Synthesis,Crystal Structure and Antitumor Activity of a New Trinuclear Ni(Ⅱ)Complex Ni3(C14H8N3O5)2(C5H5N)4
2010-11-09QIUXiaoYangXUMaoTianZHUHaiLiangLIUWeiShengZHAOWenXian
QIU Xiao-YangXU Mao-TianZHU Hai-LiangLIU Wei-ShengZHAO Wen-Xian*,
(1Department of Chemistry,Shangqiu Normal College,Shangqiu,Henan 476000)
(2State Key Laboratory of Pharmaceutical Biotechnology,Nanjing University,Nanjing 210093)
(3State Key Laboratory of Applied Organic Chemistry,Lanzhou University,Lanzhou 730000)
Synthesis,Crystal Structure and Antitumor Activity of a New Trinuclear Ni(Ⅱ)Complex Ni3(C14H8N3O5)2(C5H5N)4
QIU Xiao-Yang*,1,2XU Mao-Tian1ZHU Hai-Liang2LIU Wei-Sheng3ZHAO Wen-Xian*,1
(1Department of Chemistry,Shangqiu Normal College,Shangqiu,Henan476000)
(2State Key Laboratory of Pharmaceutical Biotechnology,Nanjing University,Nanjing210093)
(3State Key Laboratory of Applied Organic Chemistry,Lanzhou University,Lanzhou730000)
研究简报
The title complex Ni3(C14H8N3O5)2(C5H5N)4has been synthesized by the reaction of 2-hydroxy-N′-(4-nitrobenzoyl)benzohydraizide with nickel acetate in pyridine solution.Its molecular structure was characterized by elemental analysis,IR spectra and X-ray crystal structure determination.Crystal data for this compound:Monoclinic,space group P21/c,Mr=1089.00,a=0.24927(5)nm,b=0.16140(3)nm,c=0.12181(2)nm,β=94.59(3)°,V= 4.885 2(17)nm3,Z=4,Dc=1.481 Mg·m-3,F(000)=2 232,R1=0.049 7,wR2=0.106 8(observed reflections with I>2σ(I)) and R1=0.1051,wR2=0.1194 (all reflections),GOF=1.021.The complex was evaluated for their antitumor activities against two kinds of cell lines (K562 and BGC)by MTT method.A preliminary bioactivity study indicates that the complex shows distinct antitumor activity.CCDC:627252.
Ni(Ⅱ)complex;synthesis;crystal structure;antitumor activity
Transition metal complexes containing hydrazide derivative and its analogs ligands have been of great interest for many years[1-10].The complexes play an important role in the development of bioinorganic chemistry related to enzymatic reactions[2],antibacterial[3], antifungal[4],and antitumor[5].Such compounds share a common hydrazide pharmacophore as one of the structural requirement for antitumor activity.To the best of our knowledge,there is no report on the transition metal chelating ability and the antitumor activity of such ligand (Fig.1).Recently,we have described the syntheses,crystal structures and antitumor activities of several transition metal complexes with salicyhydraizide derivative ligand[11-12].As an extension of our work,the syntheses,crystal structures and antitumor activities of a new trinuclear nickel complex with bent structuresare reported herein.Trinuclearnickel complexes with bent structures are rare.As far as we know only Ni3(shi)2(Hpko)2(py)2,Ni3(p-nbzshz)2(C5H5N)4, [Ni3(L2)2(OAc)2(MeOH)(H2O)][BF4]2and[Ni3(oxen)2(en)2] [ClO4]2are reported in the literature[13-16].As expected, our investigation shows that the title complex exhibits antitumor activity against K562 and BGC,which makes it promising for transition metal complexes become novel antitumor agents.

Fig.1 Tautomeric forms of the ligand
1 Experimental
1.1 Reagent and apparatus
All chemicals were of reagent grade and were used as received.The solvents were purified using conventional methods.
Elemental analyses were performed on a CHN-ORapid instrument and were within ±0.4% of the theoretical values.IR spectra were recorded on a Nicolet AVATAR 360 series FTIR instrument using KBr pellets for spectra in the region 4 000~400 cm-1.1H NMR spectra were recorded using DPX-500 Bruker Spectrometer with CDCl3as solvent.
1.2 Synthesis of the ligand
An anhydrous ethanolic solution (20 mL)of salicylhydrazide(0.15 g,1.0 mmol)was added dropwise to the ethyl acetate solution (15 mL)of 4-nitrobenzoyl chloride(0.19 g,1.0 mmol).The reaction mixture was stirred for 4 h at 120℃.Then the solution was concentrated to half of its initial volume and cooled to room temperature.The resulting products were filtered, washed with absolute ethanol,recrystallized from absolute ethanol and then dried in a vacuum desiccator over P2O5.Yield:72%.Anal.Calc.for C14H11N3O5(%): C,55.82;H,3.68;N,13.95.Found(%):C,55.46;H, 3.78;N,13.64.1H NMR (500 MHz,CDCl3):6.96~8.39 (8H,m,Ar),10.75(1H,s,NH),11.04(1H,s,NH),11.80 (1H,s,OH),Main IR absorption (KBr,cm-1):1 692(s), 1 590(s),1 543(m)1 520(m).
1.3 Synthesis of the title complex
2-hydroxy-N′-(4-nitrobenzoyl)benzohydraizide(0.15 g,0.5 mmol)dissolved in absolute ethanol was added to an pyridine solution (1 mL)of nickel acetate(0.09 g, 0.5 mmol)at room temperature.The mixture was stirred at 50℃for 0.5 h to give a deep black solution.Suitable block-shaped black single crystals of the complex for the structure determination were obtained by slow evaporation of the solution in air.Yield:48%on the basis of the ligand.Anal.Calc.for C48H36N10Ni3O10(%): C,52.94;H,3.33;N,12.86.Found(%):C,52.85;H, 3.27;N,12.68.Main IR absorption (KBr,cm-1):1 602 (s),1 563(m),1 518(m).
1.4 X-ray crystal structure analysis
Diffraction data for the complex were collected at 298(2)K using a Bruker SMART APEXII area-detector with Mo Kα radiation(λ=0.071 073 nm).The collected data were reduced with the SAINT[17]program,and empirical absorption correction was performed using the SADABS[18]program.The structure was solved by direct methods and refined by full-matrix least-squares methods on F2by using the SHELXTL[19]software package.All of the non-hydrogen atoms were refined anisotropically.All hydrogen atoms were placed in geometrically idealized positions.The summary of the crystaldata,experimentaldetails and structure refinement parameters are recorded in Table 1.
CCDC:627252.
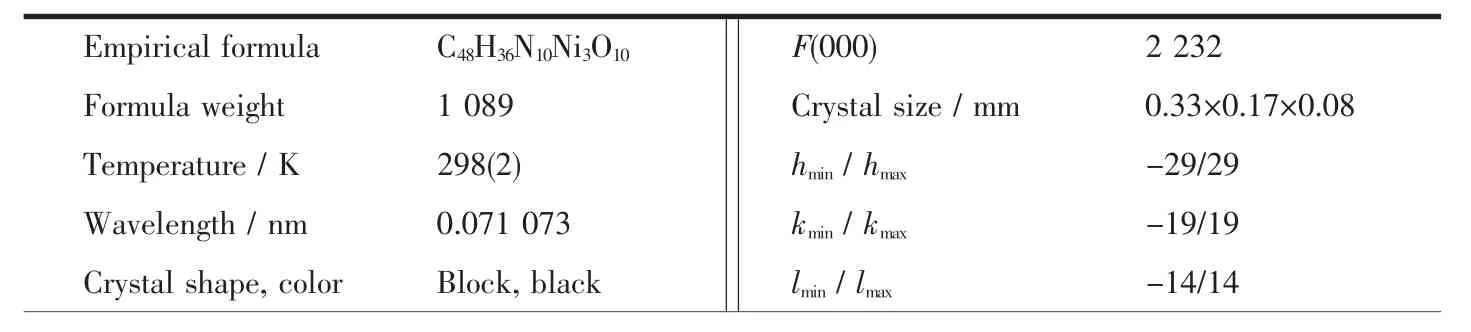
Table 1 Crystal data and structure refinement for the title complex
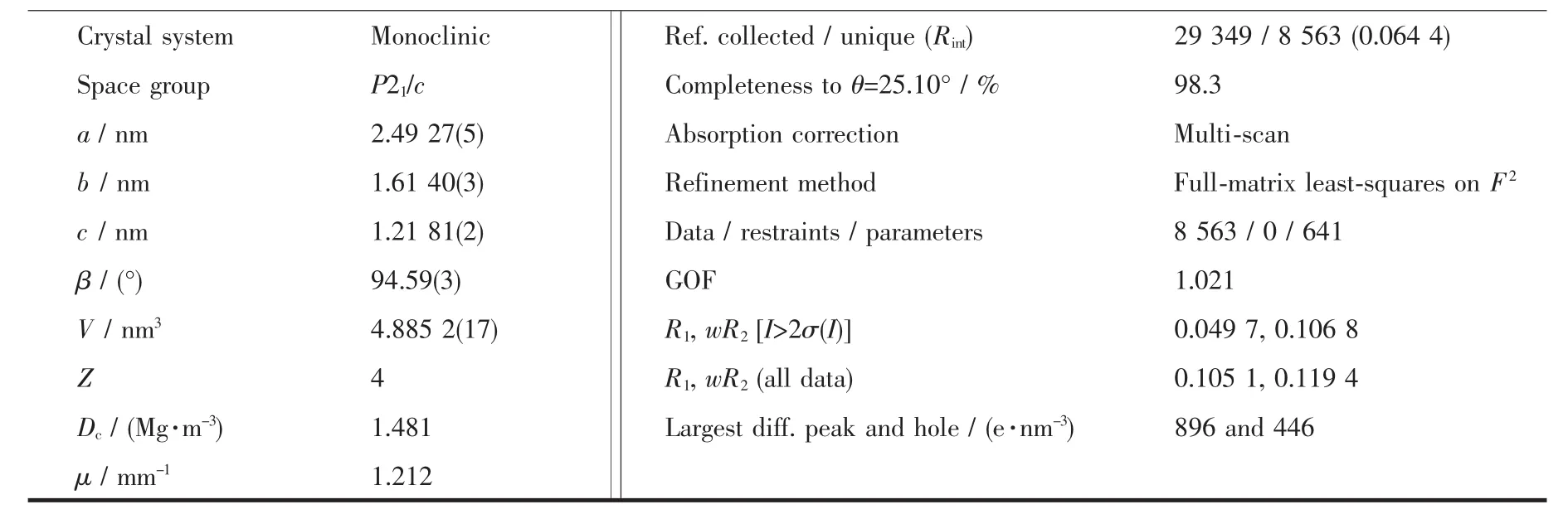
Continued Table 1
1.5 Antitumor activity determination
The antitumor activity of the title complex was assayed against two kinds of selected cancer cell lines (K562 and BGC).Cells were cultured at 37℃under a humidified atmosphere of 5%CO2in RPMI 1640 medium supplemented with 10%fetal serum and dispersed in replicate 96-well plates with 1×104cells/ well.Complex was then added.After 24,48,72 h exposure to the toxins,the cell viability was determined by the[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide](MTT)antitumor assay by measuring the absorbance at 570 nm with an ESILA plate reader.Each test was performed in triplicate.The data represent the mean of three experiments and are expressed as means±SD using Student t test.The IC50value is defined as the concentration needed for a 50%reduction in absorbance calculated from the survival curves.
2 Results and discussion
2.1 IR spectra
The main IR absorption of the ligand and its complex is recorded in Table 2.The strong band at 1 692 cm-1in the ligand may be assigned to νC=Oand disappears in the title complex,indicating participation of enol form in coordination(Fig.1).The sharp band at 1590 cm-1in the ligand underwent a shift of 12 cm-1in complexes confirming the formation of coordinate bond from hydrazide nitrogen to metal ion.From the infrared data,it is concluded that the ligand has linked with the transition metal ions through hydrazide nitrogen atoms, phenoloxygen atom and carbonyloxygen atoms behaving as a penta-dentate ligand.

Table 2 IR spectra of the ligand and the complex cm-1
2.2 Description of crystal structure
The perspective structure and the atomic numbering schemes for the title complex are shown in Fig.2.Selected bond lengths and angles are given in Table 3.

Fig.2 Crystal structure of the title complex with the atom numbering
As shown in Fig.2,the title complex is a neutral tri-nuclear nickel complex with bent structures.The structure of the complex shows that the central Ni(1)(Ⅱ)ion is coordinated by four nitrogen atoms[N(2),N(5),N(9),and(N10)]with Ni-N distances of 0.215 1(3), 0.213 4(3),0.209 7(3),and 0.210 7(3)nm,and two oxygen atoms[O(1)and O(6)]with Ni-O distances of 0.204 0(3)and 0.204 0(3)nm.The central Ni(1)ion is well described as having an octahedron configuration with N(2),N(5),N(9),N(10),O(1),and O(6).But the structures of the terminal Ni ions are different from those of the central Ni ions.The terminal Ni(2)ion is four-coordinated by two nitrogen atoms[N(4)and N(8)] with Ni-N distances of 0.182 4(3)and 0.193 7(4)nm, and two oxygen atoms[O(7)and O(8)]with Ni-O distances of 0.1844(3)and 0.1810(3)nm.The structure of the other terminal Ni(3)ion is similar to that of the Ni(2)ion.The Ni…Ni separations are 0.454 7,0.447 9, and 0.4424 nm,respectively.
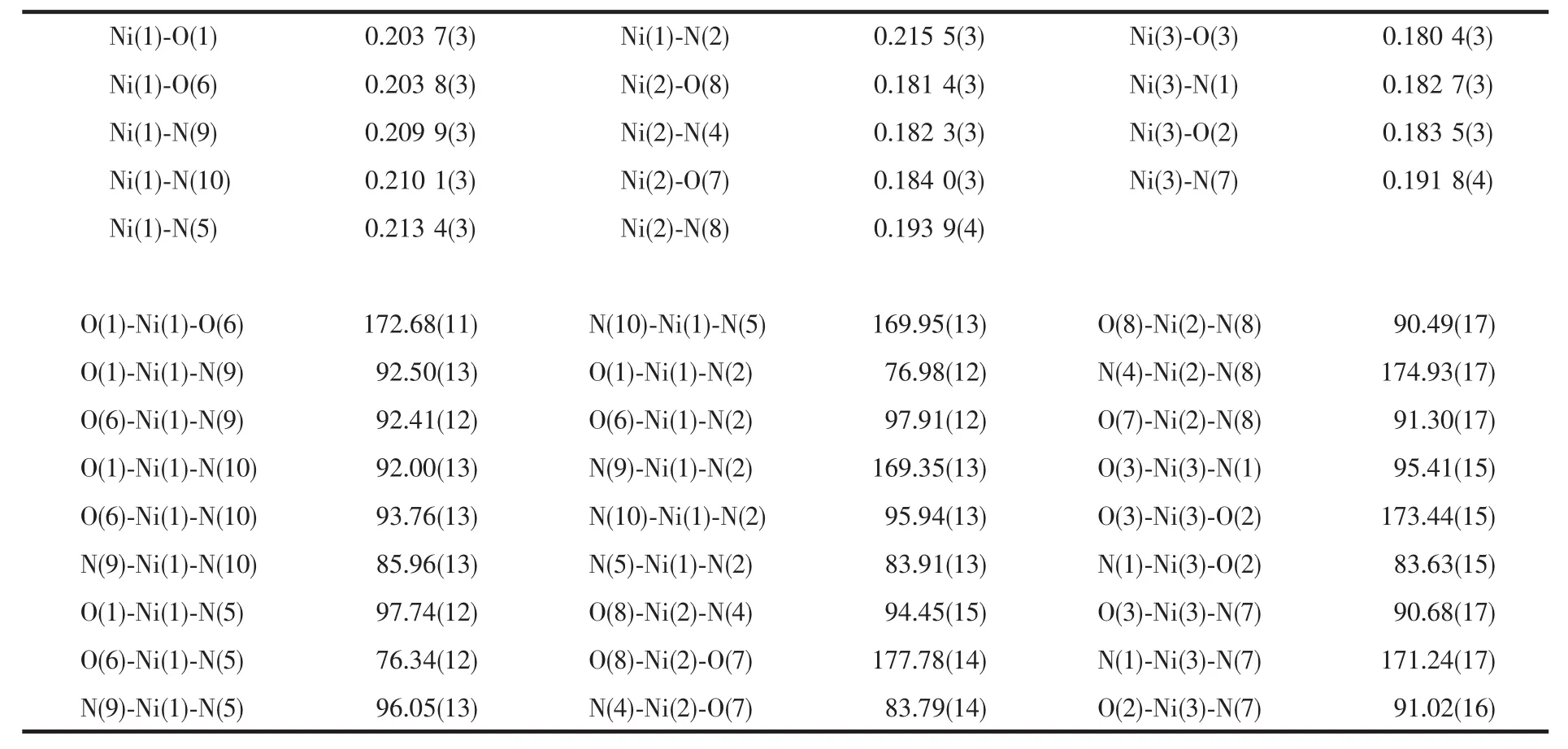
Table 3 Selected bond lengths(nm)and angles(°)of the title complex
The array of three nickel atoms in title complex is bent with a dihedral angle Ni(2)-Ni(1)-Ni(3)of 58.68°, which is smaller than that of the corresponding values of 62.36°,119.0°and 130.9°in the bent trinuclear Ni(Ⅱ)complexes with a same ligand[13]and different ligands[15-16],larger than the corresponding values of 46.50°in the bent trinuclear Ni(Ⅱ)complexes with the different ligand[14].The neighboring Ni(1)…Ni(2)and Ni(1)…Ni(3)interatomic distances are 0.454 6 nm, 0.4479 nm,respectively,which are shorter those in related complex[13].The Ni(1)…Ni(3)separation is 0.4424 nm,longer than that(0.356 nm)reported for the similar bent configuration[14]and shorter than that(0.469 3 nm) of the corresponding complex with a same ligand[13].
2.3 Antitumor activity
The title complex was evaluated for its cytotoxic activities in vitro against two kinds of cell lines(K562 and BGC)by MTT method.The 50% inhibitory concentrations(IC50)of the complexes against K562 and BGC are presented in Table 4.

Table 4 Cytotoxic activity against selected Human tumor cells of the title complex
From the data in Table 4,it suggests that the title complex shows obvious antitumor activity.On the basis of the bioassay result,the nickel complex,as an approach to enhancing inhibitory effect on proliferation of tumor cell lines[20],is worthy of further investigation. Further studies are underway to investigate the physical chemistry of the nickel complex.
3 Conclusion
In this paper,we present the syntheses,crystal structure and antitumor activity of a novel trinuclear transition metal complex with salicyhydraizide derivative ligand.Three Ni atoms in title compound exhibit a square-planar/octahedral/square-planar coordination geometry mode.The title complex reveals a curved Ni3metal arrangement with a Ni(2)-Ni(1)-Ni(3) angle of 58.68°.It should be noted that the molecular structures are remarkably different with the same ligand and samebivalenttransition metalbutdifferent solvents[13].The complex was evaluated for antitumor activities against two kinds of cell lines by MTT method.The study indicates that the complex shows distinct antitumor activity.
[1]Ainscough E W,Brodie A M,Dobbs A J,et al.Inorg.Chim. Acta,1998,267:27-38
[2]CabantchikZI,Moody-HauptS,GordeukVR.FEMSImmunol. Med.Mic.,1999,26:289-298
[3]Rodríguez-Argüelles M C,Ferrari M B,Bisceglie F,et al.J. Inorg.Biochem.,2004,98:313-321
[4]Rodríguez-Argüelles M C,Mosquera-Vázquez S,Tourón-Touceda P,et al.J.Inorg.Biochem.,2007,101:138-147
[5]Kushev D,Grünert R,Spassovska N,et al.J.Inorg.Biochem., 2003,96:469-477
[6]Buss J L,Neuzil J,Grllert N,et al.Biochem.Pharmacol., 2003,65:161-172
[7]LIANG Fang-Zhen(梁芳珍),REN Jian-Cheng(任建成),LI Kun-Cai(李坤彩),et al.Chinese.J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(7):1363-1366
[8]YANG Ming-Xing(杨明星),LIN Shen(林 深),ZHANG Xiao-Feng(张晓凤),et al.J.Mol.Sci.(Fenzi Kexue Xuebao), 2008,24(8):250-256
[9]CAI Zhu-Yu(蔡珠玉),HUANG Zun-Xing(黄尊行).Chem. Res.(Huaxue Yanjiu),2008,19(1):36-38
[10]Walcourt A,Loyevsky M,Lovejoy D B,et al.Int.J.Biochem. Cell B,2004,36:401-407
[11]Zhong X,Wei H L,Liu W S,et al.Bioorg.Med.Chem.Lett., 2007,17:3774-3777
[12]Qiu X Y,Luo Z G,Liu W S,et al.Chin.J.Struct.Chem., 2008,27(6):707-711
[13]Lin S,Yang M X,Liu S X.Polyhedron,2007,26:4793-4798
[14]Alexiou M,Tsivikas I,Dendrinou-Sanara C,et al.J.Inorg. Biochem.,2003,93:256-264
[15]Adams H,Fenton D E,McHugh P E.Inorg.Chem.Commun., 2004,7:147-150
[16]Chen Z N,Zhang H X,Yu K B,et al.Polyhedron,1998,17: 1535-1540
[17]Siemens,SMART and SAINT V4 Software Reference Manual, Siemens Analytical X-ray Systems,Inc.,Madison,Wisconsin, USA,1996.
[18]Sheldrick G M.SADABS.Program for Empirical Absorption CorrectionofAreaDetectorData,Univ.ofGöttingen,Germany, 1996.
[19]Siemens,SHELXTL,Version 5 Reference Manual,Siemens Analytical X-ray Systems,Inc.,Madison,Wisconsin,USA, 1996.
[20]FAN Zhen-Zhong(范振中),FU Xu-Chun(傅旭春),WANG Guo-Ping(王国平),et al.Chinese.J.Inorg.Chem.(Wuji Huaxue Xuebao),2005,21(9):1155-1158
三核镍配合物Ni3(C14H8N3O5)2(C5H5N)4的合成、晶体结构和抗癌活性
仇晓阳*,1,2徐茂田1朱海亮2刘伟生3赵文献*,1
(1商丘师范学院化学系,商丘 476000)
(2南京大学医药生物技术国家重点实验室,南京 210093) (3兰州大学应用有机化学国家重点实验室,兰州 730000)
镍配合物;合成;晶体结构;抗癌活性
O614.81+3
A
1001-4861(2010)02-0355-05
2009-07-27。收修改稿日期:2009-12-02。
仇晓阳,男,39岁,博士,副教授;研究方向:配位化学及生物无机化学。
国家自然科学基金项目(No.20972091),河南省教育厅项目(No.2009A150020),安徽省教育厅项目(No.KJ2008B1780)资助。*
。E-mail:qiuxiaoyang12@163.com,zhwx195812@yahoo.com.cn
猜你喜欢
杂志排行
无机化学学报的其它文章
- Synthesis and Crystal Structure of a Uranylcontaining Complex[U(CO3)3(H2O)2]·2H2O
- Synthesis,Crystal Structure and Luminescent Property of[CdCl(HL)(dpp)(H2O)]n·nH2O
- 一种新奇的d-f异双核配合物[Fe(phen)3]2[FeCe(tiron)3]·6H2O的水热合成、晶体结构和磁性
- Synthesis and Crystal Structure of a Two-Dimensional Hofmann-Type Bimetallic Complex Cu(DMF)2[Pt(CN)4]
- α-Fe2O3空心球的水热法制备及其对苯酚的吸附性能
- TiO2纳米管阵列薄膜制备及生长机理的研究
