Protective effect of paeonol on human umbilical vein endothelial cells injured by hyperlipidemic serum
2011-01-16NIUChengweiZHOUXiaohuiZHANGJinhuanXUQianCAOKai
NIU Cheng-wei,ZHOU Xiao-hui,ZHANG Jin-huan,XU Qian,CAO Kai
(1.Department of Biochemistry,2.Affiliated Hospital,Chengde Medical College,Chengde067000,China)
Protective effect of paeonol on human umbilical vein endothelial cells injured by hyperlipidemic serum
NIU Cheng-wei1,ZHOU Xiao-hui1,ZHANG Jin-huan2,XU Qian1,CAO Kai1
(1.Department of Biochemistry,2.Affiliated Hospital,Chengde Medical College,Chengde067000,China)
OBJECTIVETo investigate protective effect of paeonol(Pae)on human umbilical vein endothelial cells(HUVECs)injured by hyperlipidemic serum and explore its possible mechanism.METHODSThe injury model was induced by 20%hyperlipidemic serum incubating HUVECs for 24 h.Pae 124,247 and 495 μmol·L-1were given followed by administration of hyperlipidemic serum for 24 h.The morphological changes were observed under inverted microscope,cell survival rate was evaluated by MTT method,the nitric oxide(NO)content was measured by nitric acid reductase method and the endothelial nitric oxide synthase(eNOS)mRNA expression was determined by RT-PCR.RESULTSCompared with normal contorl group,most cells in model group split and exfoliated.However,the morphology was tending to normal level after intervention with Pae.Pae significantly improved cell viability(P<0.01).Compared with model group,the survival rate increased from(53.0±10.1)%to(68.4±9.1)%,(84.5±6.7)%and(98.1±7.5)%,respectively.The NO content and eNOS mRNA expression both increased greatly in Pae groups(P<0.01).Compared with model group,content of NO increased from 54±4 to 79±6,115±5 and(136±6)μmol·L-1,respectively.The expression level of eNOS mRNA improved from 0.215±0.060 to 0.451±0.045,0.563±0.013,0.704±0.068,respectively.CONCLUSIONPae exerts protective effect on HUVECs injured by hyperlipidemic serum by increasing eNOS mRNA expression,which might therefore improve the content of NO.
paeonol;endothelial cells;nitric oxide;nitric oxide synthase
Paeonol(Pae),the main active ingredient of Cortex Moutan,is now used as analgesic and antipyretic.There is increasing evidence that Pae hasa wide range of pharmacological effects,such as those on the cardiovascular system,and diabetes,or the anti-bacterial,antiallergic and antitumor effect.It was reported that Pae shows a significant role against AS in the cardiovascular system[4].It was also shown that Pae can regulate lipid metabolism and scavenge oxygen free radicals[5].Furthermore,Pae could protect endothelial cells and inhibit abnormalproliferationofsmoothmuscle cells[6-7].However,few published data are available on protective effects of Pae on hyperlipidemic serum-induced human umbilical vein endothelial cells(HUVECs).To accelerate the re-search in this field,further exploration is needed.
1 MATERIALS AND METHODS
1.1 Reagents and animals
Pae injection(a monomer,24 mmol·L-1,Certificated№20080703)was purchased from Ningbo Tianzhen Company.Nitric oxide(NO)kit was purchased from Biological Engineering Institute of Nanjing Jiancheng.Trizol was purchased from Invitrogen and RT-PCR kit from TaKaRa Biotechnology(Dalian,China).Primers were designed and synthesized by Sangon.Basic fibroblast growth factor(bFGF)was purchased from Sigma Aldrich(USA).
New born healthy umbilical cord was provided by the Affiliated Hospital of Chengde Medical College.
1.2 Cell culture
According to Jaffe et al[8]method,umbilical cord was obtained under sterile conditions and put into sterile DMEM.PBS,which was preheated to around 37℃,was drawn to wash umbilical vein till it was colorless.Umbilical vein was incubated with the complex enzyme of 0.1%collagenaseⅠ,0.25%trypsin,and 0.02%EDTA at 37℃for 13 min.During digestion fingers were used to knead the umbilical vein to make the enzyme solution fully accessible to vascular wall.The trypsin was inactivated with DMEM supplemented with 20%fetal calf serum(FCS).The umbilical vein was further flushed with PBS and the fluid was collected.The resulting cell suspension was diluted in DMEM which was supplemented with bFGF 20 μg·L-1,20%FCS calt and plated onto the 25 cm2cell culture flask.After forming a monolayer,HUVECs were passaged using 0.125%trypsin and 0.01%EDTA.The cultured cells were identified by morphological observation and immunohistochemistry detecting factor Ⅷ.
1.3 Preparation of hyperlipidemic serum
Twelve healthy rabbits were randomly divided into 2 groups:normal control group and model group.Rabbits in normal control group were fed with ordinary forage,the model group with high fat forage,the ingredients of high fat forage were 1%cholesterol,5%egg yolk,10%lard and 84%common feedstuff[9-10]each with 100 g in morning and with ordinary forage at other times.All animals had free access to food and water in their home cages and were sustained for 10 weeks.The blood was obtained under sterile conditions and stored at 4℃overnight.Supernatant was drawn after centrifugation at the speed of 4000×g for 15 min to prepare serum,part of which was used to measure total cholesterol(TC),triglycerides(TG)and low density lipoprotein cholesterol(LDL-C)and results were shown in Tab.1 .In this way,hyperlipidemic serum was prepared.
T
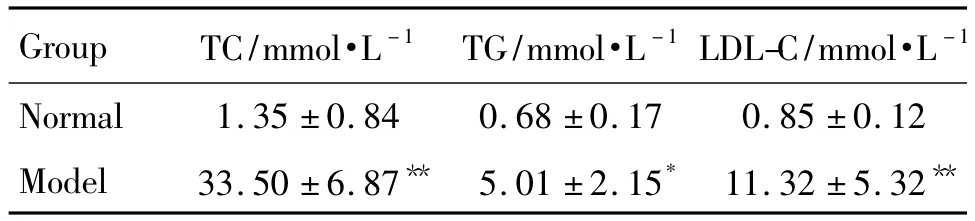
ab.1Blood lipid of rabbits fed with high fat forage
1.4 Cell grouping
HUVECswererandomlydividedinto normal control group,model group and Pae 124,247 and 495 μmol·L-1groups.The cells in normal control group were cultivated in DMEM with 20%(V/V)blank serum while the others were cultivated in DMEM with 20%(V/V)hyperlipidemic serum for 24 h,and then Pae was added for another 24 h.
1.5 Morphological observation
After intervention with Pae,HUVECs were washed with PBS and the morphological changes of HUVECs were observed in each group respectively with inverted microscope.
1.6 MTT assay for cell viability detection
In MTT assay for cell viability detection[11],HUVECs were seeded in 96-well plates at the density of 1.0×108L,each well 200 μl.When the cells reached 80%confluence,the experimental groups were divided according to the above,each group with the same well of six.After 24 h,each well was added with 20 μl MTT 5 g·L-1,then plates were incubated for 4 h.The supernatant was discarded and water-insoluble formazan was dissolved by adding 150 μl DMSO to each well.Finally,absorbance(A)was monitored at a wavelength of 490 nm.The survival rate was calculated as follows:survival rate(%)=APaeonol/ANormal×100%.
1.7 Nitric acid reductase method for NO content detection
The experiment was conducted according to the kit instructions.The concentration was determined by chromogenic assay,and the results were calculated by the following formula:NO contents=(A1-A3)÷(A2-A3)×c×n,where A1,A2 and A3 are the absorbances of the samples,standard and blank,respectively.c is the standard concentration and n is the diluted times of sample before the test.
1.8 Reverse transcription polymerase chain reaction for endothelial nitric oxide synthase(eNOS)mRNA expression
Total RNA was isolated from cells using the guanidinium thiocyanate phenol-chloroform method[12]and dissolved in diethyl pyrocarbonate(DEPC)water.RNA concentration and purity were measured with ultraviolet-visible spectrophotometer.In our experiment A260nm/A280nmof RNA samples ranged from 1.8 to 2.2,it showed that purity of RNA could meet the requirements for further experiment.
The preparation of cDNA 1 μg RNA was transcribed into cDNA:using random priming.The experiment was strictly conducted according to instructions.It was reverse-transcripted as following program:30℃10 min,42℃30 min,99℃5 min and 5℃5 min.The products were stored at-80℃.
eNOS Gene(PCR product is 348 bp in length):sense,5'-CCAGCATCCCTACTCCCACCAG-3',antisense,5'-CACCTCGGCTTCCACCTCTTG-3';β-actin gene(PCR product is 453 bp in length):sense,5'-AGCGGGAAATCGTGCGTGAC-3';antisense,5'-ACATCTGCTGGAAGGTGGAC-3'.
PCR reaction system included 4 μl RT product,4 μl 5×PCR buffer,0.2 μl primer,0.2 μl downstream primer,0.1 μl TaKaRa Ex Taq®HS,11.5 μl sterile water.
Pre-denaturation:94℃for 2 min;denaturation:94℃for 30 s,annealing temperature:63℃(eNOS)for 30 s,58℃(β-actin)for 30 s,extending temperature:72℃for 1 min.It was conducted for 30 cycles.
An aliquot of each reaction mixture was subjected to electrophoresis on a 2%agarose gel in 1×Tris-acetate-EDTA(TAE)buffer.PCR products were visualized by ethidium bromide staining and afterward photographed.eNOS mRNA band was normalized with band of relative internal reference β-actin mRNA.Relative intensity of band was analyzed by Quantity One and expressed as the ratio to β-actin mRNA band.
1.9 Statistical analysis
All results were represented asx¯±s and conducted with SPSS 13.0 statistical software.The comparison of multiple means was made with analysis of variance and the two-two comparisons between the means were done by LSD method.P<0.05 was considered statistically significant.
2 RESULTS
2.1 Effect of paeonol on morphological changes of HUVECs injured by hyperlipidemic serum
The cells were oval in shape and the border was clear in normal control group(Fig.1 A).In model group,most cells shaped like a lobe and exfoliated(Fig.1 B).In Pae 124 μmol·L-1group,part of cells became round and dilated space still occurred among cells(Fig.1 C).In Pae 247 μmol·L-1group,dilated intercellular space narrowed down between cells and the morphology was tending to normal(Fig.1 D).Cells in Pae 495 μmol·L-1group were in good state and like normal cells(Fig.1 E).
2.2 Effect of paeonol on HUVECs viability injured by hyperlipidemic serum
As shown in Tab.2 ,cell viability was assessed through the quantification of survival rate.Compared with normal controlgroup,
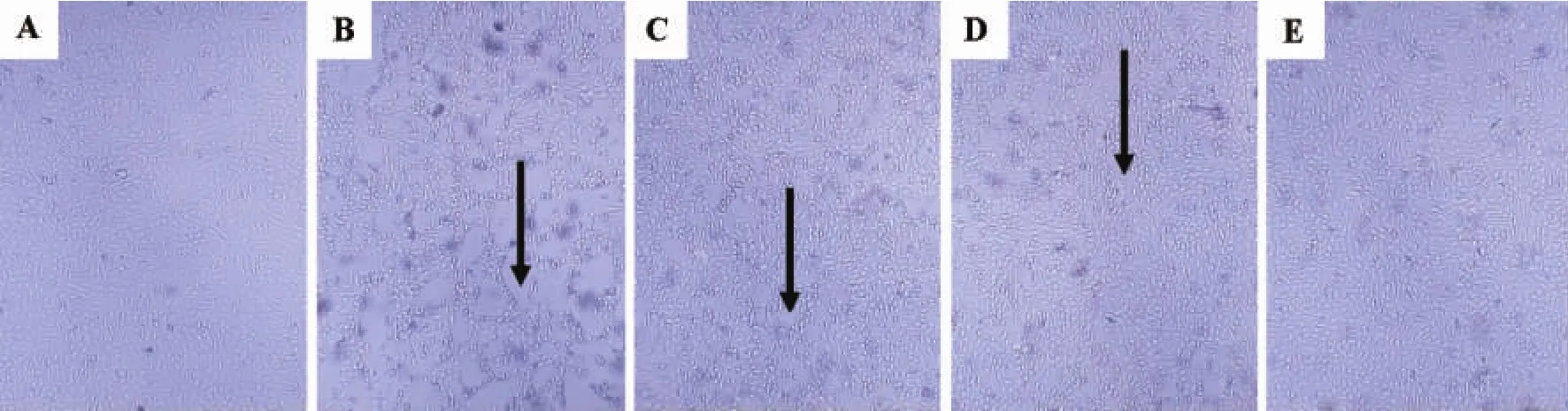
Fig.1 Effect of paeonol(Pae)on morphological changes in HUVECs injured by hyperlipidemic serum observed by inverted microscope(×40).HUVECs were induced with 20%hyperlipidemic serum for 24h,and then paeonol 124,247 and 495 μmol·L-1was added for another 24 h.A:normal control;B:model;C,D and E:Pae 124,247 and 495 μmol·L-1,respectively.Arrows show dilated intercellular space.
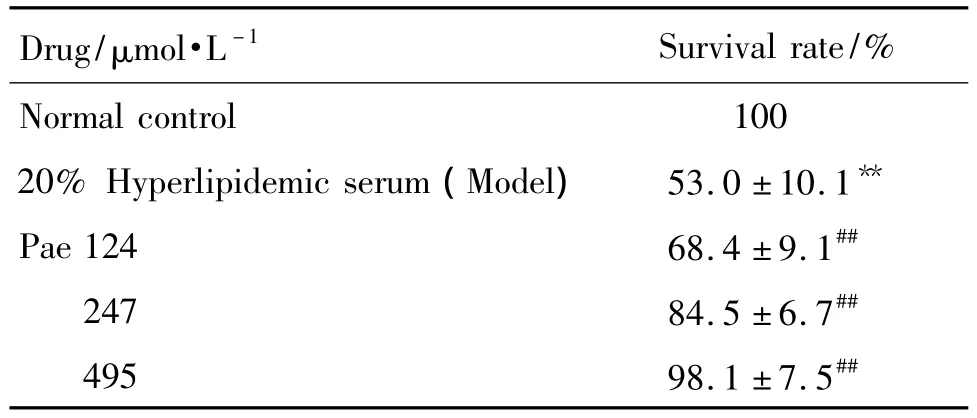
Tab.2 Effect of Pae on HUVECs viability injury by hyperlipidemic serum
treatment with 20%hyperlipidemic serum resulted in an obvious reduction of survival rate(P<0.01).Compared with model group,intervention with Pae of different concentrations significantly attenuated hyperlipidemic serum-induced survival rate loss and it increased to(68.4±9.1)%,(84.5±6.7)%and(98.1±7.5)%,respectively(P<0.01).The results demonstrated that Pae showed protective effects on hyperlipidemic serum-induced HUVECs.
2.3 Effect of Pae on NO production in HUVECs injured by hyperlipidemic serum
As shown in Tab.3 ,the production of NO in the supernatant was determined using Griess reagent.Compared with normal control group,20%hyperlipidemic serum resulted in the remarkable reduction of NO content(P<0.01),whereas Pae 124,247 and 495 μmol·L-1attenuated the hyperlipidemic serum induced decreases of NO production(P<0.01).
2.4 Effect of Pae on eNOS mRNA expression in HUVECs injured by hyperlipidemic serum
As shown in Fig.2 ,compared with normal

Tab.3 Effect of Pae on NO production in HUVECs injured by hyperlipidemic serum
control group,the expression of eNOS mRNA was decreased in hyperlipidemic serum(P<0.01).Pae markedly increased the expression of eNOS mRNA regardless of the stimulus(P<0.01).
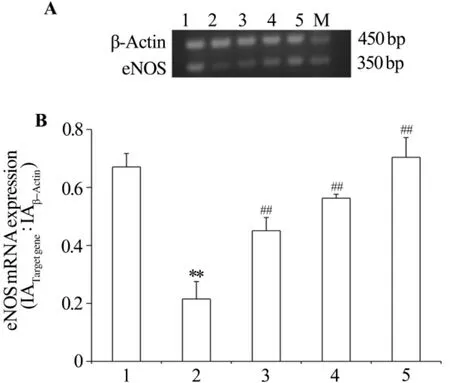
Fig.2 Effect of Pae on the expression of eNOS mRNA in HUVECs injured by hyperlipidemic serum.See Fig.1 for the treatment.A:RT-PCR result;B:semiquantitative result.M:marker;1:normal control group;2:model group;3-5:Pae 124,247 and 495 μmol·L-1groups,respectively.±s,n=6.**P<0.01,compared with normal control group;##P<0.01,compared with model group.
3 DISCUSSION
NO is the key endothelium-derived relaxing factor that plays a pivotal role in the maintenance of vascular tone and reactivity[13].It acts to limit cytokine expression and smooth muscle cell migration.In addition,NO helps to inhibit the conglomeration of blood platelets and the adhesion of monocytes to endothelial cells[14-16].Decrease in eNOS activity may cause deficiency of NO,which further lead to the occurrence of AS[17].Therefore,regulating eNOS expression and enhancing NO secretion will be important ways for controlling AS[18-20].
A typical model of endothelial cell damage is hyperlipidemic serum-induced damage.Using this model helps study pathogenesis of cardiovascular diseases.Hyperlipidemic serum results in the formation of ROS[21].ROS has been linked to aging,neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease,cancer and vascular disease.In the vasculature,oxidative stress is associated with metabolic alterations and results in disorder of eNOS expression.It has been reported that an increased ROS level in endothelial cells inhibits the expression of eNOS,which is one of the reasons patients use AS[22-23].
In this study,Pae attenuated hyperlipidemic serum-induced injury,as was evident by improvement in cell morphology,increase in cell survival rate and NO content in culture medium of HUVECs.Those results suggested that protection of endothelial cells from high lipid diet injury be one of the essential mechanisms by which Pae exerted its anti-atherosclerotic effect.
The results showed that eNOS mRNA significantly increased with the incubation of Pae,suggesting that increase of NO production be attributed to the upregulation of eNOS mRNA expression.Based on the antioxidant properties of Pae,a hypothesis is formulated that Pae acts as a scavenger of ROS[24].Pae decreased ROS induced by hyperlipidemic serum and therefore the expression of eNOS mRNA was up-regulated.Consequently,NO content increased.
In brief,our data provided clear evidence for the protective effect of Pae,which was related to improvement in cell morphology,increase in cell survival rate and NO content in culture medium of HUVECs.The primary mechanism might be associated with improving the expression of eNOS mRNA.These results provided molecular support to the idea that Pae has protective effect on HUVECs injured by hyperlipidemic serum.However,further study is needed to monitor the expression of eNOS protein in HUVECs.
[1]Schächinger V,Zeiher AM.Prognostic implications of endothelial dysfunction:does it mean anything[J]?Coron Artery Dis,2001,12(6):435-443.
[2]Levine GN,Keaney JF Jr,Vita JA.Cholesterol reduction in cardiovascular disease.Clinical benefits and possible mechanisms[J].N Engl J Med,1995,332(8):512-521.
[3]Sirtori CR,Calabresi L,Marchioli R,Rubins HB.Cardiovascular risk changes after lipid lowering medications:are they predictable[J]?Atherosclerosis,2000,152(1):1-8.
[4]Guo Q,Li YK,Wang ZG,Zhang JY,Li LD.The pharmacological progress of paeonol[J].Inf Tradit Chin Med(中医药信息),2009,26(1):20-22.
[5]Zhao X,Xu XJ,Li B,Yang J.The protective effects of paeonol on hyperlipidemic rats[J].J Chin Med Mater(中药材),2010,33(8):1312-1314.
[6]Li XL,Dai M,Chen P.The protective effects and adhesive function of Paeonol on rat arterial endothelial cells induced by hyperlipidemic serum[J].J Anhui TCM College(安徽中医学院学报),2010,29(1):53-57.
[7]Dai M,Li KF.The effects of paeonol to TNF-α content in atherosclerotic rabbits and proliferation of vascular smooth muscle cell induced by TNF-α[J].Chin J Tradit Med Technol(中国中医药科技),2007,14(1):31-32.
[8]Jaffe EA,Nachman RL,Becker CG,Minick CR.Culture of human endothelial cells derived from umbilical veins.Identification by morphologic and immunologic criteria[J].J Clin Invest,1973,52(11):2745-2756.
[9]Ma Y.The effects of valsartan on hyperlipidemic rabbits[J].Pharmaceutical Res,2008,17(15):14-15.
[10]Wang CB,Gao DZ.Expression of NF-κB,VCAM-1 and VEGF in aorta of hyperlipidemic rabbits and influence by fluvastatin[J].Chin J Cardiovasc Rev(中国心血管病研究),2008,6(5):379-382.
[11]Jiang N,Guo YL,Chen HB.Interventional effect of phycocyanin on mitochondrial member potential and activity of PC12 cells after hypoxia/reoxygenation[J].Neural Regen Res(Eng),2006,1(2):137-139.
[12]Chomczynski P,Sacchi N.Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction[J].Anal Biochem,1987,162(1):156-159.
[13]Furchgott RF,Zawadzki JV.The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine[J].Nature,1980,288(5789):373-376.
[14]De Caterina R,Libby P,Peng HB,Thannickal VJ,Rajavashisth TB,Gimbrone MA Jr,et al.Nitric oxide decreases cytokine-induced endothelial activation.Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines[J].J Clin Invest,1995,96(1):60-68.
[15]Behrendt D,Ganz P.Endothelial function.From vascular biology to clinical applications[J].Am J Cardiol,2002,90(10C):40L-48L.
[16]Kinlay S,Libby P,Ganz P.Endothelial function and coronary artery disease[J].Curr Opin Lipidol,2001,12(4):383-389.
[17]Knowles JW,Reddick RL,Jennette JC,Shesely EG,Smithies O,Maeda N.Enhanced atherosclerosis and kidney dysfunction in eNOS(-/-)Apoe(-/-)mice are ameliorated by enalapril treatment[J].J Clin Invest,2000,105(4):451-458.
[18]Ignarro LJ,Buga GM,Wood KS,Byrns RE,Chaudhuri G.Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide[J].Proc Natl Acad Sci USA,1987,84(24):9265-9269.
[19]Huang PL,Huang Z,Mashimo H,Bloch KD,Moskowitz MA,Bevan JA,et al.Hypertension in mice lacking the gene for endothelial nitric oxide synthase[J].Nature,1995,377(6546):239-242.
[20]Hyndman ME,Parsons HG,Verma S,Bridge PJ,Edworthy S,Jones C,et al.The T-786-->C mutation in endothelial nitric oxide synthase is associated with hypertension[J].Hypertension,2002,39(4):919-922.
[21]Azumi H,Inoue N,Ohashi Y,Terashima M,Mori T,Fujita H,et al.Superoxide generation in directional coronary atherectomy specimens of patients with angina pectoris:important role of NAD(P)H oxidase[J].Arterioscler Thromb Vasc Biol,2002,22(11):1838-1844.
[22]Ungvari Z,Csiszar A,Kaley G.Vascular inflammation in aging[J].Herz,2004,29(8):733-740.
[23]Hansson GK.Inflammation,atherosclerosis,and coronary artery disease[J].N Engl J Med,2005,352(16):1685-1695.
[24]Stocker R,Keaney JF Jr.Role of oxidative modifications in atherosclerosis[J].Physiol Rev,2004,84(4):1381-1478.
丹皮酚对高脂血清损伤的人内皮细胞的保护作用
牛成伟1,周晓慧1,张金环2,徐倩1,曹凯1
(承德医学院1.生化教研室,2.附属医院,河北承德067000)
目的观察丹皮酚对高脂血清损伤的人脐静脉内皮细胞(HUVECs)的保护作用,并探讨其作用机制。方法20%高脂血清作用于HUVECs细胞24 h制备高脂血清损伤模型。丹皮酚组细胞加入20%高脂血清24 h后再分别加入丹皮酚124,247和495 μmol·L-1。采用倒置显微镜观察HUVECs形态学变化;MTT法检测细胞存活率;用硝酸还原酶法检测一氧化氮(NO)含量;用RT-PCR法检测内皮型一氧化氮合酶(eNOS)mRNA的表达。结果与正常对照组相比,模型组中大部分细胞出现片状分离和脱落,然而丹皮酚干预后细胞形态趋于正常。丹皮酚能够显著提高细胞存活率(P<0.01)。经丹皮酚124,247和495 μmol·L-1干预后,细胞存活率从模型组的(53.0±10.1)%依次升高至(68.4±9.1)%,(84.5±6.7)%,(98.1±7.5)%。丹皮酚能够显著提高NO含量和eNOS mRNA水平(P<0.01)。NO含量从模型组的(54±4)μmol·L-1依次升高至79±6,115±5和(136±6)μmol·L-1;eNOS mRNA的表达水平由模型组的0.215±0.060增加至0.451±0.045,0.563±0.013,0.704±0.068。结论丹皮酚可能通过上调高脂血清损伤人脐静脉内皮细胞eNOS的表达促进NO的合成,从而发挥其对高脂血清损伤内皮细胞的保护作用。
丹皮酚;内皮细胞;一氧化氮;一氧化氮合酶
河北省科技厅资助项目(07276101D-47)
曹凯,E-mail:caokai58@163.com,Tel:(0314)2290999
2010-12-02接受日期:2011-04-13)
R963,R285.5
A
1000-3002(2011)05-0413-06
10.3867/j.issn.1000-3002.2011.05.001
Atherosclerosis(AS)is the main pathological basis of cardiovascular disease and remains the most important risk factor for cardiovascular disease.Hypothesis explains AS as that initiation with some injury to the endothelial cells lining the artery walls causes endothelial dysfunction,which medicates the chronic inflammation and finally induces formation of AS.Endothelial dysfunction plays a critical role in the pathogenesis of AS.Moreover,recent experimental and clinical data have shown that endothelial dysfunction precedes the formation of arteriosclerotic lesions and is associated with an increased risk for future cardiovascular events[1],which suggests a crucial role of endothelial dysfunction for the clinical outcome in patients.Of all high risk factors,more evidence has emerged pointing to hyperlipidemia as an independent risk factor in the induction of endothelial dysfunction[2-3].
The project supported by Science and Technology Bureau of Hebei Province(07276101D-47)
Biography:NIU Cheng-wei(1982-),master degree,main research field is cardiovascular pharmacology.
CAO Kai,Tel:(0314)2290999,E-mail:caokai58@163.com
(本文编辑:付良青)
