fig. 4.
3.4 Glucokinase activity
Glucokinase activity was measured as an indicator of glucose metabolism, as it is the first key enzyme for glucose to be catabolized through glycolytic and pentose phosphate pathway. Table 3 shows the specific activities of glucokinase ofsp. PCC 7120 under photoautotrophic and mixotrophic conditions. It is found that the activities are comparable to that previously reported for[39]. The activity of glucokinase is higher under mixotrophic conditions, being 1.25- and 2.03-fold of that under photoautotrophic condition at glucose concentrations of 3 and 9 g·L-1, respectively. It was reported that glucokinase activity was significantly elevated in mixotrophic and heterotrophic cultures ofsp. PCC 6803 [43]. Sundaram. [44] noticed in their work that the activity of glucose-6-phosphate dehydrogenase insp. PCC 7120 was 6.4% higher in the presence of exogenous 10 mmol·L-1glucose than in photoautotrophic culture. The increased activities of glucokinase and glucose-6-phosphate dehydrogenase in mixotrophic culture are consistent with the utilization of glucose and the glucose-improved cell growth, indicating thatsp. PCC 7120 can regulate cellular metabolism of exogenous glucose at least at enzymatic levels.
3.5 Kinetics of cell growth in the photobioreactor
Photoautotrophic culture and mixotrophic culture ofsp. PCC 7120 in the airlift photobioreactor are depicted in Fig. 5. The growth profiles in two nutritional modes exhibit similarity. However, the specific growth rate is higher in mixotrophic culture, and the maximal cell density in mixotrophic growth (1.93 g·L-1) is 19% higher than that in photoautotrophic growth. The consumption of glucose coincides well with the rapid cell growth in mixotrophic process.

Figure 5 Photoautotrophic and mixotrophic (glucose 3 g·L-1) culture ofsp. PCC 7120 in the airlift photobioreactor
□ photoautotrophic growth;▲ mixotrophic growth;● glucose
In both photoautotrophic and mixotrophic cultures in the photobioreactor, light supply can be assumed to be the major factor to influence cell growth considering no growth in the absence of light, the supply of 3% CO2and the limited utilization of glucose. The Monod model can be adopted to relate the specific growth rate with the average light intensity in the reactor. Since no cell growth is observed with light intensity below a threshold in either culture, a constant0is introduced into the light intensity item of the model, which is considered as the threshold of the average light intensity necessary for cell growth and an overall display of compensation irradiance for cells. The modified form of the Monod model is thus presented as
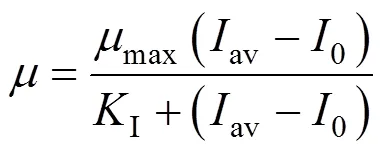
wheremaxis the maximum specific growth rate andIthe saturation constant.


Figure 6 Average light intensity in the airlift photobioreactor
The results by using the modified Monod model to fit photoautotrophic and mixotrophic growth in the photobioreactor (Fig. 5) are shown in Fig. 7. It is seen that the model gives an appropriate description of cell growth. Table 4 lists the estimated parameters of the kinetic model from the data of photoautotrophic and mixotrophic growth. The maximum specific growth rate of mixotrophic growth is greater than that of photoautotrophic growth, and the0value and the saturation constant for mixotrophic growth are lower than those for photoautotrophic growth. These results suggest that mixotrophic cells can start to grow at a lower average light intensity and achieve a higher specific growth rate when adopting the same light intensity as in photoautotrophic culture. That is,sp. PCC 7120 may depend to a lesser degree on light in mixotrophic growth and can realize a better cell growth compared with photoautotrophic culture due to the participation of glucose and possibly the more efficient use of light.

Figure 7 Photoautotrophic and mixotrophic growth ofsp. PCC 7120 in the airlift photobioreactor and their fitted results by the modified Monod model

Table 4 Comparison of estimated parameters of the modified Monod model from data of photoautotrophicand mixotrophic growth of Anabaena sp. PCC 7120 in the airlift photobioreactor
4 CONCLUSIONS
This paper reported some characteristic features of mixotrophic growth and physiology ofsp. PCC 7120 with glucose as the exogenous carbon substrate. The existence of glucose was able to improve cell growth evidently. Mixotrophy modified the composition of cellular photosynthetic pigments and the energy flow between two photosystems. The activity of glucose assimilation was further stimulated in mixotrophic growth. Cell growth in the airlift photobioreactor and relevant kinetic analysis revealed that mixotrophic culture can realize a more advantageous growth than photoautotrophic culture for this important cyanobacterial strain.
ACKNOWLEDGEMENTS
The authors wish to thank Miss Ning Shao andMiss Pengpeng Zhang for their
1 Marquez, F.J., Nishio, N., Nagai, S., Sasaki, K., “Enhancement of biomass and pigment production during growth ofin mixotrophic culture”,...., 62, 159-164 (1995).
2 Chen, F., “High cell density culture of microalgae in heterotrophic growth”,., 14, 421-426 (1996).
3 Chen, F., Zhang, Y., “High cell density mixotrophic culture ofon glucose for phycocyanin production using a fed-batch system”,.., 20, 221-224 (1997).
4 Droop, M.R., “Heterotrophy of carbon”, In: Algal Physiology and Biochemistry, University of California Press, Berkeley and Los Angeles, 530-559 (1974).
5 Smith, A.J., “Modes of cyanobacterial carbon metabolism”, In: The Biology of Cyanobacteria, Blackwell Scientific Publications, Oxford, London, Edinburgh, Boston and Melbourne, 47-85 (1982).
6 Tuchman, N.C., “The role of heterotrophy in algae”, In: Algal Ecology, Academic Press, San Diego, 299-319 (1996).
7 Lee, Y.K., Ding, S.Y., Hoe, C.H., Low, C.S., “Mixotrophic growth ofin outdoor enclosed photobioreactor”,..., 8, 163-169 (1996).
8 Martinez, M.E., Camacho, F., Jimenez, J.M., Espinola, J.B., “Influence of light intensity on the kinetic and yield parameters ofmixotrophic growth”,., 32, 93-98 (1997).
9 Ip, P.F., Wong, K.H., Chen, F., “Enhanced production of astaxanthin by the green microalgain mixotrophic culture”,., 39, 1761-1766 (2004).
10 Liang, Y., Sarkany, N., Cui, Y., “Biomass and lipid productivities ofunder autotrophic, heterotrophic and mixotrophic growth conditions”,.., 31, 1043-1049 (2009).
11 Kobayashi, M., Kakizono, T., Yamaguchi, K., Nishio, N., Nagai, S., “Growth and astaxanthin formation ofin heterotrophic and mixotrophic conditions”,..., 74, 17-20 (1992).
12 Zhang, X.W., Gong, X.D., Chen, F., “Kinetic models for astaxanthin production by high cell density mixotrophic culture of the microalga”,...., 23, 691-696 (1999).
13 Jeon, Y.C., Cho, C.W., Yun, Y.S., “Combined effects of light intensity and acetate concentration on the growth of unicellular microalga”,.., 39, 490-495 (2006).
14 Shamala, T.R., Drawert, F., Leupold, G., “Studies ongrowth. I. Effect of autotrophic and mixotrophic conditions on the growth of”,.., 24, 1287-1299 (1982).
15 Combres, C., Laliberte, G., Reyssac, J.S., Delanoue, J., “Effect of acetate on growth and ammonium uptake in the microalga”,.., 91, 729-734 (1994).
16 Xie, J., Zhang, Y., Li, Y., Wang, Y., “Mixotrophic cultivation of”,..., 13, 343-347 (2001).
17 Rippka, R., “Photoheterotrophy and chemoheterotrophy among unicellular blue-green algae”,.., 87, 93-98 (1972).
18 Stal, L.J., Moezelaar, R., “Fermentation in cyanobacteria”,.., 21, 179-211 (1997).
19 Marquez, F.J., Sasaki, K., Kakizono, T., Nishio, N., Nagai, S., “Growth characteristics ofin mixotrophic and heterotrophic conditions”,..., 76, 408-410 (1993).
20 Chen, F., Zhang, Y., Guo, S., “Growth and phycocyanin formation ofin photoheterotrophic culture”,.., 18, 603-608 (1996).
21 Chen, T., Zheng, W., Yang, F., Bai, Y., Wong, Y.S., “Mixotrophic culture of high selenium-enrichedon acetate and the enhanced production of photosynthetic pigments”,.., 39, 103-107 (2006).
22 Wang, Y., Li, Y., Shi, D., Shen, G., Ru, B., Zhang, S., “Characteristics of mixotrophic growth ofsp. in an enclosed photobioreactor”,., 24, 1593-1597 (2002).
23 Yu, H., Jia, S., Dai, Y., “Growth characteristics of the cyanobacteriumin photoautotrophic, mixotrophic and heterotrophic cultivation”,..., 21, 127-133 (2009).
24 Haury, J.F., Spiller, H., “Fructose uptake and influence on growth of and nitrogen fixation by”,.., 147, 227-235 (1981).
25 Rozen, A., Arad, H., Schonfeld, M., Tel-Or, E., “Fructose supports glycogen accumulation, heterocysts differentiation, N2fixation and growth of the isolated cyanobiont”,.., 145, 187-190 (1986).
26 Rozen, A., Schonfeld, M., Tel-Or, E., “Fructose-enhanced development and growth of the N2-fixing cyanobiont”,.., 43c, 408-412 (1988).
27 Valiente, E.F., Nieva, M., Avendano, M.C., Maeso, E.S., “Uptake and utilization of fructose byATCC 29413. Effect on respiration and photosynthesis”,., 33, 307-313 (1992).
28 Vonshak, A., Cheung, S.M., Chen, F., “Mixotrophic growth modifies the response of()(cyanobacteria) cells to light”,.., 36, 675-679 (2000).
29 Rippka, R., Deruelles, J., Waterbury, J.B., Herdman, M., Stanier, R.Y., “Generic assignments, strain histories and properties of pure cultures of cyanobacteria”,..., 111, 1-61 (1979).
30 Bryant, D.A., The Molecular Biology of Cyanobacteria, Kluwer Academic Publishers, The Netherlands (1994).
31 Kaneko, T., Nakamura, Y., Wolk, C.P., Kuritz, T., Sasamoto, S., Watanabe, A., Iriguchi, M., Ishikawa, A., Kawashima, K., Kimura, T., Kishida, Y., Kohara, M., Matsumoto, M., Matsuno, A., Muraki, A., Nakazaki, N., Shimpo, S., Sugimoto, M., Takazawa, M., Yamada, M., Yasuda, M., Tabata, S., “Complete genomic sequence of the filamentous nitrogen-fixing cyanobacteriumsp. strain PCC 7120”,., 8, 227-253 (2001).
32 Archer, S.D., McDonald, K.A., Jackman, A.P., “Effect of light irradiance on the production of sulfolipids from7120 in a fed-batch photobioreactor”,..., 67, 139-152 (1997).
33 Morales, E., Rodriguez, M., Garcia, D., Loreto, C., Marco, E., “Effect of pH and CO2on growth, pigments and exopolysaccharides production from cyanobacteriasp. PCC 7120”,, 27, 373-378 (2002).
34 Ren, L., Shi, D.J., Dai, J.X., Ru, B.G., “Expression of the mouse metallothionein-I gene conferring cadmium resistance in a transgenic cyanobacterium”,.., 158, 127-132 (1998).
35 Liu, F.L., Zhang, H.B., Shi, D.J., Shang, Z.D., Lin, C., Shao, N., Peng, G.H., Zhang, X.Y., Zhang, H.X., Wu, J.Y., Wang, J., Xu, X.D., Jiang, Y.H., Zhong, Z.P., Zhao, S.J., Wu, M., Zeng, C.K., “Construction of shuttle, expression vector of human tumor necrosis factor alpha (hTNF-a) gene and its expression in a cyanobacterium,sp. PCC7120”,., 42, 25-33 (1999).
36 Castenholz, R.W., “Culturing methods for cyanobacteria”, In: Methods in Enzymology, Vol. 167, Cyanobacteria, Academic Press, San Diego, 68-93 (1988).
37 Williams, J.G.K., “Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in6803”, In: Methods in Enzymology, Vol. 167, Cyanobacteria, Academic Press, San Diego, 766-778 (1988).
38 Slein, M.W., Cori, G.T., Cori, C.F., “A comparative study of hexokinase from yeast and animal tissues”,..., 186, 763-780 (1950).
39 Pearce, J., Carr, N.G., “The incorporation and metabolism of glucose byvariabilis”,..., 54, 451-462 (1969).
40 Markwell, M.A.K., Haas, S.M., Bieber, L.L., Tolbert, N.E., “A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples”,.., 87, 206-210 (1978).
41 Hirata, S., Taya, M., Tone, S., “Characterization ofcell cultures in batch and continuous operations under a photoautotrophic condition”,..., 29, 953-959 (1996).
42 Scherer, S., “Do photosynthetic and respiratory electron transport chains share redox proteins”,.., 15, 458-462 (1990).
43 Knowles, V.L., Plaxton, W.C., “From genome to enzyme: analysis of key glycolytic and oxidative pentose-phosphate pathway enzymes in the cyanobacteriumsp. PCC 6803”,., 44, 758-763 (2003).
44 Sundaram, S., Karakaya, H., Scanlan, D.J., Mann, N.H., “Multiple oligomeric forms of glucose-6-phosphate dehydrogenase in cyanobacteria and the role of OpcA in the assembly process”,, 144, 1549-1556 (1998).
** To whom correspondence should be addressed. E-mail: yugc@tsinghua.edu.cn
2010-04-06,
2010-09-18.
a grant from the State Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences.
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Dynamic Simulation and Analysis of Industrial Purified TerephthalicAcid Solvent Dehydration Process*
- Preparation of p-Hydroxybenzaldehyde by Hydrolysis of DiazoniumSalts Using Rotating Packed Bed*
- Liquid-solid Equilibria in Quinary System Na+, K+, Mg2+//Cl-, at 25 °C*
- Pervaporation Separation of Butanol-Water Mixtures UsingPolydimethylsiloxane/Ceramic Composite Membrane*
- Reaction Kinetics of Biodiesel Synthesis from Waste Oil Using a Carbon-based Solid Acid Catalyst
- Enzyme-catalyzed Synthesis of Vitamin E Succinate Using aChemically Modified Novozym-435*
